Introduction
Gastric carcinoma, a gastrointestinal malignancy, is
the second leading cause of cancer-associated mortality worldwide
(1). There are more new gastric
cancer cases every year in China than in any other country
(2). Despite advances in the
diagnosis and treatment strategies, the 5-year overall survival
rate of gastric cancer is <25%, which makes the prognosis for
gastric cancer patients one of the poorest among all malignant
cancer types (3).
Gastric cancer is a type of highly heterogeneous
malignancy in terms of histology and genetics; therefore, it is
difficult to forecast the clinical outcomes using traditional
histological classification (4).
Current clinical treatments for gastric cancer, such as surgery,
radiotherapy and chemotherapy, have limited efficacy, thus
highlighting the importance of advanced treatment strategies to
prolong patient survival. The occurrence and development of gastric
cancer are identified to be a multistage process with multigene
regulation involving oncogenes and tumor suppressor genes. It has
been reported recently that certain novel oncogenes and suppressor
genes contribute to the early diagnosis and targeted therapy of
gastric cancer (5,6). An in-depth study on the molecular
mechanisms of gastric carcinogenesis and progression is important
to develop novel therapeutic targets and to improve clinical
outcomes (5,7–10).
A number of cellular processes are involved in the
regression or progression of gastric cancer. Among them, the
insulin-like growth factor (IGF) signaling pathway plays a
significant role. The IGF system, as an important modifier of tumor
cell proliferation, growth and survival, is composed of IGF-I and
-II and their receptors, the IGF-I receptor and
IGF-II/mannose-6-phosphate receptor, and a family of six
high-affinity IGF binding proteins (11). IGF-I and IGF-II are known to stimulate
growth, differentiation and the proliferative abilities of various
tumor cells. IGF dysregulation is involved in tumor development,
and increased IGF activity has been detected in certain tumors
(5). IGF-II acts as an autocrine
growth factor in certain tumors and tumor cell lines, and
contributes to tumorigenesis. The higher expression level and rate
of IGF-II compared with IGF-I in local tumor tissues has been
reported in several studies (12–17),
revealing the more significant role of IGF-II in gastric
carcinogenesis and development in comparison to IGF-I.
IGF binding protein (IGFBP)-6 is a member of a
family that includes six high-affinity IGFBPs, which participate in
the regulation of the activities of IGF and IGF-independent effects
(18). IGFBP-6 is unique among the
IGFBPs due to its 20- to 100-fold higher binding affinity for
IGF-II than IGF-I (19–25), making it a relatively distinctive
IGF-II inhibitor. IGFBP-6 can inhibit IGF-II actions in
vitro and in vivo by preventing IGF-II from binding to
receptors on the surface of tumor cells, directly inhibiting the
tumorigenic properties of IGF-II in an IGF-II-dependent manner
(18,26). In addition, a number of studies have
reported that it may also inhibit growth and modulate cell
migration and apoptosis in an IGF-II-independent manner via
interactions with several plasma, extracellular matrix and cell
surface molecules (18,24,26,27).
However, to date, there have been no studies on the
expression information and prognostic importance of IGFBP-6 in
primary gastric adenocarcinoma. Therefore, the expression level of
IGFBP-6 was assessed using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), western blotting and
immunohistochemical (IHC) staining in the present study.
Additionally, the association between IGFBP-6, clinicopathological
features and prognostic significance was investigated in gastric
adenocarcinoma patients.
Patients and methods
Approval and consent
The Research Ethics Committee of the Sun Yat-sen
University Cancer Center (SYSUCC; Guangzhou, Guangdong, China)
approved the present study, and each patient provided written
informed consent prior to the study.
Patients and tissue specimens
This study retrospectively analyzed
clinicopathological information from 263 gastric adenocarcinoma
patients who underwent surgery between October 2003 and October
2005 at SYSUCC. The patients were selected according to the
following criteria: i) Histopathologically proven gastric
adenocarcinoma; ii) received gastrectomy with D1/D2
lymphadenectomy; iii) complete follow-up information; iv) no
chemotherapy or radiotherapy administered prior to surgery; v) no
synchronous malignancies, remnant or recurrent gastric cancer; and
vi) no perioperative mortality. Every patient underwent radical
gastrectomy with D2 lymphadenectomy, which was performed by by
experienced surgeons using standard procedures in line with the
Japanese Gastric Cancer Association guidelines (28). The study consisted of 181 men and 82
women, with a median age of 60 years (range, 17–85 years). The
histological classification was graded based on the World Health
Organization classification criteria (29). Tumor-Node-Metastasis (TNM) staging was
estimated according to the Union for International Cancer Control
(UICC) seventh edition (30).
Fresh gastric cancer tumor tissue and matched normal
tissue were obtained from a cohort of 48 consecutive gastric cancer
patients who underwent gastrectomy at SYSUCC between February 2004
and February 2005. The postoperative tissue samples were immersed
in RNAlater (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and were stored at −80°C until RNA extraction. The tumor
tissue and matched normal tissue (obtained >2 cm away from the
tumor edge) were confirmed pathologically.
RNA extraction and RT-qPCR
The total RNA of the specimens was extracted
according to the manufacturer's instructions using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). To avoid DNA
contamination, RNAse-free DNAse I was added. RNA quality and
quantity were determined using a NanoDrop spectrophotometer (Thermo
Fisher Scientific, Inc.). First-strand cDNA was synthesized by
reverse transcription using M-MLV reverse transcriptase (Promega
Corporation, Madison, WI, USA) and 2µg total RNA. The cDNA was then
subjected to RT-qPCR to evaluate the relative expression levels of
Image result for glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
as an internal control) and IGFBP-6. The Applied Biosystems ABI
7900HT machine was used to measure the binding of SYBR Green I to
double-stranded DNA in gene specific amplification. The 15-µl
reactions contained cDNA, SYBR Green master mix (Invitrogen; Thermo
Fisher Scientific, Inc.) and each pair of oligonucleotide primers.
The sequences of the sense and antisense primers were as follows:
IGFBP6 forward, 5′-TGGAGCTGTCATCACTCAAC-3′ and reverse,
5′-GCCAACACCAACACTCTTTC-3′; and GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′ and reverse,
5′-CCCAATACGACCAAATCCGTT-3′. The PCR cycling conditions were as
follows: 95°C for 10 min, and 45 cycles of melting at 95°C for 30
sec, followed by 60°C for 1 min. The relative quantity and
regression curves of all specimens were calculated by the
instrument's software (SDS 2.0). GAPDH was selected as the internal
control gene for normalizing the relative expression of the target
gene to the geometric mean. qPCR was performed in triplicate in
96-well plates and repeated twice separately. The normalized final
data were analyzed using the 2−∆∆Cq method (31).
Western blotting
The tumor and non-tumor tissues from the gastric
cancer patients were homogenized in radioimmunoprecipitation assay
lysis buffer. Following centrifugation (4,000 × g) at 4°C for 15
min, the total proteins were collected and the protein
concentrations were determined. The protein samples, in equal
amounts of 30 µg, were transferred to polyvinylidene fluoride
membranes through 12% SDS-PAGE gels. Next, the membranes were
incubated with IGFBP-6 polyclonal antibody (catalog no. ab109765;
1:1,000 dilution) or anti-β-actin antibody (catalog no. ab16039;
1:2,000 dilution) (both Abcam, Cambridge, UK), overnight at 4°C,
after immersion in 5% skimmed milk for 60 min to block non-specific
binding sites. Subsequent to being washed, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(catalog no. ab6759; 1:5,000 dilution; Abcam) for 2 h at room
temperature, followed by washing again and analysis using an
electrochemiluminescence system (Pierce; Thermo Fisher Scientific,
Inc.).
Immunohistochemistry
All specimens from the patients were processed with
formalin-fixation and paraffin-embedding, followed by the formation
of 2-μm thick slides, which were subsequently deparaffinized and
rehydrated. Subsequently, the sections were soaked in
ethylenediaminetetraacetic acid (pH 8.0), followed by heating for
antigen retrieval. After blocking the endogenous peroxidase with
hydrogen peroxide solution, the sections were soaked in IGFBP-6
antibody (catalog no. SC-6007; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at a dilution of 1:500, overnight at 4°C. The
sections were then washed and immersed with secondary antibody
(OriGene Technologies, Inc., Beijing, China) for 30 min at room
temperature. Finally, the reaction signals were visualized with
diaminobenzidine tetrahydrochloride, and all of the sections were
counterstained with hematoxylin.
Semi-quantitative methods
Two experienced pathologists who were blinded to the
clinical parameters evaluated these specimens. Deeper discussion
was required to reach a consensus when faced with discrepancies.
The total IGFBP-6 immunostaining score was estimated based on the
positive cell percentage (the percentage of tumor cells positively
staining versus the total cells) and the staining intensity, as
described previously (32–34). The positive cell percentages were
identified as follows: <5% (negative), score of 0; 5–25%
(sporadic), score of 1; 25–50% (focal), score of 2; and >50%
(diffuse), score of 3. The staining intensities of no staining (−),
weakly-stained (+), moderately-stained (++) and strongly-stained
(+++) were scored as 0, 1, 2 and 3, respectively. The total IGFBP-6
IHC expression score was the product of the aforementioned factors,
which ranged from 0 to 9. IGFBP-6 expression was grouped into low
expression (scores of 0–3) and high expression (scores of 4–9).
Statistical analysis
Associations between the clinicopathological
features and IGFBP-6 expression were evaluated with the
χ2 test and Pearson's index. The overall survival curves
and survival differences were analyzed by the Kaplan-Meier method
and log-rank test. Multivariate analysis was assessed using the Cox
proportional-hazard regression model. The predictive ability of the
two models including and excluding IGFBP-6 expression and other
prognostic clinicopathological variables were compared based on
Akaike information criterion (AIC) and Harrell's concordance index
(c-index). The minimum AIC value indicates the optimum predictive
prognosis model with the least clinicopathological data loss and
inefficiency. Harrell's c-index was an index to evaluate the
predictive accuracy, with a value much closer to 1.0 implying
higher accuracy. All data were analyzed statistically using the
SPSS 18.0 software package (SPSS, Inc., Chicago, IL, USA), and
statistical significance was considered to be indicated by
P<0.05 in a two-tailed test.
Results
RT-qPCR of IGFBP-6 mRNA
expression
The IGFBP-6 transcriptional levels were detected by
RT-qPCR in 48 pairs of post-operative samples, including tumor
tissue and normal tissue, from gastric cancer patients. The IGFBP-6
mRNA levels were significantly decreased in the tumor tissue of 36
(75.0%) patients compared with those in the normal tissue
(P=0.0073; Fig. 1).
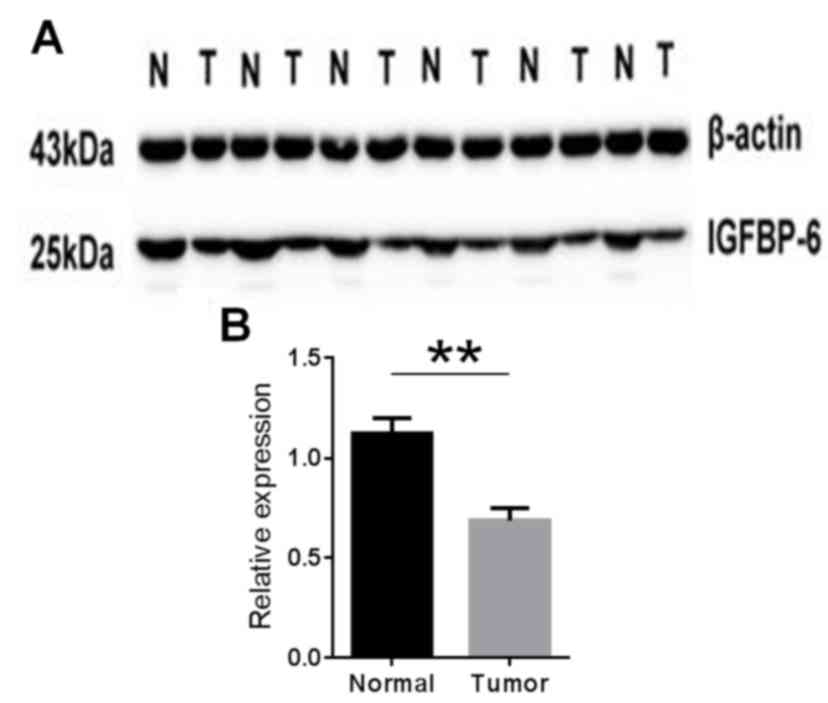
Western blotting of IGFBP-6
expression
The IGFBP-6 protein expression level in gastric
cancer tissue specimens was detected by western blotting. The
results showed IGFBP-6 expression was decreased in the tumor tissue
compared with the matched normal tissue in 33 (68.8%) of the 48
patients (P=0.016; Fig. 2). These
findings were consistent with those of the RT-qPCR.
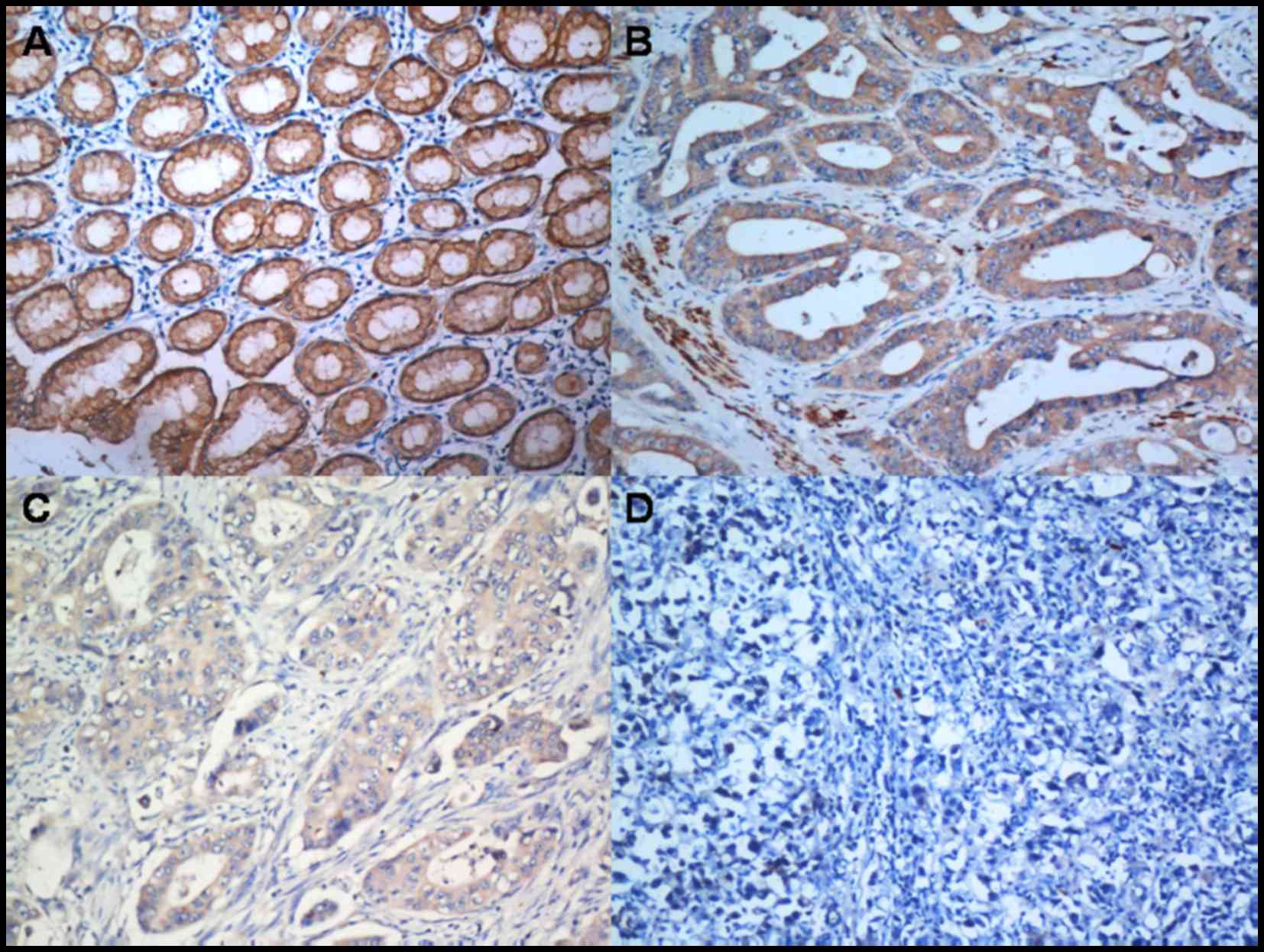
Correlation between IGFBP-6 expression
and clinicopathological characteristics
To further determine the role of IGFBP-6, the
expression level of IGFBP-6 protein in paraffin-embedded tissue
slides, including tumor tissue and matched normal tissue, were
examined by immunohistochemistry in 263 gastric cancer patients
(Fig. 3). IGFBP-6 protein was
localized positively in the cytoplasm upon IHC staining.
As aforementioned, all patients were categorized
into two groups according to the IGFBP-6 expression levels. The
correlations between IGFBP-6 expression and the clinicopathological
features are listed in Table I.
IGFBP-6 expression was significantly decreased in
poorly-differentiated (G3) carcinomas compared with
well-differentiated (G1) and moderately-differentiated (G2)
carcinomas (P=0.001). There were significant associations between
the reduced expression of IGFBP-6 and larger tumor size
(P<0.001) and the presence of a palliative gastrectomy
(P=0.015). Reduced IGFBP-6 expression was also significantly
associated with an increased depth of tumor invasion (T3/4a/4b;
P<0.001) and lymph node metastasis (P<0.001). Additionally,
the majority of the patients in stage III (72.4%) and stage IV
(80.7%) exhibited a reduced IGFBP-6 expression level
(P<0.001).
 | Table I.Association between IGFBP-6
expression and the clinicopathological features of patients with
gastric cancer. |
Table I.
Association between IGFBP-6
expression and the clinicopathological features of patients with
gastric cancer.
|
|
| IGFBP-6
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients, n | Low (n=170) | High (n=93) | P-value |
|---|
| Age, years |
0.912 |
|
≤60 | 160 | 103 | 57 |
|
|
>60 | 103 | 67 | 36 |
|
| Gender |
0.096 |
|
Male | 181 | 111 | 70 |
|
|
Female | 82 | 59 | 23 |
|
| Tumor size, cm |
<0.001a |
|
≤5.0 | 179 | 102 | 77 |
|
|
>5.0 | 84 | 68 | 16 |
|
| Histological
grade |
0.001a |
|
Well-differentiated
(G1)/moderately-differentiated (G2) | 70 | 34 | 36 |
|
|
Poorly-differentiated
(G3) | 193 | 136 | 57 |
|
| Location |
0.228 |
|
Proximal | 150 | 104 | 46 |
|
|
Distant | 105 | 59 | 46 |
|
|
Total |
8 |
7 |
1 |
|
| Radical
resection |
0.015a |
|
Yes | 228 | 141 | 87 |
|
| No | 35 | 29 |
6 |
|
| Tumor invasion
(T) |
<0.001a |
|
T1+T2 | 51 | 14 | 37 |
|
|
T3+T4a/T4b | 212 | 156 | 56 |
|
| Nodal status
(N) |
<0.001a |
| No | 69 | 31 | 38 |
|
|
Yes | 194 | 139 | 55 |
|
| Metastasis status
(M) |
0.070 |
| M0 | 237 | 149 | 88 |
|
| M1 | 26 | 21 |
5 |
|
| TNM staging |
<0.001a |
| I | 29 |
1 | 28 |
|
| II | 63 | 43 | 20 |
|
|
III | 145 | 105 | 40 |
|
| IV | 26 | 21 |
5 |
|
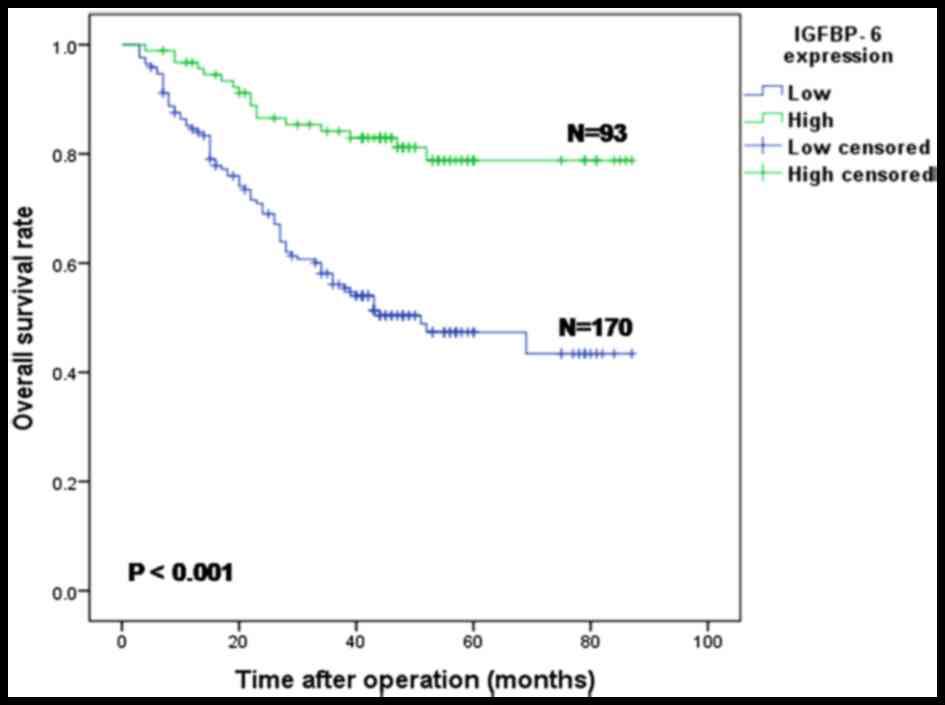
Correlation between IGFBP-6 expression
and patient survival
The median survival time and overall survival rate
of all 263 patients were 58 months (3–87 months) and 56.0%,
respectively. Compared with the IGFBP-6 low expression group, the
overall survival rate (78.8 vs. 43.4%) and the 5-year overall
survival rate (78.8 vs. 47.4%) in the high expression group were
significantly improved (both P<0.001; Fig. 4).
Univariate and multivariate
analyses
The associations between IGFBP-6 expression and
other clinicopathological features and the prognosis of gastric
cancer patients were examined by univariate and multivariate
analyses. According to the results of the univariate analysis, the
following 9 factors were significantly associated with overall
survival: Age (P=0.012), tumor location (P=0.001), tumor size
(P<0.001), histological grade (P=0.002), radical resection
(P<0.001), IGFBP-6 expression (P<0.001), T stage
(P<0.001), N stage (P<0.001) and M stage (P<0.001) based
on the UICC TNM staging, seventh edition (Table II). Next, two Cox regression models
were constructed, which contained the factors of IGFBP-6 expression
or no IGFBP-6 expression, in order to realize the role of IGFBP-6
expression in prognostic prediction. Model A (without IGFBP-6
expression) showed that tumor location (P=0.010), tumor size
(P=0.001), histological grade (P=0.012), tumor infiltration degree
(P=0.018), lymph node metastases (P<0.001) and distant
metastases (P=0.008) were independent prognostic predictors in
gastric cancer patients (Table II).
Model B (with IGFBP-6 expression) identified that tumor location
(P=0.008), tumor size (P=0.007), IGFBP-6 expression (P=0.014),
tumor infiltration degree (P=0.046), lymph node metastases
(P<0.001) and distant metastases (P=0.004) were independent
prognostic prediction factors in gastric cancer patients (Table II). Compared with the IGFBP-6 high
expression group, the relative risk of mortality was 2.104 times
higher in the IGFBP-6 low expression group (hazard ratio, 2.104;
95% confidence interval, 1.162–3.807; P=0.014). Furthermore, AIC
and Harrell's c-index were used to evaluate the predictive accuracy
of the two prognostic models. The results showed that the model
including IGFBP-6 expression (AIC, 924.881; c-index, 0.878)
possessed superior prognostic value compared with the model
excluding IGFBP-6 expression (AIC, 947.164; c-index, 0.825).
 | Table II.Univariate and multivariate survival
analyses of clinicopathological variables for 263 gastric carcinoma
patients. |
Table II.
Univariate and multivariate survival
analyses of clinicopathological variables for 263 gastric carcinoma
patients.
| A, Model without
IGFBP6 expression |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 1.513 | 0.994–2.303 | 0.054 |
|
|
|
| Age, years (≥60 vs.
<60) | 1.674 | 1.120–2.504 |
0.012a | 1.437 | 0.953–2.166 | 0.083 |
| Location
(distal/proximal/total) | 0.475 | 0.311–0.725 |
0.001a | 0.574 | 0.375–0.878 |
0.010a |
| Size, cm (>5 vs.
≤5) | 2.392 | 1.595–3.588 |
<0.001a | 1.985 | 1.302–3.025 |
0.001a |
| Histological grade
(G3 vs. G2/G1) | 2.549 | 1.420–4.577 |
0.002a | 2.142 | 1.186–3.867 |
0.012a |
| Radical resection
(no vs. yes) | 4.288 | 2.694–6.824 |
<0.001a | 1.139 | 0.454–2.859 | 0.781 |
| T (T4/T3 vs.
T2/T1) | 5.812 | 2.359–14.317 |
<0.001a | 3.130 | 1.217–8.047 |
0.018a |
| N (yes vs. no) | 4.095 | 2.123–7.899 |
<0.001a | 3.940 | 2.002–7.753 |
<0.001a |
| M (M1 vs. M0) | 5.218 | 3.145–8.656 |
<0.001a | 3.887 | 1.432–10.554 |
0.008a |
|
| B, Model containing
IGFBP6 expression |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Gender (male vs.
female) | 1.513 | 0.994–2.303 | 0.054 |
|
|
|
| Age, years (≥60 vs.
<60) | 1.674 | 1.120–2.504 |
0.012a | 1.457 | 0.965–2.198 | 0.073 |
| Location
(distal/proximal/total) | 0.475 | 0.311–0.725 |
0.001a | 0.562 | 0.367–0.860 |
0.008a |
| Size, cm (>5 vs.
≤5) | 2.392 | 1.595–3.588 |
<0.001a | 1.795 | 1.170–2.753 |
0.007a |
| Histological grade
(G3 vs. G2/G1) | 2.549 | 1.420–4.577 |
0.002a | 1.819 | 0.996–3.321 | 0.052 |
| Radical resection
(no vs. yes) | 4.288 | 2.694–6.824 |
<0.001a | 1.023 | 0.407–2.574 | 0.961 |
| IGFBP-6 expression
(low vs. high) | 3.949 | 2.237–6.971 |
<0.001a | 2.104 | 1.162–3.807 |
0.014a |
| T (T4/T3 vs.
T2/T1) | 5.812 | 2.359–14.317 |
<0.001a | 2.637 | 1.018–6.831 |
0.046a |
| N (yes vs. no) | 4.095 | 2.123–7.899 |
<0.001a | 3.772 | 1.914–7.435 |
<0.001a |
| M (M1 vs. M0) | 5.218 | 3.145–8.656 |
<0.001a | 4.359 | 1.600–11.876 |
0.004a |
Discussion
It has been shown that IGF-I and IGF-II are involved
in cancer development, acting as strong mitogenic agents in
different cell types (35–38). Certain studies have shown that IGF-II
is an autocrine growth factor in a number of tumors; IGF-II was
overexpressed in numerous tumors, including those in ovarian
cancer, functioning by binding to the IGF-I receptor (IGF-IR) and
insulin receptor (IR-A) (39).
Meanwhile, malignant cells that predominantly express IR-A have
been shown to overexpress IGF-II (40–43). As
IGF-II could bind to IGF-IR and IR-A, it has been hypothesized that
when IGF-II binds to these receptors, the signals of mitosis and
antiapoptosis may be activated. In certain regional tissues with
receptors and IGF-II overexpression, the effect of IGF-II is thus
substantially amplified (39). A
powerful autocrine or paracrine growth stimulatory cycle based on
all these key factors is supposed to contribute to the development
of cancer (44). Consistent results
from in vitro studies have suggested that IGF-II is likely
to increase the cellular ability of migration and invasion by
acting on IR-A (43). These findings
indicate that IGF-II is critical in the tumorigenesis, progression
and prognosis of numerous malignancies.
As a relatively specific inhibitor of IGF-II
(20–26), IGFBP-6 could interfere with the
process of cell proliferation, cell growth, cell differentiation
and cell adhesion in numerous types of cells (18). However, IGFBP-6 not only inhibits
IGF-II activity directly, but also develops IGF-II intrinsic
functions, such as cell growth suppression and apoptosis
stimulation (19,25,27,31).
Previous studies demonstrated that IGFBP-6 overexpression in
rhabdomyosarcoma significantly reduced tumor growth in nude mice
(24,45) and stimulated the programmed cell death
of cancer cells (46). IGFBP-6 was
reported to inhibit IGF-II-induced proliferation in colon cancer
cells, but not IGF-I-induced proliferation (47). These in vivo and in
vitro findings hinted that IGFBP-6 suppresses tumor growth. A
number of studies have demonstrated that the development and
progression of a tumor is a multi-factor and multi-step process
that involves various cytokines, proteins and their corresponding
receptors, proteases and chemokines, which are affected by the
tumor microenvironment and genetic and epigenetic factors (48,49).
IGFBP-6 has been shown to regulate the function of IGFs,
particularly IGF-II, which is involved in tumor progression.
Therefore, it is plausible that the loss of IGFBP-6 expression
causes cell dedifferentiation and colony formation, which
accelerate metastasis and tumor invasion. However, to date, IGFBP-6
expression information and its prognostic significance in primary
gastric adenocarcinoma patients have not been evaluated.
The expression of IGFBP-6 at the gene and protein
levels was analyzed using three different methods in the present
study. First, IGFBP-6 expression at the mRNA and protein levels in
post-operative gastric adenocarcinoma samples was investigated by
RT-qPCR and western blotting. The results revealed that IGFBP-6
expression was decreased in tumor tissue compared with that in
matched normal tissue at the mRNA (P=0.0073) and protein (P=0.016)
levels. These preliminary data coincide with the aforementioned
hypothesis that lower expression of IGFBP-6 may play a vital role
in gastric tumorigenesis and development, and function as a tumor
suppressor.
Furthermore, immunohistochemistry confirmed the
expression of IGFBP-6 in the tumor tissues of the gastric
adenocarcinoma patients. IGFBP-6 was expressed in the cytoplasm,
and the staining intensity varied among the different tissues. The
results demonstrated that low IGFBP-6 expression was associated
with a larger tumor size, poorly-differentiated adenocarcinoma
(G3), whole stomach cancer, radical resection, deeper depth of
tumor infiltration (T3 and T4a/b) and lymph node metastasis. Low
IGFBP-6 expression was more likely to be detected in stage III
(73%) and stage IV (81%) patients, findings that were consistent
with the experimental results of RT-qPCR and western blotting.
These findings indicated that IGFBP-6 could be viewed as a
potential suppressor participating in the occurrence and
development of gastric cancer.
According to the results from the Kaplan-Meier
survival analysis, patients with higher expression levels of
IGFBP-6 experienced significantly improved survival outcomes
compared with those with lower expression levels of IGFBP-6.
Univariate analysis demonstrated that decreased IGFBP-6 expression
was significantly associated with the OS rate and the 5-year OS
rate in gastric cancer patients. Multivariate analysis showed that
IGFBP-6 expression (P=0.014), combined with certain other
conventional clinicopathological factors, including tumor location
(P=0.008), tumor size (P=0.007), tumor differentiated degree
(P=0.046), lymph node metastasis status (P<0.001) and distant
metastasis status (P=0.004), were independent prognostic factors.
Furthermore, the AIC and c-index values manifested that the
prognosis model that included IGFBP-6 expression (AIC, 924.881;
c-index, 0.878) possessed a stronger predictive ability than the
model excluding expression (AIC, 947.164; c-index, 0.825) in this
study. These results indicated that the decreased expression of
IGFBP-6 may be useful to identify an unfavorable prognosis in
gastric cancer patients and may consequently be viewed as a
potentially novel prognostic factor. Additionally, these results
indicated that the incorporation of certain gene expression levels,
such as those of IGFBP-6, in traditional clinicopathological
prognostic models may be of benefit to better predict the survival
outcomes of patients.
Although the possible association between the
decreased expression of IGFBP-6 and other cellular factors, and the
exact mechanism of the tumor suppression function of IGFBP-6 have
not been further investigated in the present study, the comparative
quantity of post-operative samples from gastric cancer patients who
underwent uniformly standard treatment enhances the value of the
present results. All the data are conducive to understanding
gastric cancer in more depth.
In conclusion, the data from the present study
demonstrated that the decreased expression of IGFBP-6 predicts poor
clinical outcomes in gastric cancer patients. Nevertheless, the
exact molecular mechanisms and relative signal channel of IGFBP-6
involved in gastric cancer must be investigated further in future
studies. A greater and more thorough comprehension of the
functional mechanism of IGFBP-6 in the genesis and development of
tumors is beneficial to improving clinical outcomes and developing
more effective treatment strategies in gastric cancer patients.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Guangdong Province (2015A030313089), the
Medical Science and Technology Research Foundation of Guangdong
Province (B2014160), the Major Program of Collaborative Innovation
of Guangzhou (201508030042) and the Science and Technology Project
of Fujian Province (2014J01284).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nobili S, Bruno L, Landini I, Napoli C,
Bechi P, Tonelli F, Rubio CA, Mini E and Nesi G: Genomic and
genetic alterations influence the progression of gastric cancer.
World J Gastroenterol. 17:290–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY,
Kuo ML, Chang KJ and Hsieh FJ: Gene expression profile predicts
patient survival of gastric cancer after surgical resection. J Clin
Oncol. 23:7286–7295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: A review. Gastric Cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HS, Cho SB, Lee HE, Kim MA, Kim JH,
Park DJ, Kim JH, Yang HK, Lee BL and Kim WH: Protein expression
profiling and molecular classification of gastric cancer by the
tissue array method. Clin Cancer Res. 13:4154–4163. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oue N, Hamai Y, Mitani Y, Matsumura S,
Oshimo Y, Aung PP, Kuraoka K, Nakayama H and Yasui W: Gene
expression profile of gastric carcinoma: Identification of genes
and tags potentially involved in invasion, metastasis, and
carcinogenesis by serial analysis of gene expression. Cancer Res.
64:2397–2405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hippo Y, Taniguchi H, Tsutsumi S, Machida
N, Chong JM, Fukayama M, Kodama T and Aburatani H: Global gene
expression analysis of gastric cancer by oligonucleotide
microarrays. Cancer Res. 62:233–240. 2002.PubMed/NCBI
|
|
10
|
Yasui W, Oue N, Sentani K, Sakamoto N and
Motoshita J: Transcriptome dissection of gastric cancer:
Identification of novel diagnostic and therapeutic targets from
pathology specimens. Pathol Int. 59:121–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iosef C, Vilk G, Gkourasas T, Lee KJ, Chen
BP, Fu P, Bach LA, Lajoie G, Gupta MB, Li SS and Han VK:
Insulin-like growth factor binding protein-6 (IGFBP-6) interacts
with DNA-end binding protein Ku80 to regulate cell fate. Cell
Signal. 22:1033–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minniti CP, Luan D, O'Grady C, Rosenfeld
RG, Oh Y and Helman LJ: Insulin-like growth factor II
overexpression in myoblasts induces phenotypic changes typical of
the malignant phenotype. Cell Growth Differ. 6:263–269.
1995.PubMed/NCBI
|
|
13
|
Osborne CK, Coronado EB, Kitten LJ,
Arteaga CI, Fuqua SA, Ramasharma K, Marshall M and Li CH:
Insulin-likegrowth factor-II (IGF-II): A potential
autocrine/paracrine growth factor forhuman breast cancer acting via
the IGF-I receptor. Mol Endocrinol. 3:1701–1709. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thompson MA, Cox AJ, Whitehead RH and
Jonas HA: Autocrine regulation of human tumor cell proliferation by
insulin-like growth factor II: An in-vitro model. Endocrinology.
126:3033–3042. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christofori G, Naik P and Hanahan D: A
second signal supplied by insulin-like growth factor II in
oncogene-induced tumorigenesis. Nature. 369:414–418. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cullen KJ, Allison A, Martire I, Ellis M
and Singer C: Insulin-like growth factor expression in breast
cancer epithelium and stroma. Breast Cancer Res Treat. 22:21–29.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho MN, Delgado CH, Owens GA and Steller
MA: Insulin-like growth factor-II participates in the biphasic
effect of a gonadotropin-releasing hormone agonist on ovarian
cancer cell growth. Fertil Steril. 67:870–876. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Firth SM and Baxter RC: Cellular actions
of the insulin-like growth factor binding proteins. Endocr Rev.
23:824–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiefer MC, Schmid C, Waldvogel M,
Schläpfer I, Futo E, Masiarz FR, Green K, Barr PJ and Zapf J:
Characterization of recombinant human insulin-like growth factor
binding proteins 4, 5, and 6 produced in yeast. J Biol Chem.
267:12692–12699. 1992.PubMed/NCBI
|
|
20
|
Bach LA: IGFBP-6 five years on; not so
‘forgotten’? Growth Horm IGF Res. 15:185–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bach LA, Hsieh S, Brown AL and Rechler MM:
Recombinant human insulin-like growth factor (IGF)-binding
protein-6 inhibits IGF-II-induced differentiation of L6A1
myoblasts. Endocrinology. 135:2168–2176. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dake BL, Boes M, Bach LA and Bar RS:
Effect of an insulin-like growth factor binding protein fusion
protein on thymidine incorporation in neuroblastoma and
rhabdomyosarcoma cell lines. Endocrinology. 145:3369–3374. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallicchio MA, Kaun C, Wojta J, Binder B
and Bach LA: Urokinase type plasminogen activator receptor is
involved in insulin-like growth factor-induced migration of
rhabdomyosarcoma cells in vitro. J Cell Physiol. 197:131–138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gallicchio MA, Kneen M, Hall C, Scott AM
and Bach LA: Overexpression of insulin-like growth factor binding
protein-6 inhibits rhabdomyosarcoma growth in vivo. Int J Cancer.
94:645–651. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EJ, Kang YH, Schaffer BS, Bach LA,
MacDonald RG and Park JH: Inhibition of Caco-2 cell proliferation
by all-trans retinoic acid: Role of insulin-like growth factor
binding protein-6. J Cell Physiol. 190:92–100. 2001. View Article : Google Scholar
|
|
26
|
Mohan S and Baylink DJ: IGF-binding
proteins are multifunctional and act via IGF-dependent and
-independent mechanisms. J Endocrinol. 175:19–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sueoka N, Lee HY, Wiehle S, Cristiano RJ,
Fang B, Ji L, Roth JA, Hong WK, Cohen P and Kurie JM: Insulin-like
growth factor binding protein-6 activates programmed cell death in
non-small cell lung cancer cells. Oncogene. 19:4432–4436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sano T: Evaluation of the gastric cancer
treatment guidelines of the Japanese Gastric Cancer Association.
Gan To Kagaku Ryoho. 37:582–586. 2010.(In Japanese). PubMed/NCBI
|
|
29
|
Berlth F, Bollschweiler E, Drebber U,
Hoelscher AH and Moenig S: Pathohistological classification systems
in gastric cancer: Diagnostic relevance and prognostic value. World
J Gastroenterol. 20:5679–5684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wittekind C: 2010 TNM system: On the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Lv L, Pan K, Zhang Y, Zhao JJ,
Chen JG, Chen YB, Li YQ, Wang QJ, He J, et al: Reduced expression
of transcription factor AP-2α is associated with gastric
adenocarcinoma prognosis. PLoS One. 6:e248972011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YF, Wang DD, Zhao BW, Wang W, Yuan SQ,
Huang CY, Chen YM, Zheng Y, Keshari RP, et al: Poor prognosis of
gastric adenocarcinoma with decreased expression of AHRR. PLoS One.
7:e435552012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang CY, Chen YM, Zhao JJ, Chen YB, Jiang
SS, Yan SM, Zhao BW, Pan K, Wang DD, Lv L, et al: Decreased
expression of transcription elongation factor A-like 7 is
associated with gastric adenocarcinoma prognosis. PLoS One.
8:e546712013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Badry OM, Romanus JA, Helman LJ, Cooper
MJ, Rechler MM and Israel MA: Autonomous growth of a human
neuroblastoma cell line is mediated by insulin-like growth factor
II. J Clin Invest. 84:829–839. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stewart CE and Rotwein P: Growth,
differentiation, and survival: Multiple physiological functions for
insulin-like growth factors. Physiol Rev. 76:1005–1026.
1996.PubMed/NCBI
|
|
37
|
Toretsky JA and Helman LJ: Involvement of
IGF-II in human cancer. J Endocrinol. 149:367–372. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
LeRoith D, Baserga R, Helman L and Roberts
CT Jr: Insulin-like growth factors and cancer. Ann Intern Med.
122:54–59. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu L, Katsaros D, Wiley A, de la Longrais
IA Rigault, Risch HA, Puopolo M and Yu H: The relationship of
insulin-like growth factor-II, insulin-like growth factor binding
protein-3, and estrogen receptor-alpha expression to disease
progression in epithelial ovarian cancer. Clin Cancer Res.
12:1208–1214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Denley A, Wallace JC, Cosgrove LJ and
Forbes BE: The insulin receptor isoform exon 11-(IR-A) in cancer
and other diseases: A review. Horm Metab Res. 35:778–785. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frasca F, Pandini G, Scalia P, Sciacca L,
Mineo R, Costantino A, Goldfine ID, Belfiore A and Vigneri R:
Insulin receptor isoform A, a newly recognized, high-affinity
insulin-like growth factor II receptor in fetal and cancer cells.
Mol Cell Biol. 19:3278–3288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalli KR, Falowo OI, Bale LK, Zschunke MA,
Roche PC and Conover CA: Functional insulin receptors on human
epithelial ovarian carcinoma cells: Implications for IGF-II
mitogenic signaling. Endocrinology. 143:3259–3267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sciacca L, Mineo R, Pandini G, Murabito A,
Vigneri R and Belfiore A: In IGF-I receptor-deficient
leiomyosarcoma cells autocrine IGF-II induces cell invasion and
protection from apoptosis via the insulin receptor isoform A.
Oncogene. 21:8240–8250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vella V, Pandini G, Sciacca L, Mineo R,
Vigneri R, Pezzino V and Belfiore A: A novel autocrine loop
involving IGF-II and the insulin receptor isoform-A stimulates
growth of thyroid cancer. J Clin Endocrinol Metab. 87:245–254.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bach LA: Insulin-like growth factor
binding protein-6: The ‘forgotten’ binding protein? Horm Metab Res.
31:226–234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuo YS, Tang YB, Lu TY, Wu HC and Lin CT:
IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis
through regulating EGR-1 expression. J Pathol. 222:299–309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leng SL, Leeding KS, Whitehead RH and Bach
LA: Insulin-like growth factor (IGF)-binding protein-6 inhibits
IGF-II-induced but not basal proliferation and adhesion of LIM 1215
colon cancer cells. Mol Cell Endocrinol. 174:121–127. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saeki N, Ono H, Sakamoto H and Yoshida T:
Genetic factors related to gastric cancer susceptibility identified
using a genome-wide association study. Cancer Sci. 104:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|