Introduction
In previous decades, thiosemicarbazones (TSCs) have
been a focus of chemists and biologists due to their wide range of
pharmacological effects, such as antibacterial, antiviral,
antifungal, and antitumor activity (1–3). Among
TSCs, (N)-heterocyclic TSCs have been extensively investigated as
potential anticancer agents, and 3-aminopyridine-2-carboxaldehyde
TSC (3-AP or triapine) is currently undergoing phase II clinical
trials (4). It has been confirmed
that TSCs play essential antitumor roles through numerous
mechanisms, including ribonucleotide reductase inhibition (5,6),
metal-dependent radical damage (4),
DNA binding (7) and inhibition of
protein synthesis (8). However, it
should be noted that the biological activities of TSCs often show a
high dependence on their substituents (1,4).
Therefore, there is increasing interest in structural modification
of TSC derivatives, with the aim of improving the pharmaceutical
profile of existing candidates or identifying novel derivatives. At
present, the majority of studies have focused on the
structure-activity association of TSCs bearing six-member
heterocycles (9–14). However, the antitumor effects and the
underlying mechanisms of TSCs containing five-member heterocycles
(15), particularly pyrrole, remain
poorly understood. It is well known that a large amount of pyrrole
constitutes, such as sunitinib, are a class of promising anticancer
agents (16). Our previous work has
also shown that certain acylhydrazones bearing a pyrrole unit have
antitumor activity towards hepatocellular carcinoma HepG2 cells
(17). In addition, certain pyrrole
imines can bind to DNA effectively (18). Therefore, a series of TSCs derived
from formyl-pyrrole were synthesized in the present study.
Materials and methods
Reagents
Ethyl-5-formyl-1H-pyrrole-2-carboxylate,
4-substituted thiosemicarbazide, ethanol and methanol were
purchased from Beijing Chemical Works (Beijing, China). Elemental
analyses (concentration of each compound, 1.25×10−5
mol/l) were performed at the Microanalytical Laboratory of the
Department of Chemistry of Lanzhou University (Lanzhou, China)
using the CHN-O-Rapid analyzer (Foss Heraeus GmbH, Hanau, Germany).
1H nuclear magnetic resonance (NMR) spectra were
recorded on a Bruker AV400 NMR spectrometer (Bruker Corporation,
Billerica, MA, USA) in d6-dimethyl sulfoxide (DMSO)
solution, with tetramethylsilane as an internal standard. The
infrared (IR) spectra (ν=4,000–400 cm−1) were determined
by the KBr pressed disc method on a Bruker V70 FT-IR (Bruker
Corporation) spectrophotometer. The single crystal X-ray
diffraction measurements for 1a·CH3OH, 1b and 1e were
determined on a Bruker SMART APEX IICCD diffractometer (Bruker
Corporation) equipped with a graphite-monochromatized Mo-Kα
radiation (λ=0.71073 Å). Semi-empirical absorption correction was
applied to the intensity data using the SADABS program (19). The structures were solved by direct
methods and refined by full matrix least-square on
F2 using the SHELXTL-97 program (20). All non-hydrogen atoms were refined
anisotropically. All H atoms were positioned geometrically and
refined using a riding model.
General synthesis procedure of TSCs
1a-e
Ethyl 5-formyl-1H-pyrrole-2-carboxylate (0.167 g, 1
mmol) and differently 4-substituted thiosemicarbazide (1 mmol) were
mixed in ethanol (5 ml). Subsequent to 0.05 ml of acetic acid being
added, the resultant mixture was kept refluxing for ~4 h. The
progress of the reaction was monitored by thin layer
chromatography. Following completion of the reaction, the separated
solid was filtered and recrystallized in methanol. The single
crystals suitable for X-ray diffraction measurements were obtained
during the recrystallization process.
1a: Yellow powder. Yield 66%. Melting point (M.p.)
205-208°C. IR (max, cm−1): 1,683 (C=O); 1,594 (C=N); and
760 (C=S). 1H NMR (400 MHz, d6-DMSO, ppm):
1.22–1.25 (t, 3H, 8′-CH3, J=8.0 Hz); 4.19–4.25 (q, 2H,
7′-CH2, J=8.0 Hz); 6.46–6.48 (q, 1H, 3′-CH, J=4.0 Hz);
6.73–6.75 (q, 1H, 4′-CH, J=4.0 Hz); 7.80 (s, 1H, 1′-CH); 8.19 (s,
1H, 4-NH); 8.39–8.40 (d, 1H, 4-NH); 11.42 (s, 1H, pyrrole-NH); and
12.09 (s, 1H, 2-NH). Analysis calculated for
C9H12N4O2S: C, 44.99%;
H, 5.03%; and N, 23.32%; found: C, 45.12%; H, 5.13%; and N,
23.47%.
1b: Yellow powder. Yield 75%. M.p. 220–221°C. IR
(max, cm−1): 1,705 (C=O); 1,616 (C=N); and 754 (C=S).
1H NMR (400 MHz, d6-DMSO, ppm): 1.22–1.26 (t, 3H,
8′-CH3, J=8.0 Hz); 3.00–3.01 (t, 3H, 4-CH3);
4.20–4.26 (q, 2H, 7′-CH2, J=8.0 Hz); 6.49–6.50 (q, 1H,
3′-CH, J=4.0 Hz); 6.75–6.76 (q, 1H, 4′-CH, J=4.0 Hz); 7.82 (s, 1H,
1′-CH); 8.73–8.77 (q, 1H, 4-NH); 11.50 (s, 1H, pyrrole-NH); and
11.95 (s, 1H, 2-NH). Analysis calculated for
C10H14N4O2S: C, 47.23%;
H, 5.55%; and N, 22.03%; found: C, 46.99%; H, 5.42%; and N,
22.15%.
1c: Yellow powder. Yield 77%. M.p. 206–208°C. IR
(max, cm−1): 1,680 [C=O stretching (str.)]; 1,611 (C=N
str.); and 763 (C=S). 1H NMR (400 MHz; d6-DMSO,
ppm): 1.10–1.14 (t, 3H, 4-CH3, J=8.0 Hz); 1.22–1.26 (t,
3H, 8′-CH3, J=8.0 Hz); 3.53–3.60 (m, 4H,
4-CH2); 4.20–4.26 (q, 2H, 7′-CH2, J=8.0 Hz);
6.50–6.51 (q, 1H, 3′-CH, J=4.0 Hz); 6.75–6.77 (q, 1H, 4′-CH, J=4.0
Hz); 7.83 (s, 1H, 1′-CH); 8.77–8.80 (t, 1H, 4-NH); 11.44 (s, 1H,
pyrrole-NH); and 11.94 (s, 1H, 2-NH). Analysis calculated for
C11H16N4O2S: C, 49.24%;
H, 6.01%; and N, 20.88%; found: C, 49.30%; H, 5.92%; and N,
20.95%.
1d: Yellow powder. Yield 63%. M.p. 167–169°C. IR
(max, cm−1): 1,673 (C=O); 1,615 (C=N); and 757 (C=S).
1H NMR (400 MHz; d6-DMSO, ppm): 1.18–1.20 (d, 6H,
4-CH3, J=8.0 Hz); 1.23–1.26 (t, 3H, 8′-CH3,
J=8.0 Hz); 4.20–4.25 (q, 2H, 7′-CH2, J=8.0 Hz);
4.44–4.53 (m, 1H, 4-CH); 6.52–6.53 (q, 1H, 3′-CH, J=4.0 Hz);
6.76–6.78 (q, 1H, 4′-CH, J=4.0 Hz); 7.85 (s, 1H, 1′-CH); 8.24–8.26
(d, 1H, 4-NH); 11.40 (s, 1H, pyrrole-NH); and 11.98 (s, 1H, 2-NH).
Analysis calculated for
C12H18N4O2S: C, 51.04%;
H, 6.43%; and N, 19.84%; found: C, 50.89%; H, 6.52%; and N,
19.73%.
1e: Yellow powder. Yield 58%. M.p. 195–197°C. IR
(max, cm−1): 1,675 (C=O); 1,598 (C=N); and 764 (C=S).
1H NMR (400 MHz; d6-DMSO, ppm): 1.22–1.26 (t, 3H,
8′-CH3, J=8.0 Hz); 4.20–4.26 (q, 2H, 7′-CH2,
J=8.0 Hz); 6.56–6.58 (q, 1H, 3′-CH, J=4.0 Hz); 6.77–6.79 (q, 1H,
4′-CH, J=4.0 Hz), 7.37–7.50 (m, 5H, 4-phenyl); 7.93 (s, 1H, 1′-CH);
10.31 (s, 1H, 4-NH); 11.85 (s, 1H, pyrrole-NH); and 12.11 (s, 1H,
2-NH). Analysis calculated for
C15H16N4O2S: C, 56.94%;
H, 5.10%; and N, 17.71%; found: C, 56.85%; H, 5.21%; and N,
17.68%.
Cell culture
The human non-small cell lung adenocarcinoma PC-9,
esophageal squamous carcinoma Eca-109 and gastric adenocarcinoma
SGC-7901 cell lines were obtained from the Cell Culture Center of
the Basic Institute of Medical Sciences, Peking Union Medical
College (Beijing, China), and were routinely maintained at Central
Laboratory of the Affiliated Yixing Hospital of Jiangsu University
(Yixing, China). All three cell lines were cultured in RPMI-1640
medium containing 10% fetal calf serum. Cell culture reagents were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). All cells were maintained in a humidified incubator at 37°C
with a 5% CO2 atmosphere.
MTT assay
MTT assay was applied to evaluate the potential
anticancer abilities of TSCs 1a-e. MTT assay kits were purchased
from Beyotime Institute of Biotechnology (Haimen, China). Briefly,
the three tumor cell lines (PC-9, Eca-109 and SGC-7901) were plated
in 96-well plates in triplicate at a density of at 1×104
cells/well and grown to 75% confluency. Subsequent to treatment
with 1–2,500 µM concentrations of the TSCs for 72 h, the media were
replaced with 10 µl of fresh media containing 0.45 mg/ml MTT
reagent. The cells were incubated for 1 h at 37°C in a 5%
CO2 atmosphere to allow for formation of formazan
crystals. The formazan crystals were dissolved by addition of 100
µl DMSO during a 4-h incubation at 37°C and 5% CO2.
Colorimetric change was measured on a spectrophotometer at an
absorbance of 570 nm. Data was expressed as percentage viability
relative to vehicle. At least three independent experiments were
performed.
Terminal dexynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) staining
TUNEL assay kits were purchased from Kaiji BioTech
(Nanjing, China), and TUNEL staining was applied according to the
manufacturer's protocol. Briefly, the PC-9, Eca-109 and SGC-7901
cells (3×105) were seeded into 24-well plates and
incubated overnight at 37°C in a humidified atmosphere containing
5% CO2. The cells were treated by the TSCs with each
corresponding IC25 dose for 24 h. Cells were fixed in 4%
formaldehyde for 20 min at room temperature (15–25°C) and then
rinsed with PBS for 30 min. Subsequently, the cells were incubated
with 3% hydrogen peroxide in methanol for 10 min at room
temperature followed by washing twice with PBS for 25 min. The TSCs
were incubated with 0.1% Triton X-100 and 0.1% sodium citrate
(Shanghai Shenggong Co., Ltd., Shanghai, China) in water for 30 min
at room temperature. For the negative control, TdT was not added to
the sample, and for the positive control, cells were treated with
DNase I (Tiangen Biotech Co., Ltd., Beijing, China). Subsequent to
washing twice with PBS, pretreated specimens were incubated with 50
µl TdT labeling reaction buffer at 4°C overnight in dark and then
in a humidified atmosphere at 37°C for another 2–3 h. Subsequently,
the slides were incubated with 50 µl streptavidin-HRP for 60 min,
followed by detection with 50 µl diaminobenzidine reagent for 10
min. The cells seeded were observed at ×400 magnification, and
images were captured under an optical microscope. The cells stained
brown were TUNEL positive, and therefore showed apoptotic
characteristics.
Western blot analysis
The PC-9, Eca-109 and SGC-7901 cell lines were
seeded into 6-well plates at a density of 1×106
cells/well and incubated overnight at 37°C in a humidified
atmosphere containing 5% CO2. Cells were treated by the
TSCs with each corresponding half maximal inhibitory concentration
(IC50) dose for 24 h. Following treatment, all the cell
samples were washed twice with PBS and lysed in sample buffer. The
protein concentration of each sample was determined by
bicinchoninic acid protein assay with a commercial kit (cat no.
P0009; Beyotime Institute of Biotechnology). The samples were
separated by SDS-PAGE, transferred to polyvinylidene fluoride
membranes by electroblotting, blocked using 5% dried skimmed milk
overnight at 4°C, and probed overnight at 4°C with primary
antibodies against caspase-3, Bax, Bcl-2 and GAPDH, whcih were
diluted 1:1,000, 1:1,000, 1:1,000 and 1:2,000, respectively, in 5%
bovine serum albumin (cat. no. 10711454001; Roche Applied Science,
Penzberg, Germany). Polyclonal antibodies against caspase-3 (cat.
no. 3004), and the apoptosis regulators Bax (cat. no. 3032) and
Bcl-2 (cat. no. 3195) were obtained from BioVision, Inc. (Milpitas,
CA, USA). Anti-GAPDH (cat. no. E7EUT5) polyclonal antibody was
purchased from Abmart, Inc. (Shanghai, China). The membranes were
incubated with secondary immunoglublin G (IgG) antibodies
conjugated to alkaline phosphatase (AP) for 2 h at room
temperature, followed by two washes with PBS, incubation with an
enhanced chemiluminescence reagent (Lumi Phos™ WB; Thermo Fisher
Scientific, Inc.) and visualization on autoradiography film. The
AP-conjugated anti-mouse IgG (cat. no. A0258) and AP-conjugated
anti-rabbit IgG (cat. no. A0239) secondary antibodies were obtained
from Beyotime Institute of Biotechnology and were diluted 1:1,000
in PBS. Densitometry values were determined using UN-SCAN-IT
software version 6.0 (Silk Scientific, Inc., Orem, UT, USA) in a
ScanJet G4010 flatbed scanner (HP, Inc., Palo Alto, CA, USA).
Statistical analysis
All data were analyzed by SAS 6.12 software (SAS
Institute, Cary, NC, USA) and the results were expressed as the
mean ± standard deviation. To compare the differences between the
groups, statistical significance was analyzed using a one-way
analysis of variance followed by post-hoc comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Synthesis
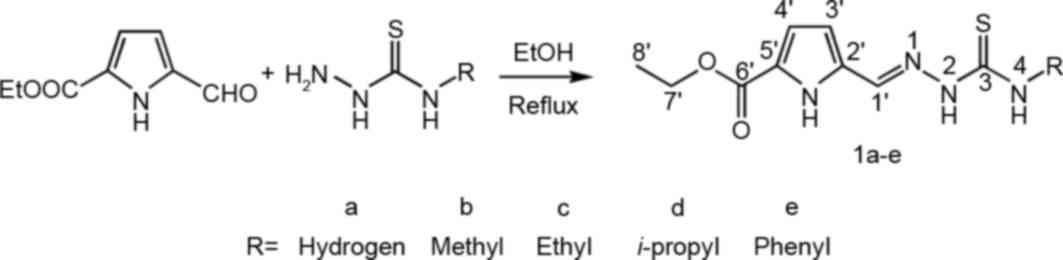
The TSCs 1a-e were synthesized by the condensation
of formyl-1H-pyrrole and 4-substituted thiosemicarbazide in
moderate yield. The reaction scheme is shown in Fig. 1. All TSCs were soluble in ethanol,
methanol, acetonitrile and chloroform, in addition to DMSO and DMF,
but the TSCs were insoluble in water and ethyl ether. The
analytical data for C, H and N confirmed the composition of the
TSCs and the stoichiometry proposed. Furthermore, all TSCs have
similar IR spectra. The strong bands at ~1,680 cm−1 are
attributable to stretch vibrations of the carbonyl group [ν(C=O)]
(17). The peak at ~1610
cm−1 should be assigned to the ν(C=N), and the peak at
~760 cm−1 to ν(C=S) (15).
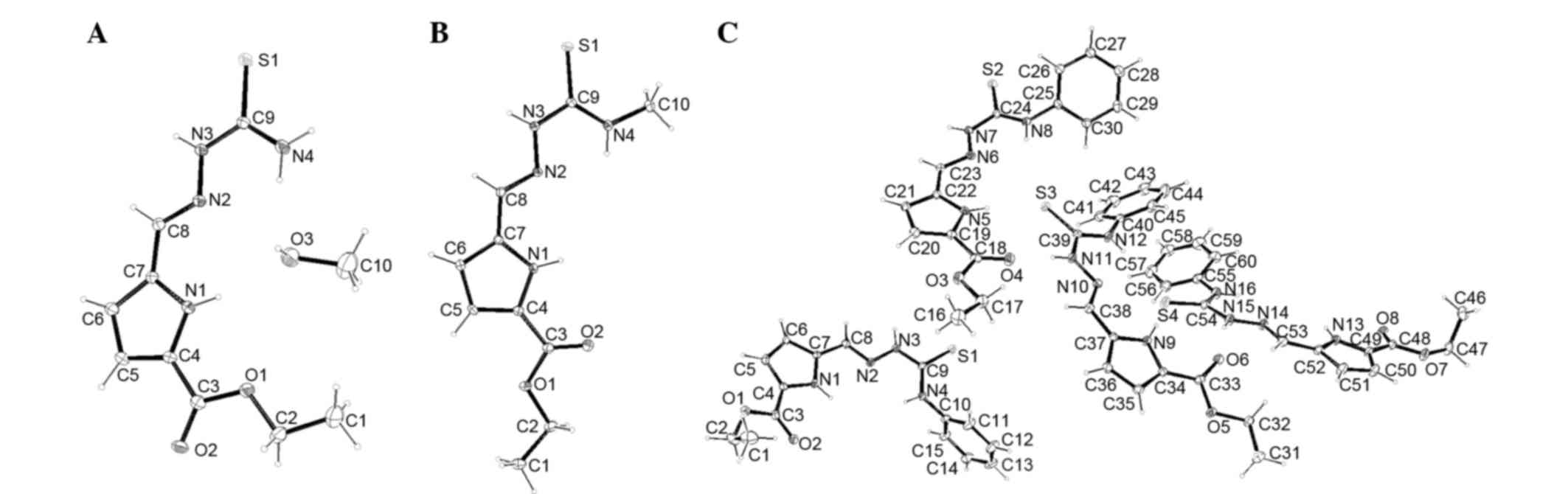
Single crystal X-ray diffraction was found to be
particularly informative in identifying the compound structure.
Details of the crystal parameters, data collection and refinements
for 1a·CH3OH, 1b and 1e are summarized in Table I, selected bond lengths and angles are
provided in Table II. Fig. 2 shows perspective views of
1a·CH3OH, 1b and 1e. Different from 1a·CH3OH
and 1b, there are four independent TSCs molecules in the asymmetric
unit of 1e. The bond distances are extremely similar in all
structures. The S-C bond varies between 1.666 (4) and 1.6907 (19) Å, confirming that each TSC molecule is
in a ketone form (15). The imine C=N
double bond in each structure has an E configuration. The N-C=S and
CH=N-N bond lengths are in the ranges of 1.328 (4)-1.352 (4) Å
and 1.273 (4)-1.286 (4) Å, respectively, which are in agreement
with other previously known TSCs in the literature (15,21). The
Cambridge Crystallographic Data Centre serial numbers for each
crystal were as follows: 1032751, 1a·CH3OH; 1032752, 1b;
and 1032753, 1e.
 | Table I.Crystal data and structure
refinements for 1a·CH3OH, 1b and 1e. |
Table I.
Crystal data and structure
refinements for 1a·CH3OH, 1b and 1e.
| Data |
1a·CH3OH | 1b | 1e |
|---|
| Empirical
formula |
C10H16N4O3S |
C10H14N4O2S |
C15H16N4O2S |
| Formula weight | 272.33 | 7352.74 | 7355.96 |
| T (K) | 296 (2) | 296 (2) | 296 (2) |
| Space group | Monoclinic | Monoclinic | Triclinic |
| Crystal system | P2(1)/c | C2/c | P-1 |
| a, Å | 6.564 (4) | 13.6777 (13) | 13.8242 (19) |
| b, Å | 23.947 (15) | 8.1410 (7) | 15.147 (2) |
| c, Å | 9.555 (6) | 23.051 (2) | 16.839 (2) |
| α, deg | 90 | 90 | 110.226 (3) |
| β, deg | 104.980 (12) | 106.930 (2) | 104.337 (3) |
| γ, deg | 90 | 90 | 90.795 (3) |
|
V/Å3 | 1450.9 (15) | 2455.5 (4) | 3186.3 (8) |
| Z | 4 | 8 | 8 |
| Dc, g
cm−3 | 1.247 | 1.376 | 1.319 |
| µ,
mm−1 | 0.230 | 0.260 | 0.215 |
| Size, mm | 0.20×0.16×0.15 | 0.32×0.25×0.20 | 0.25×0.20×0.07 |
| F(000) | 576 | 1072 | 1328 |
| Limiting
indices |
|
|
|
|
h | −7≤h≤6 |
−11≤h≤16 |
−16≤h≤13 |
|
k |
−28≤k≤28 | −9≤k≤9 |
−14≤k≤18 |
|
l |
−11≤l≤10 |
−27≤l≤18 |
−19≤l≤20 |
| θ for data
collection, deg | 1.70–25.70 | 1.85–24.85 | 1.44–25.44 |
| reflns
collected/unique | 6886/2503 | 6071/2169 | 16426/11148 |
|
R(int) | 0.0689 | 0.0276 | 0.0485 |
| Goodness-of-fit on
F2 | 1.027 | 1.047 | 1.030 |
| final R
indices |
R1=0.0584 |
R1=0.0346 |
R1=0.0590 |
| [I>2σ(I)] |
wR2=0.1487 |
wR2=0.0819 |
wR2=0.0825 |
| R indices |
R1=0.1192 |
R1=0.0462 |
R1=0.1102 |
| (all data) |
wR2=0.1897 |
wR2=0.0888 |
wR2=0.1698 |
| Largest peak and
hole/e Å−3 | 0.313 and
−0.354 | 0.152 and
−0.189 | 0.269 and
−0.216 |
 | Table II.Selected bond lengths in
1a·CH3OH, 1b and 1e. |
Table II.
Selected bond lengths in
1a·CH3OH, 1b and 1e.
| A,
1a·CH3OH |
|---|
|
|---|
| Bond | Bond length, Å |
|---|
| S(1)-C(9) | 1.696 (4) |
| N(3)-C(9) | 1.343 (4) |
| N(2)-C(8) | 1.273 (4) |
|
| B, 1b |
|
|
| Bond | Bond length, Å |
|
| S(1)-C(9) | 1.691
(19) |
| N(3)-C(9) | 1.347 (2) |
| N(2)-C(8) | 1.278 (2) |
|
| C, 1e |
|
| Bond | Bond length, Å |
|
| S(1)-C(9) | 1.665 (4) |
| N(3)-C(9) | 1.352 (4) |
| N(2)-C(8) | 1.286 (4) |
| S(2)-C(24) | 1.677 (4) |
| N(7)-C(24) | 1.345 (4) |
| N(6)-C(23) | 1.285 (4) |
| S(3)-C(39) | 1.675 (4) |
| N(12)-C(39) | 1.328 (4) |
| N(10)-C(38) | 1.277 (4) |
| S(4)-C(54) | 1.666 (4) |
| N(16)-C(54) | 1.339 (5) |
| N(14)-C(53) | 1.273 (5) |
Cytotoxic activity
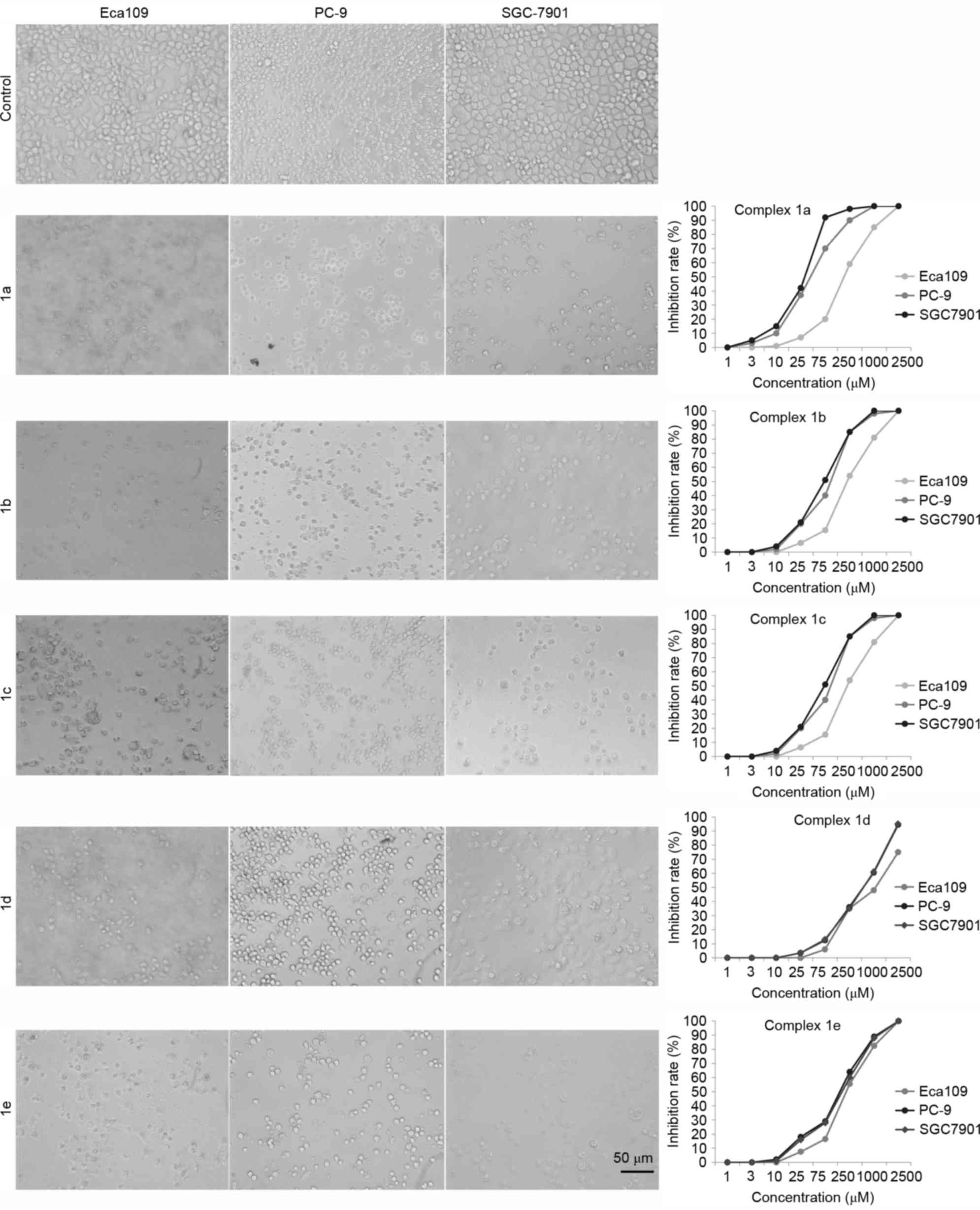
The effect of TSCs on the proliferation of tumor
cells was explored by MTT assay. The three tumor cells were
incubated with five TSCs in RPMI-1640 at a concentration of 0–2,500
µM, and the cell viability was assessed with MTT assay at 24 h. It
was demonstrated that the five TSCs exhibited a
concentration-dependent cytotoxic profile in all three cancer cell
lines. The IC50 values obtained for all tested TSCs are
presented in Table III. The
morphological examination also showed that the proliferation of the
cells was significantly inhibited, and the cells exhibited
morphological change, such as cell shrinkage and cell detachment
(Fig. 3). The IC50 values
for each TSC on all the cells were statistically different and TSCs
exhibited a greater effect against SGC-7901 cell lines, indicating
that the TSCs bearing pyrrole units may be more effective on the
SGC-7901 cell line. In addition, 1a shows the best cytotoxic
activity in all tested cell lines among the five tested TSCs,
suggesting that 1a may have more significant cytotoxic activities
than that of other TSCs.
 | Table III.IC50 values of compoundes
1a-e against the three human cancer cell lines subsequent to
incubation for 72 h. |
Table III.
IC50 values of compoundes
1a-e against the three human cancer cell lines subsequent to
incubation for 72 h.
|
| IC50,
µmol/l |
|---|
|
|
|
|---|
| compounds | PC-9 | Eca109 | SGC-7901 |
|---|
| 1a |
44.87 |
157.75 |
33.52 |
| 1b | 102.08 |
250.60 |
73.88 |
| 1c | 424.78 |
660.84 |
36.60 |
| 1d | 469.39 | 1,082.89 | 460.41 |
| 1e | 131.65 |
228.28 | 141.04 |
Cell apoptosis
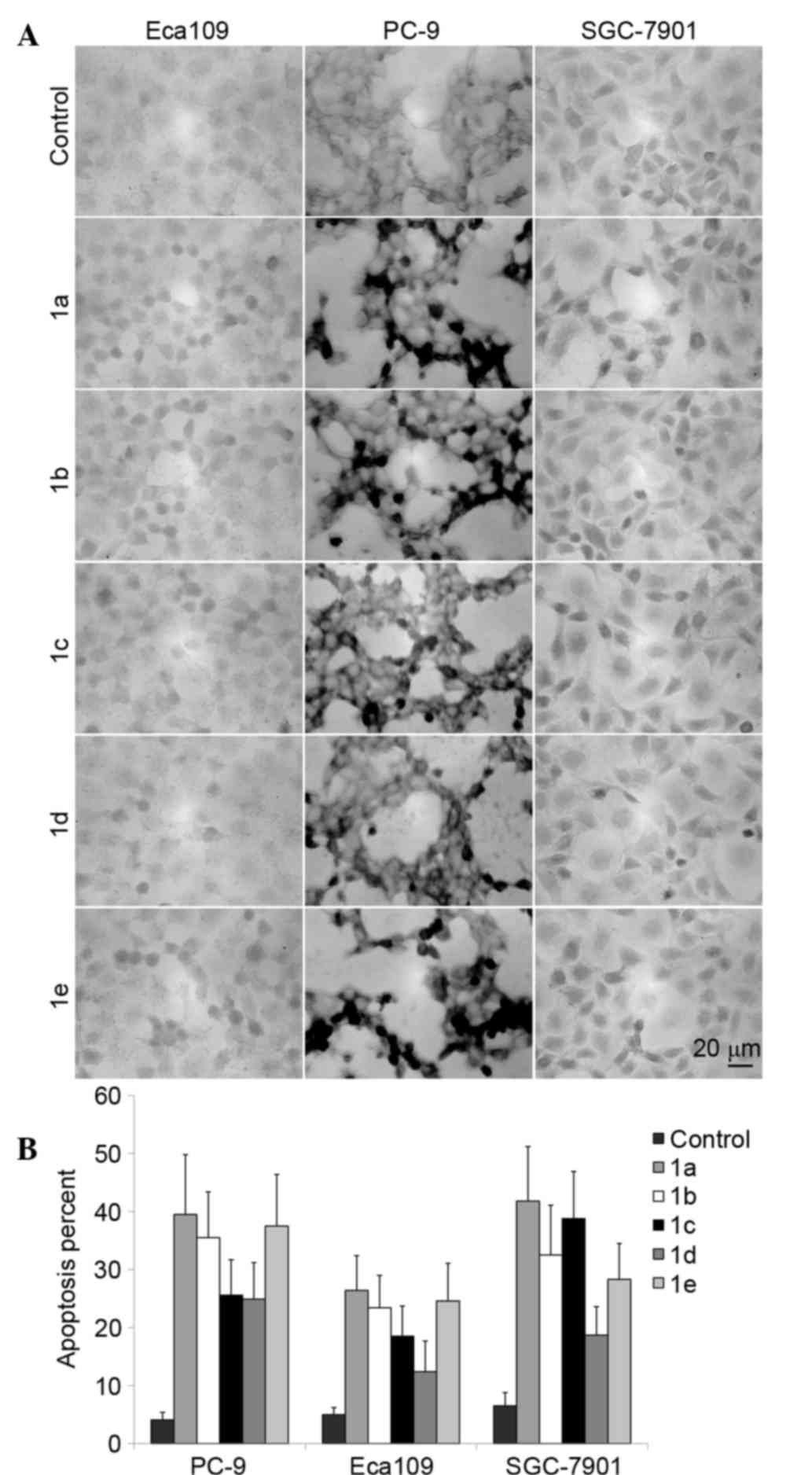
The cell apoptosis induced by TSCs in the three
tumor cell lines was analyzed by TUNEL staining. As shown in
Fig. 4, among the five TSCs
candidates, 1a shows the strongest induction of tumor cell
apoptosis among the five TSCs, corresponding to its cytotoxic
activity.
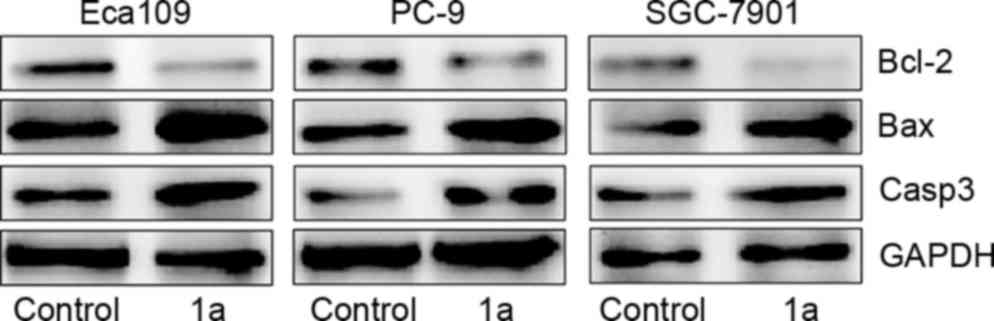
Changes in Bax, Bcl-2 and caspase-3 protein levels
in cancer cells. In order to explore the potential mechanisms of
the apoptosis induced by TSCs in tumor cells, western blotting was
performed to measure the expression of Bax, Bcl-2 and caspase-3. As
shown in Fig. 5, the expression level
of the apoptosis proteins was significantly different following
treatment with 1a, which decreased the expression of the
anti-apoptotic factor Bcl-2 and increased the expression of the
pro-apoptotic factor Bax and caspase-3 (P<0.05, Student's
t-test). These results may improve the understanding of the
pharmacological mechanism of the compounds in the treatment of
cancer.
Discussion
The five TSCs exhibited a concentration-dependent
cytotoxic effect in the human tumor cell lines assessed in the
present study. The IC50 values for 1a were significantly
decreased compared with the other TSCs in all tested cells. The
steric effect of N(4) substitutes in
TSCs may explain the trends. 1a has a smaller sterical
hindrancecompared with the other TSCs, which allows 1a to interact
with biomolecules efficiently and is responsible for improved
antitumor activity. Since the 1a isoform had more powerful
anti-tumor effects, it was selected for the subsequent TUNEL
staining and western blot analysis to clarify the possible involved
mechanisms. Apoptosis, a program of cellular suicide, is a form of
programmed cellular death that occurs through activation of the
cell-intrinsic suicide machinery and is considered an important
mechanism in the action of numerous anticancer drugs (22,23). As
shown in Fig. 4, the TUNEL assay
confirmed that the number of apoptotic cells induced by 1a is
increased compared with the other TSCs, which is consistent with
the result of cytotoxic activities.
As previously reported, the Bcl-2 family proteins
are key regulators of the apoptotic pathway (24). When Bcl-2 is produced in excess, cells
are protected from apoptosis. By contrast, when Bax expression is
high, the cells proceed into apoptosis. It has been suggested that
the alteration in the balance between Bcl-2 and Bax is critical to
determine the susceptibility of cells to apoptosis (25). Caspases, a family of cysteine
proteases, are the central regulators of apoptosis. Caspase-3 is
the main downstream effector caspase that performs essential roles
in degrading the majority of key cellular components in apoptotic
cells (26,27). In the present study, western blot
analysis was performed to determine whether Bax/Bcl-2 and caspase-3
were involved in the process of apoptosis induced by 1a in the
three human cancer cell lines. Concomitantly, 1a significantly
induced cancer cell apoptosis accompanied by increasing the
expression of Bax/Bcl-2 ratio and activation of caspase-3.
Overall, the present study suggests that TSCs
bearing pyrrole units possess the potential for development as
novel agents for human cancer therapy. Additional experiments are
required to explore the effects and potential mechanisms of such
TSCs in vivo. As predicted, the present study demonstrated
that such compounds show considerable antitumor activity against
three human-derived cancer model cell lines, namely the human lung
cancer PC-9, human esophageal cancer Eca-109 and human gastric
cancer SGC-7901 cell lines. In addition, complex TSC 1a
significantly induced cancer cell apoptosis, accompanied by
increased Bax/Bcl-2 ratio and activation of caspase-3. In
conclusion, TSCs bearing pyrrole units possess the potential for
development as novel agents for human cancer therapy.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (grant no. 81201908, 21404033),
Fund of the Natural Science Foundation of Jiangsu (grant no.
BK20141122), and Development Fund of Clinical Science and
Technology of Jiangsu University (grant no. JLY20140065).
References
|
1
|
Huang H, Chen Q, Ku X, Meng L, Lin L, Wang
X, Zhu C, Wang Y, Chen Z, Li M, et al: A series of
alpha-heterocyclic carboxaldehyde thiosemicarbazones inhibit
topoisomerase IIalpha catalytic activity. J Med Chem. 53:3048–3064.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jansson PJ, Sharpe PC, Bernhardt PV and
Richardson DR: Novel thiosemicarbazones of the ApT and DpT series
and their copper complexes: Identification of pronounced redox
activity and characterization of their antitumor activity. J Med
Chem. 53:5759–5769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soares MA, Lessa JA, Mendes IC, Da Silva
JG, Dos Santos RG, Salum LB, Daghestani H, Andricopulo AD, Day BW,
Vogt A, et al: N(4)-Phenyl-substituted 2-acetylpyridine
thiosemicarbazones: Cytotoxicity against human tumor cells,
structure-activity relationship studies and investigation on the
mechanism of action. Bioorg Med Chem. 20:3396–3409. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enyedy ÉA, Primik MF, Kowol CR, Arion VB,
Kiss T and Keppler BK: Interaction of Triapine and related
thiosemicarbazones with iron (III)/(II) and gallium (III): A
comparative solution equilibrium study. Dalton Trans. 40:5895–5905.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kowol CR, Trondl R, Arion VB, Jakupec MA,
Lichtscheidl I and Keppler BK: Fluorescence properties and cellular
distribution of the investigational anticancer drug triapine
(3-aminopyridine-2-carboxaldehyde thiosemicarbazone) and its zinc
(II) complex. Dalton Trans. 39:704–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeglis BM, Divilov V and Lewis JS: Role of
metalation in the topoisomerase IIα inhibition and
antiproliferation activity of a series of
α-heterocyclic-N4-substituted thiosemicarbazones and their Cu (II)
complexes. J Med Chem. 54:2391–2398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramachandran E, Thomas SP, Poornima P,
Kalaivani P, Prabhakaran R, Padma VV and Natarajan K: Evaluation of
DNA binding, antioxidant and cytotoxic activity of mononuclear Co
(III) complexes of 2-oxo-1,2-dihydrobenzo [h]
quinoline-3-carbaldehyde thiosemicarbazones. Eur J Med Chem.
50:405–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raja DS, Paramaguru G, Bhuvanesh NS,
Reibenspies JH, Renganathan R and Natarajan K: Effect of terminal
N-substitution in 2-oxo-1,2-dihydroquinoline-3-carbaldehyde
thiosemicarbazones on the mode of coordination, structure,
interaction with protein, radical scavenging and cytotoxic activity
of copper (II) complexes. Dalton Trans. 40:4548–4559. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li MX, Zhang LZ, Yang M, Niu JY and Zhou
J: Synthesis, crystal structures, in vitro biological evaluation of
zinc (II) and bismuth (III) complexes of 2-acetylpyrazine
N(4)-phenylthiosemicarbazone. Bioorg Med Chem Lett. 22:2418–2423.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li MX, Zhang LZ, Zhang D, Ji BS and Zhao
JW: Synthesis, crystal structures, and biological evaluation of
manganese (II) and nickel (II) complexes of
4-cyclohexyl-1-(1-(pyrazin-2-yl)ethylidene)thiosemicarbazide. Eur J
Med Chem. 46:4383–4390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li MX, Zhang D, Zhang LZ and Niu JY:
Synthesis, crystal structures, and biological activities of
2-thiophene N(4)-methylthiosemicarbazone and its unusual
hexanuclear silver(I) cluster. Inorg Chem Commun. 13:1268–1271.
2010. View Article : Google Scholar
|
|
12
|
Matesanz AI, Leitao I and Souza P:
Palladium (II) and platinum (II) bis (thiosemicarbazone) complexes
of the 2,6-diacetylpyridine series with high cytotoxic activity in
cisplatin resistant A2780cisR tumor cells and reduced toxicity. J
Inorg Biochem. 125:26–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matesanz AI and Souza P: Unprecedented
Pt(II) complex of an asymmetric 2,6-diacetylpyridine
bis(4N-substituted thiosemicarbazone) ligand. Inorg Chem Commun.
27:5–8. 2013. View Article : Google Scholar
|
|
14
|
Palanimuthu D, Shinde SV, Somasundaram K
and Samuelson AG: In vitro and in vivo anticancer activity of
copper bis (thiosemicarbazone) complexes. J Med Chem. 56:722–734.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ilies DC, Pahontu E, Shova S, Gulea A and
Rosu T: Synthesis, characterization and crystal structures of
nickel(II), palladium(II) and copper(II) complexes with
2-furaldehyde-4-phenylthiosemicarbazone. Polyhedron. 51:307–315.
2013. View Article : Google Scholar
|
|
16
|
Vine KL, Matesic L, Locke JM, Ranson M and
Skropeta D: Cytotoxic and anticancer activities of isatin and its
derivatives: A comprehensive review from 2000–2008. Anticancer
Agents Med Chem. 9:397–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye XP, Zhu TF, Wu WN, Ma TL, Zhang ZP,
Wang Y and Jia L: Syntheses, characterizations and biological
activities of two Cu(II) complexes with acylhydrazone ligand
bearing pyrrole unit. Inorg Chem Commun. 47:60–62. 2014. View Article : Google Scholar
|
|
18
|
Wang Y, Yang ZY and Chen ZN: Synthesis,
characterization and DNA-binding properties of four Zn (II)
complexes with bis (pyrrol-2-yl-methyleneamine) ligands. Bioorg Med
Chem Lett. 18:298–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheldrick GM: SADABS, program for
empirical absorption correction of area detector data. University
of Göttingen; Germany: 1996
|
|
20
|
Sheldrick GM: SHELX-97, program for
crystal structure solution and refinement. University of Göttingen;
Germany: 1997
|
|
21
|
Yang M, Lu YL, Li MX, Xu XW and Chen L:
Synthesis, crystal structures and biological evaluation of
2-benzoylpyridine N(4)-cyclohexylthiosemicarbazone and its
binuclear copper(II) complex. Inorg Chem Commun. 35:117–121. 2013.
View Article : Google Scholar
|
|
22
|
Nouri K and Yazdanparast R: Proliferation
inhibition, cell cycle arrest and apoptosis induced in HL-60 cells
by a natural diterpene ester from Daphne mucronata. Daru.
19:145–153. 2011.PubMed/NCBI
|
|
23
|
Farnebo M, Bykov VJ and Wiman KG: The p53
tumor suppressor: A master regulator of diverse cellular processes
and therapeutic target in cancer. Biochem Biophys Res Commun.
396:85–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shu D, Qing Y, Tong Q, He Y, Xing Z, Zhao
Y, Li Y, Wei Y, Huang W and Wu X: Deltonin isolated from Dioscorea
zingiberensis inhibits cancer cell growth through inducing
mitochondrial apoptosis and suppressing Akt and mitogen activated
protein kinase signals. Biol Pharm Bull. 34:1231–1239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagaraj NS, Anilakumar KR and Singh OV:
Diallyl disulfide causes caspase-dependent apoptosis in human
cancer cells through a Bax-triggered mitochondrial pathway. J Nutr
Biochem. 21:405–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han YH, Kim SZ, Kim SH and Park WH:
Pyrogallol inhibits the growth of lung cancer Calu-6 cells via
caspase-dependent apoptosis. Chem Biol Interact. 177:107–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|