Introduction
Cholangiocarcinoma is the second most common type of
primary liver cancer worldwide, following hepatocellular carcinoma
(HCC) (1). A higher number of
mortalities are ascribed to cholangiocarcinoma, compared with HCC,
in England and Wales since mid-1990 (1,2).
Cholangiocarcinoma is rare in the majority of Western countries,
and the rate of incidence ranges from 0.35/100,000 in Canada to
3.36/100,000 in Italy (2).
Conversely, the reported incidences are significantly higher in
certain areas of Asia, including ~5.7–85.0/100,000 in Thailand,
~7.45–7.55/100,000 in China, ~7.10–8.75/100,000 in Korea and
~3.05–3.40/100,000 in Japan (2). In
Taiwan, a modest incidence of cholangiocarcinoma at 4.7/100,000 has
been reported (2).
Cholangiocarcinoma emerges from the dysregulated
proliferation of bile duct epithelial cells, known as
cholangiocytes, and is notorious for its poor prognosis and
response to chemotherapy (3).
Clinically, cholangiocarcinoma is comprised of a group of tumors
with markedly heterogeneous morphology, histology and clinical
presentation (3). Cholangiocarcinoma
may be classified as intrahepatic and extrahepatic types (1–3). The
extrahepatic tumor is further classified into perihilar (Klatskin
tumor) and distal forms (3). However,
in certain cancer registries and epidemiological studies, perihilar
cholangiocarcinoma has been considered as an intrahepatic tumor
(4).
The etiology of cholangiocarcinoma remains largely
unknown (4). Risk factors for
cholangiocarcinoma include old age, primary sclerosing cholangitis,
biliary tree stones and structural anomalies of bile ducts and
liver flukes; however, combined, they account for <30% of
cholangiocarcinoma cases (1).
Numerous molecular changes have been identified in
cholangiocarcinoma, including the inactivation of tumor suppressor
genes [tumor protein 53, anaphase-promoting complex, mothers
against decapentaplegic homolog 4 (SMAD4) and cyclin
dependent kinase inhibitor 2A], somatic mutations or the
upregulation of oncogenes [e.g. Kirsten rat sarcoma (KRas),
c-Myc and human epidermal growth factor receptor 2
(ERBB2)], and other chromosomal anomalies (2).
Surgical resection is applicable to <40% of all
intrahepatic cholangiocarcinoma cases (1,5).
Transcatheter arterial chemoembolization (TACE), radiofrequency
ablation and combination chemotherapy (gemcitabine + cisplatin),
have been used for the treatment of unresectable and recurrent
cholangiocarcinoma (1,2). A number of clinicopathological and
genetic parameters have been identified as poor prognostic factors
following surgical resection, including lymph node metastasis
(1), positive resection margin
(6), perineural invasion (7) and KRas mutations (8). No cancer staging systems and standard of
care guidelines have been globally accepted (9,10).
However, four tumor characteristics, including vascular invasion,
tumor number, lymph node metastasis and distant metastasis, are the
major determinants in two staging systems developed independently
in Japan and the United States to address the post-resection
survival of intrahepatic cholangiocarcinoma (9,10). Three
staging systems are available for perihilar cholangiocarcinoma,
including the American Joint Committee on Cancer
Tumor-Node-Metastasis (TNM) system (11), the Bismuth-Corlette staging system
(12) and the Blumgart modifications
(13). All of these staging systems
correlated poorly with post-resection survival in an earlier
validation study (14).
Previously, through the use of the genome-wide
association method followed by prospective validation, it was
revealed that the germline genotypes of polypeptide
N-acetylgalactosaminyltransferase 14 (GALNT14) may serve as
response predictors for chemotherapy in HCC (15). A leading single nucleotide
polymorphism, rs9679162, was identified to be associated with
chemotherapy response, time-to-tumor progression and overall
survival in a previous study of patients with HCC at Barcelona
Clinic Liver Cancer (BCLC) Stage C (16,17). The
genotypes were also identified to correlate with the therapeutic
response in TACE-treated patients with HCC at BCLC Stage B
(18). It was revealed that the gene
product of GALNT14 was an enzyme catalyzing O-glycosylation
of numerous proteins, including the death receptors (DRs) 4 and 5
(19). O-glycosylation of DR 4/5
increased their sensitivity to extrinsic apoptotic signals
(19). Furthermore, germline
mutations in GALNT14 were associated with an increased risk
of hereditary neuroblastoma (20) and
GALNT14 was recently identified as an embryonic lethal gene
based on studies in consanguineous families (21). Therefore, the association between the
GALNT14 genotype and tumor behavior may not be restricted to
HCC. The present study examined the association between the
prognosis of patients with resected cholangiocarcinoma and the
GALNT14 genotype.
Materials and methods
Patients
Under approval of the Institutional Review Board of
Chang Gung Memorial Hospital (Taoyuan, Taiwan ROC), surgical tissue
samples from 112 patients with cholangiocarcinoma, resected between
January 1999-December 2008, were retrieved from the hospital's
tissue bank, without any specific selection criteria. Written
informed consent was obtained from all patients enrolled in the
present study. Patients' clinical data were subsequently collected
(see Table I), including age, sex,
hepatitis B virus (HBV) surface antigen (HBsAg), anti-hepatitis C
antibody (anti-HCV), cirrhosis, Eastern Co-operative Oncology Group
performance status, biliary tree stones, cholangitis, tumor
characteristics (location, invasion to vessels, perineural
invasion, periductal invasion, lymph node metastasis, tumor number
and size), histology, extrahepatobiliary invasion, resection margin
and the extent of surgical resection. Pre-surgery biochemical data
was collected, including on carcinoembryonic antigen (CEA),
carbohydrate antigen 19–9 (CA-19-9), bilirubin, aspartate
transaminase (AST) and alanine transaminase (ALT).
 | Table I.Clinical and tumor characteristics of
the 112 patients included in the study. |
Table I.
Clinical and tumor characteristics of
the 112 patients included in the study.
| Parameters | Values |
|---|
| Age, years, mean ±
SD | 60.2±10.7 |
| Sex, male (%) | 62 (55.4) |
| HBsAg, positive
(%) | 26 (23.2) |
| Anti-HCV, positive
(%) | 15 (13.4) |
| Cirrhosis, positive
(%) | 19 (17.0) |
| ECOG stage |
|
| 0 | 68 (60.7) |
| 1 | 44 (39.3) |
| Biliary tree stones,
yes (%) | 24 (21.4) |
| Stone-unrelated
cholangitis, yes (%) | 46 (41.0) |
| Tumor
characteristics |
|
| Perihilar, yes
(%) | 25 (22.3) |
| Invasion to vessel,
yes (%) | 30 (26.8) |
| Perineural invasion,
yes (%) | 48 (42.9) |
| Periductal invasion,
yes (%) | 45 (40.2) |
| Lymph node
involvement, yes (%) | 33 (29.5) |
| Tumor number |
|
| 1 | 101 (90.2) |
| 2 | 6 |
| 3 | 1 |
|
>3 | 4 |
| Tumor size, cm, mean
± SD | 6.0±3.2 |
| Histology |
|
| Well
differentiated, yes (%) | 25 (22.3) |
| Mixed
hepatocellular carcinoma, yes (%) | 14 (12.5) |
| Extrahepatobiliary
invasion, yes (%) | 46 (41.1) |
| Resection margin
involvement, yes (%) | 44 (39.3) |
| More than one
segment of resection, | 98 (87.5) |
| yes (%) |
|
| Biochemistry |
|
| CEA,
ng/ml, mean ± SD | 41.0±104.4 |
|
CA-19-9, U/ml, mean ± SD |
8,648.5±26,889.6 |
|
Bilirubin, mg/dl, mean ±
SD | 1.8±3.1 |
| AST,
U/l, mean ± SD | 56.3±69.5 |
| ALT,
U/l, mean ± SD | 62.9±83.4 |
| GALNT14
genotype |
|
| TT
(%) | 35 (31.3) |
| TG
(%) | 55 (49.1) |
| GG
(%) | 22 (19.6) |
GALNT14 genotyping
GALNT14 genotyping was performed on thawed
surgical tissue samples, which were freshly cryopreserved at −70°C
immediately following surgery. DNA was extracted from the tissues
using QIAamp DNA Mini and Blood Mini kits (Qiagen GmbH, Hilden,
Germany) following the manufacturers' protocol. Polymerase chain
reactions were performed using a pair of primers
(5′-TCACGAGGCCAACATTCTAG-3′ and 5′-TTAGATTCTGCATGGCTCAC-3′) to
amplify the DNA fragment containing GALNT14-rs9679162 (95°C,
1 min; 55°C, 1 min; 72°C, 1 min; 30 cycles), followed by direct
sequencing using the conventional Sanger sequencing method
(22). To ensure the accuracy of
genotyping for each sample, polymerase chain reaction was performed
two times and bidirectional sequencing was carried out.
Statistical analysis
Parametric data is presented as mean ± standard
deviation. Dichotomous data is presented as percentage. The
genotype counts of GALNT14-rs9679162 in HapMap Chinese Han
Beijing (CHB) and Metropolitan Denver (CHD) cohorts were retrieved
from the public domain (http://hapmap.ncbi.nlm.nih.gov/). These counts were
compared with those obtained from the present study. Associations
between the GALNT14 rs9679162 genotypes and clinical factors
were analyzed using univariate and multivariate linear regressions.
Genotype distributions were compared using the Cochran-Armitage
Trend test or χ2 test. Loss of follow up was considered
as censored data. Post-resection overall survival was analyzed
using log-rank tests, Kaplan-Meier plots and the Cox proportional
hazards model, where the censorship data occurred prior to the
earliest events were dropped automatically by default of the SPSS
Statistics 13.0 statistical software (SPSS Inc., Chicago, IL, USA).
Statistical significance in the Cox proportional hazards model was
evaluated using Wald tests. All tests were two-tailed. P<0.05
was considered to indicate a statistically significant
difference.
Results
Germline GALNT14 genotypes and tumor
characteristics associated with overall survival in resected
cholangiocarcinoma
Clinicopathological parameters of 112 patients with
surgically resected cholangiocarcinoma are summarized in Table I. Major features of this cohort were
as follows: HBsAg-negative (76.8%), anti-HCV-negative (86.6%),
non-cirrhotic (83%), non-HCC-cholangiocarcinoma-mixed histology
(87.5%) and intrahepatic (77.7%). The frequency of the
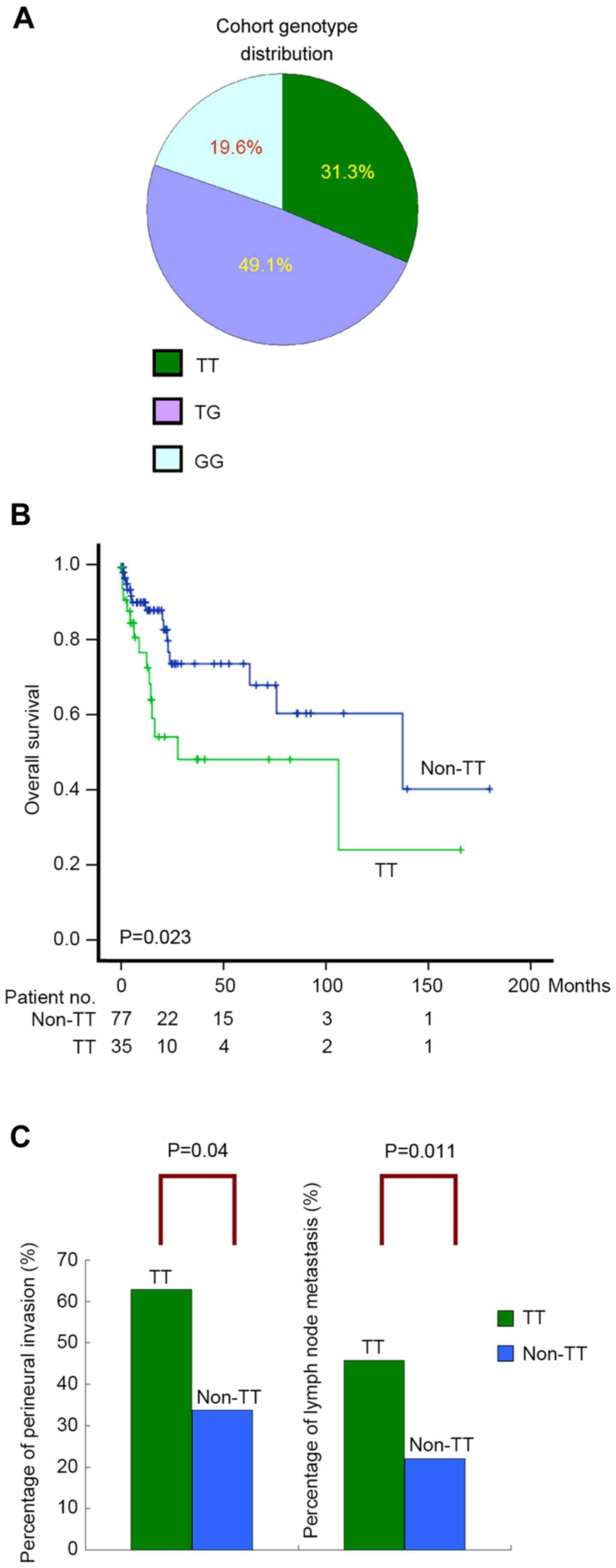
GALNT14-rs9679162 genotypes ‘TT’, ‘TG’ and ‘GG’ were 31.3%,
49.1% and 19.6%, respectively (Fig.
1A), which did not deviate significantly from the ethnic
reference genotype distribution of the HapMap Chinese Han Beijing
(CHB) and Metropolitan Denver (CHD) cohorts (Cochran-Armitage Trend
test, P=0.59 and P=0.46, respectively) (23).
Subsequently, clinicopathological parameters and
GALNT14 genotypes were determined to be correlated with
overall survival using the Cox proportional hazards model. In
previous clinical studies, the genotype-prognosis association was
revealed to be based on comparison of the ‘TT’ and ‘non-TT’
genotypes (including ‘TG’ and ‘GG’) (12–14);
therefore, the same genotype classification was used in the current
study. The median post-resection follow-up time was 14 months
(range, 1–180). Age, sex, liver cirrhosis, cholangitis and biliary
tree stones did not demonstrate significant associations with
overall survival post-resection. By contrast, five tumor
characteristics (vessel invasion, perineural invasion, lymph node
metastasis, largest tumor size and resection margins), two
tumor-associated serum biomarkers (CEA and CA-19-9 levels) and
GALNT14 genotypes, revealed significant associations in
univariate analysis (Table II).
Patients stratified by the ‘TT’ and ‘non-TT’ genotypes demonstrated
distinguishable survival curves in the Kaplan-Meier plot (log-rank,
P=0.023; Fig. 1B). Subsequently,
multivariate analysis was performed on these associated factors,
excluding CEA and CA-19-9, which had not been assessed in the
majority of patients. It was revealed that vascular and perineural
invasions are two independent factors associated with overall
survival (P=0.002 and P=0.001, respectively), whereas
GALNT14 genotypes were not independently associated with
overall survival.
 | Table II.Analysis of clinicopathological and
genotypic parameters for overall survival in 112 patients with
cholangiocarcinoma receiving surgery. |
Table II.
Analysis of clinicopathological and
genotypic parameters for overall survival in 112 patients with
cholangiocarcinoma receiving surgery.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Parameters | No. of
patients | Mean overall
survival time (95% CI) | Hazard ratio (95%
CI) | P-value | Adjusted hazard
ratio (95% CI) | P-value |
|---|
| Age (years) |
|
|
| 0.732 |
|
|
|
≤60 | 57 | 98.5
(69.4–127.4) |
|
|
|
|
|
>60 | 55 | 89.9
(68.6–111.6) | 1.138
(0.544–2.544) |
|
|
|
| Sex |
|
|
| 0.964 |
|
|
|
Female | 50 | 81.8
(57.3–106.3) |
|
|
|
|
|
Male | 62 | 108.6
(78.3–139.3) | 0.983
(0.472–2.472) |
|
|
|
| HBsAg |
|
|
| 0.055 |
|
|
|
Negative | 86 | 73.2
(54.1–92.1) |
|
|
|
|
|
Positive | 26 | 138.2
(103.3–173.3) | 0.346
(0.117–1.117) |
|
|
|
| Anti-HCV |
|
|
| 0.278 |
|
|
|
Negative | 97 | 95.2
(71.6–118.6) |
|
|
|
|
|
Positive | 15 | 64.7
(51.1–78.1) | 0.450
(0.106–1.106) |
|
|
|
| Cirrhosis |
|
|
| 0.341 |
|
|
| No | 93 | 96.1
(72.1–120.1) |
|
|
|
|
|
Yes | 19 | 74.1
(54.7–93.7) | 0.558
(0.168–1.168) |
|
|
|
| ECOG stage |
|
|
| 0.062 |
|
|
| 0 | 68 | 109.5
(83.8–135.8) |
|
|
|
|
|
>0 | 44 | 37.7
(20.9–54.9) | 2.135
(0.964–4.964) |
|
|
|
| Biliary tree
stones |
|
|
| 0.092 |
|
|
| No | 88 | 106.4
(81.9–130.9) |
|
|
|
|
|
Yes | 24 | 37.9
(13.1–62.1) | 2.130
(0.883–5.883) |
|
|
|
| Cholangitis
(stone-unrelated) |
|
|
| 0.081 |
|
|
| No | 66 | 76.1
(53.6–98.6) |
|
|
|
|
|
Yes | 46 | 118.6
(84.8–152.8) | 0.491
(0.221–1.221) |
|
|
|
| Tumor
characteristics Location |
|
|
| 0.157 |
|
|
|
Intrahepatic | 87 | 102.8
(74.9–130.9) |
|
|
|
|
|
Perihilar | 25 | 68.2
(38.4–97.4) | 1.742
(0.808–3.808) |
|
|
|
| Invasion to
vessels |
|
|
| 0.010c |
| 0.002c |
| No | 82 | 109.2
(83.9–134.9) |
|
|
|
|
|
Yes | 30 | 39.8
(25.5–54.5) | 2.838
(1.285–6.285) |
| 3.853
(1.624–9.624) |
|
| Perineural
invasion |
|
|
|
<0.001c |
| 0.001c |
| No | 64 | 141.6
(115.0–168.0) |
|
|
|
|
|
Yes | 48 | 51.6
(29.8–73.8) | 4.812
(2.126–10.126) |
| 5.086
(1.894–13.894) |
|
| Periductal
invasion |
|
|
| 0.997 |
|
|
| No | 67 | 99.3
(71.2–127.2) |
|
|
|
|
|
Yes | 45 | 85.3
(59.5–111.5) | 1.002
(0.472–2.472) |
|
|
|
| Lymph node
involvement |
|
|
|
<0.001c |
| 0.308 |
| No | 79 | 119.3
(92.1–146.1) |
|
|
|
|
|
Yes | 33 | 45.5
(15.6–75.6) | 3.756
(1.796–7.796) |
| 1.645
(0.632–4.632) |
|
| Tumor number |
|
|
|
|
|
|
| 1 | 101 | 105.8
(82.1–129.1) |
|
|
|
|
|
>1 | 11 | 68.1
(27.9–108.9) | 1.361
(0.471–3.471) |
|
|
|
| Largest tumor size
(cm) |
|
|
| 0.008a |
| 0.113 |
|
≤5.25 | 56 | 116.5
(86.5–146.5) |
|
|
|
|
|
>5.25 | 56 | 69.0
(38.8–99.8) | 2.839
(1.306–6.306) |
| 2.147
(0.835–5.835) |
|
| Histology |
|
|
| 0.654 |
|
|
| Well
differentiated | 25 | 65.0
(49.0–81.0) |
|
|
|
|
|
Moderate/poorly
differentiated | 87 | 96.3
(71.5–121.5) | 1.249
(0.472–3.472) |
|
|
|
| Mixed
hepatocellular carcinoma |
|
|
| 0.126 |
|
|
| No | 98 | 85.1
(64.1–106.1) |
|
|
|
|
|
Yes | 14 |
| 0.037
(0.001–2.001) |
|
|
|
| Extrahepatobiliary
invasion |
| b |
| 0.268 |
|
|
| No | 66 | 102.3
(75.5–129.5) |
|
|
|
|
|
Yes | 46 | 85.9
(59.9–111.9) | 1.532
(0.720–3.720) |
|
|
|
| Resection
margin |
|
|
| 0.015c |
| 0.180 |
| R0 | 68 | 113.3
(87.4–139.4) |
|
|
|
|
| R1 | 44 | 37.8
(26.1–49.1) | 2.654
(1.207–5.207) |
| 1.736
(0.776–3.776) |
|
| Resection
method |
|
|
| 0.993 |
|
|
|
Mono-segmental | 14 | 58.1
(33.8–82.8) |
|
|
|
|
| >1
segment | 98 | 101.7
(77.6–125.6) | 1.005
(0.346–2.346) |
|
|
|
| Biochemistry CEA
(ng/ml)a |
|
|
|
<0.001c |
|
|
|
≤5.1 | 36 | 133.8
(102.5–165.5) |
|
|
|
|
|
>5.1 | 33 | 47.6
(20.6–74.6) | 6.069
(2.332–15.332) |
|
|
|
| CA-19-9
(U/ml)a |
|
|
|
<0.001c |
|
|
|
≤107 | 41 | 139.4
(111.0–167.0) |
|
|
|
|
|
>107 | 29 | 29.7
(15.8–43.8) | 14.531
(4.195–50.195) |
|
|
|
| Bilirubin
(mg/dl) |
|
|
| 0.371 |
|
|
|
≤0.9 | 59 | 97.2
(71.5–123.5) |
|
|
|
|
|
>0.9 | 53 | 100.1
(67.3–133.3) | 1.396
(0.642–2.642) |
|
|
|
| AST (U/l) |
|
|
| 0.356 |
|
|
|
≤31.5 | 56 | 103.0
(73.6–132.6) |
|
|
|
|
|
>31.5 | 56 | 92.2
(62.8–121.8) | 1.415
(0.677–2.677) |
|
|
|
| ALT (U/l) |
|
|
| 0.087 |
|
|
|
≤32.0 | 58 | 115.6
(84.7–146.7) |
|
|
|
|
|
>32.0 | 54 | 79.7
(51.6–107.6) | 1.926
(0.908–4.908) |
|
|
|
| Chemotherapy |
|
|
| 0.577 |
|
|
| No | 72 | 15.6
(2.2–29.2) |
|
|
|
|
|
Yes | 40 | 33.2
(6.7–59.7) | 0.807
(0.380–1.380) |
|
|
|
| GALNT14
genotype |
|
|
| 0.027c |
| 0.897 |
|
Non-TT | 77 | 113.6
(86.0–141.0) |
|
|
|
|
| TT | 35 | 71.6
(39.1–104.1) | 2.282
(1.098–4.098) |
| 0.948
(0.424–2.424) |
|
Germline GALNT14 genotypes are
independently correlated with perineural invasion and lymph node
metastasis in resected cholangiocarcinoma
The genotype-prognosis association was exclusively
observed in the univariate analysis, and not in the multivariate
analysis, which suggested that the genotype may have an
unrecognized association with the tumor characteristics. In
consideration of this, the present study further investigated the
correlations between genotypes and the evaluated
clinicopathological parameters, using univariate and multivariate
linear regressions. Amongst all the clinicopathological parameters,
two tumor characteristics, perineural invasion and lymph node
metastasis, were determined to be independently associated with the
GALNT14 genotype ‘TT’ (multivariate analysis, P=0.035 and
P=0.005, respectively; Table III).
The percentage of perineural invasion was significantly higher in
patients with the ‘TT’ genotype, compared with those with a
‘non-TT’ genotype (P=0.004; Fig. 1C).
Similarly, the frequency of lymph node metastasis was significantly
higher in patients with the ‘TT’ genotype, compared with the
‘non-TT’ genotype (P=0.011; Fig.
1C).
 | Table III.Linear regression analysis for
correlation between clinicopathological factors and the
GALNT14 ‘TT’ genotype. |
Table III.
Linear regression analysis for
correlation between clinicopathological factors and the
GALNT14 ‘TT’ genotype.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameters | β | 95% CI of β | P-value | β | 95% CI of β | P-value |
|---|
| Sex, male | 0.059 | −0.117–0.117 | 0.510 |
|
|
|
| Age, years | −0.003 | −0.011–0.011 | 0.438 |
|
|
|
| HBsAg-positive | −0.006 | −0.214–0.214 | 0.952 |
|
|
|
|
Anti-HCV-positive | −0.053 | −0.310–0.310 | 0.684 |
|
|
|
| ECOG status | −0.103 | −0.281–0.281 | 0.255 |
|
|
|
| Cirrhosis | 0.067 | −0.166–0.166 | 0.568 |
|
|
|
| Biliary tree
stones | 0.080 | −0.133–0.133 | 0.461 |
|
|
|
| Cholangitis
(stone-unrelated) | 0.068 | −0.112–0.112 | 0.456 |
|
|
|
| Tumor
characteristics |
|
|
|
|
|
|
|
Perihilar | 0.113 | −0.097–0.097 | 0.288 |
|
|
|
|
Invasion to vessels | 0.120 | −0.077–0.077 | 0.231 |
|
|
|
|
Perineural invasion | 0.255 | 0.085–0.085 |
0.004a | 0.185 | 0.014–0.014 |
0.035a |
|
Periductal invasion | −0.002 | −0.181–0.181 | 0.979 |
|
|
|
| Lymph
node involvement | 0.060 | 0.027–0.027 |
0.001a | 0.050 | 0.015–0.015 |
0.005a |
| Tumor
number >1 | 0.158 | −0.135–0.135 | 0.289 |
|
|
|
| Tumor
size (cm) | 0.013 | −0.014–0.014 | 0.339 |
|
|
|
|
Moderate/poor
differentiation | −0.113 | −0.322–0.322 | 0.288 |
|
|
|
| Mixed
hepatocellular carcinoma | −0.031 | −0.295–0.295 | 0.819 |
|
|
|
|
Extrahepatobiliary
invasion | 0.134 | −0.042–0.042 | 0.135 |
|
|
|
|
Resection margin involved | 0.009 | −0.170–0.170 | 0.918 |
|
|
|
| >1
segment of resection | 0.031 | −0.234–0.234 | 0.819 |
|
|
|
| Biochemistry |
|
|
|
|
|
|
| CEA,
ng/ml | 0.001 | 0.000–0.000 | 0.050 |
|
|
|
|
CA-19-9, ×1,000 U/ml | 0.004 | −0.001–0.001 | 0.103 |
|
|
|
|
Bilirubin, mg/dl | 0.002 | −0.026–0.026 | 0.870 |
|
|
|
| AST,
U/l |
−0.00000257 | −0.001–0.001 | 0.997 |
|
|
|
| ALT,
U/l | 0.000 | −0.001–0.001 | 0.667 |
|
|
|
Subsequently, the genotype distributions (‘TT’ vs.
‘non-TT’) were investigated in subgroups of patients stratified by
the presence or absence of the two aggressive characteristics,
perineural invasion and lymph node metastasis. The distribution of
patients with perineural invasion deviated significantly from the
reference cohorts, HapMap-CHB and CHD (CHB, P=0.049; CHD, P=0.034),
where the ‘TT’ type was particularly enriched (23). No such deviations were identified in
patients without perineural invasion (CHB, P=0.144; CHD, P=0.236).
Similarly, the genotype distribution in patients with lymph node
metastasis also deviated significantly from the ethnic references
(CHB, P=0.046; CHD, P=0.032). No such deviations were identified in
patients without lymph node metastasis (CHB, P=0.337; CHD,
P=0.501).
Discussion
Aggressive growth of cholangiocarcinoma occurred
sporadically with no known major predisposition etiology (3,4).
Therefore, it was conjectured in the present study that personal
genetic background may contribute to onset, progression and
malignant phenotypes. The present study demonstrated that the
GALNT14 genotype ‘TT’ was independently associated with two
known predictors of unfavorable prognosis in cholangiocarcinoma:
Perineural invasion and lymph node metastasis. The ‘TT’ genotypes
were revealed to be particularly enriched in patients with these
aggressive phenotypes, as compared with the ethnic references. Such
enrichment may be due to patients with the ‘TT’ type being more
likely to develop these two aggressive tumor characteristics. In
the survival analysis, the association between the GALNT14
genotype and overall survival was only observed in univariate
analysis, and not in multivariate analysis. It is possible that the
tumor characteristics-prognosis association in the multivariate
analysis concealed the underlying genotype-prognosis association.
As the genotype is determined at birth, while perineural invasion
and lymph node metastasis are identified at the time of surgical
treatment, a causal association may be inferred that the genotype
first affected the development of these two tumor characteristics,
which subsequently altered the postoperative prognosis (Fig. 1).
The present study was an extension of previous
studies on HCC and cholangiocarcinoma, as a result of their
similarities and differences (3,15,17,18,23,24).
Cholangiocarcinoma arises from bile duct epithelial cells, whereas
HCC originates from hepatocytes (3,24).
Cholangiocarcinoma and HCC have fundamental differences in their
oncogenic pathways (3,24). HCC is primarily caused by viral
hepatitis, including chronic HBV rather than HCV, dependent on the
region of the world (25). By
contrast, even in Taiwan, which is a HBV hyperendemic region, the
percentage of HBsAg-positivity among patients with
cholangiocarcinoma is low (23.2%), as compared with in patients
with HCC (>60%) (1,2,26). An
additional difference is that HCC often develops from a cirrhotic
background; however, the majority of patients with
cholangiocarcinoma in the present study were non-cirrhotic (83%)
(26). Despite these differences,
certain HCC and cholangiocarcinoma cases have overlapping histology
patterns, demonstrating mixed tissue types (3). As GALNT14 encodes an enzyme that
catalyzes the O-glycosylation of numerous proteins, it is possible
that differential O-glycosylation environments associated with
various GALNT14 genotypes may result in the distinct tumor
characteristics of cholangiocarcinoma and HCC (19). Further studies focusing on the
underlying molecular mechanisms are required to clarify this
point.
In conclusion, patients with the GALNT14
genotype ‘TT’ are associated with two aggressive tumor
characteristics: Perineural invasion and lymph node metastasis.
This genotype was therefore associated with an unfavorable overall
survival.
Acknowledgements
The present study was supported by the Chang Gung
Memorial Hospital (grant nos. CMRPG1B0571, CIRPG3B0032, CLRPG3C0011
and CLRPG3C0012). The authors thank Miss Hui-Chin Chen, Dr Ji-Wei
Lin, Miss Yen-Ling Chuang, Miss Wen-Hsin Kuo, Miss Hsiao-Chih Yu,
Miss Hsiu-Ting Wang and Miss May-Ling Tsao from the Liver Research
Center, Chang Gung Memorial Hospital for their excellent technical
assistance.
References
|
1
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and
treatment. Hepatology. 48:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bragazzi MC, Carpino G, Venere R, Semeraro
R, Gentile R, Gaudio E and Alvaro D: Cholangiocarcinoma:
Epidemiology and risk factors. Transl Gastrointest Cancer. 1:21–32.
2012.
|
|
5
|
Shaib YH, Davila JA, Henderson L, McGlynn
KA and El-Serag HB: Endoscopic and surgical therapy for
intrahepatic cholangiocarcinoma in the United States: A
population-based study. J Clin Gastroenterol. 41:911–917. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng CT, Chu YY, Yeh CN, Huang SC, Chen
MH, Wang SY, Tsai CY, Chiang KC, Chen YY, Ma MC, et al: Peritumoral
SPARC expression and patient outcome with resectable intrahepatic
cholangiocarcinoma. Onco Targets Ther. 8:1899–1907. 2015.PubMed/NCBI
|
|
7
|
Shirai K, Ebata T, Oda K, Nishio H,
Nagasaka T, Nimura Y and Nagino M: Perineural invasion is a
prognostic factor in intrahepatic cholangiocarcinoma. World J Surg.
32:2395–2402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen TC, Jan YY and Yeh TS: K-ras mutation
is strongly associated with perineural invasion and represents an
independent prognostic factor of intrahepatic cholangiocarcinoma
after hepatectomy. Ann Surg Oncol. 19 Suppl 3:S675–S681. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nathan H, Aloia TA, Vauthey JN, Abdalla
EK, Zhu AX, Schulick RD, Choti MA and Pawlik TM: A Proposed staging
system for intrahepatic cholangiocarcinoma. Ann Surg Oncol.
16:14–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okabayashi T, Yamamoto J, Kosuge T,
Shimada K, Yamasaki S, Takayama T and Makuuchi M: A new staging
system for mass-forming intrahepatic cholangiocarcinoma: Analysis
of preoperative and postoperative variables. Cancer. 92:2374–2383.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaiteerakij R, Harmsen WS, Marrero CR,
Aboelsoud MM, Ndzengue A, Kaiya J, Therneau TM, Sanchez W, Gores GJ
and Roberts LR: A new clinically based staging system for perihilar
cholangiocarcinoma. Am J Gastroenterol. 109:1881–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paul A, Kaiser GM, Molmenti EP, Schroeder
T, Vernadakis S, Oezcelik A, Baba HA, Cicinnati VR and Sotiropoulos
GC: Klatskin tumors and the accuracy of the Bismuth-Corlette
classification. Am Surg. 77:1695–1699. 2011.PubMed/NCBI
|
|
13
|
Matsuo K, Rocha FG, Ito K, D'Angelica MI,
Allen PJ, Fong Y, Dematteo RP, Gonen M, Endo I and Jarnagin WR: The
Blumgart preoperative staging system for hilar cholangiocarcinoma:
Analysis of resectability and outcomes in 380 patients. J Am Coll
Surg. 215:343–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zervos EE, Osborne D, Goldin SB,
Villadolid DV, Thometz DP, Durkin A, Carey LC and Rosemurgy AS:
Stage does not predict survival after resection of hilar
cholangiocarcinomas promoting an aggressive operative approach. Am
J Surg. 190:810–815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang KH, Lin CC and Yeh CT: GALNT14 SNP
as a potential predictor of response to combination chemotherapy
using 5-FU, mitoxantrone and cisplatin in advanced HCC.
Pharmacogenomics. 12:1061–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin WR, Hsu CW, Chen YC, Chang ML, Liang
KH, Huang YH and Yeh CT: GALNT14 genotype, α-fetoprotein and
therapeutic side effects predict post-chemotherapy survival in
patients with advanced hepatocellular carcinoma. Mol Clin Oncol.
2:630–640. 2014.PubMed/NCBI
|
|
17
|
Yeh CT, Liang KH, Lin CC, Chang ML, Hsu CL
and Hung CF: A single nucleotide polymorphism on the GALNT14 gene
as an effective predictor of response to chemotherapy in advanced
hepatocellular carcinoma. Int J Cancer. 134:1214–1224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang KH, Lin CL, Chen SF, Chiu CW, Yang
PC, Chang ML, Lin CC, Sung KF, Yeh C, Hung CF, et al: GALNT14
genotype effectively predicts the therapeutic response in
unresectable hepatocellular carcinoma treated with transcatheter
arterial chemoembolization. Pharmacogenomics. 17:353–366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wagner KW, Punnoose EA, Januario T,
Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF,
Totpal K, et al: Death-receptor O-glycosylation controls tumor-cell
sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med.
13:1070–1077. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Mariano M, Gallesio R, Chierici M,
Furlanello C, Conte M, Garaventa A, Croce M, Ferrini S, Tonini GP
and Longo L: Identification of GALNT14 as a novel neuroblastoma
predisposition gene. Oncotarget. 6:26335–26346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shamseldin HE, Tulbah M, Kurdi W, Nemer M,
Alsahan N, Al Mardawi E, Khalifa O, Hashem A, Kurdi A, Babay Z, et
al: Identification of embryonic lethal genes in humans by
autozygosity mapping and exome sequencing in consanguineous
families. Genome Biol. 16:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ihle MA, Fassunke J, König K, Grünewald I,
Schlaak M, Kreuzberg N, Tietze L, Schildhaus HU, Büttner R and
Merkelbach-Bruse S: Comparison of high resolution melting analysis,
pyrosequencing, next generation sequencing and immunohistochemistry
to conventional Sanger sequencing for the detection of p.V600E and
non-p.V600E BRAF mutations. BMC Cancer Cancer Cancer. 14:132014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang KH, Yang PC and Yeh CT: Genotyping
the GALNT14 gene by joint analysis of two linked single nucleotide
polymorphisms using liver tissues for clinical and geographical
comparisons. Oncol Lett. 8:2215–2220. 2014.PubMed/NCBI
|
|
24
|
Inokawa Y, Inaoka K, Sonohara F, Hayashi
M, Kanda M and Nomoto S: Molecular alterations in the
carcinogenesis and progression of hepatocellular carcinoma: Tumor
factors and background liver factors. Oncol Lett. 12:3662–3668.
2016.PubMed/NCBI
|
|
25
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeh CT, Huang YH, Liang KH, Chang ML, Hsu
CW, Chen YC, Chen TC, Yeh TS and Lee WC: Segregation of signaling
proteins as prognostic predictors for local recurrence and distant
metastasis in hepatocellular carcinoma. Int J Oncol. 44:491–504.
2014.PubMed/NCBI
|