Introduction
In recent years, the indications for laparoscopic
surgery have been expanded to include radical curative resection of
early to advanced colorectal cancer and palliative surgery for
stage IV disease (1–6). In Japan, laparoscopy-assisted colorectal
surgery (pure LACS) is widely used. However, pure LACS has several
disadvantages, such as requiring at least 2 physicians who are
familiar with the procedure and prolonging the operating time, as
well as needing more staff and limiting the availability of
operating theaters. Previously, it was reported that pure LACS
achieves the same or better outcomes as conventional laparotomy
(CL) with regard to wound infection, hospital stay, and survival,
together with superior cosmetic results (7–10). In
Europe and the USA, hand-assisted laparoscopic surgery (HALS) (HH)
is more widely used than pure LACS. HH is characterized by: i)
Providing the operator with palpation/tactile sensation, and
allowing full grasping manipulation with the left hand and the
possibility of smoothly removing even large and heavy tumors; ii) a
shorter operating time than for pure LACS; and iii) a more rapid
learning curve than for pure LACS (8,9,11–17).
In Japan, various surgical procedures are employed
for colorectal cancer, including pure LACS (30–40%), CL (~50%), and
other methods such as HALS and microincisional surgery (18). HALS is often regarded as being an
optimal medium between CL and pure LACS (8,9,18–23). In
Japan, HALS initially became popular for a short period of time
during the introduction of pure LACS in 2000, possibly as a method
of training for other procedures, prior to showing a marked decline
in this function. Consequently, at present, single-center reviews
of HALS are performed in countries other than Japan (9,24).
Previously, 212 patients with primary colorectal cancer (stages
I–III) underwent radical curative resection by hand-assisted
laparoscopic surgery (HALS) (n=98) or conventional laparotomy (CL)
(n=114) and were compared with respect to 3-year relapse-free
survival (3Y-RFS) and 3-year overall survival (3Y-OS) (25). However this type of surgery has
limitations. In the present study, 5-year data on the clinical
outcomes of HALS and CL for colorectal cancer were analyzed at a
single institution in Japan.
Patients and methods
Patients
In total, 850 patients underwent radical curative
resection of primary colorectal cancer between April 2002 and
December 2012. Aggressive introduction of HALS colorectal cancer
surgery commenced in July 2007. Of the patients that were followed
up over a period of 5 years, 114 patients (stage I, 27; stage II,
44; and stage III, 43) who received radical curative resection via
CL prior to the introduction of HALS in July 2007, were carefully
selected as historical controls (CL group), and were compared with
96 patients (stage I, 40; stage II, 28; and stage III, 28) who
underwent resection by HALS (HALS group). The two groups were
matched for stage and received the same postoperative adjuvant
chemotherapy regimen and follow-up protocol. HALS and CL were
performed in patients with a performance status of 0–2, and who
exhibited no serious cardiopulmonary disease, no obvious
preoperative lateral lymph node metastasis or multiple organ
involvement, and no pelvic cavity disease (4,24–27).
In the CL group, standard midline laparotomy was
performed with a ≥30 cm incision, while 2-port HALS (colon, 5 mm/5
mm) or 3-port HALS (rectum and sigmoid colon, 5 mm/12 mm/5 mm) was
performed in conjunction with a 45–55 mm longitudinal midline upper
umbilical (colon)/umbilical (rectum/sigmoid colon) incision
(4,26,27). At
least 12 lymph nodes were collected from patients in the two groups
following D2 or D3 resection according to the Japanese
classification (28–30). The postoperative adjuvant chemotherapy
regimens were as follows: No chemotherapy in stage I, only oral
anticancer agents (UFT/PSK) in stage II, and modified
5-fluorouracil (5-FU)/leucovorin (LV) or modified FOLFIRI
(5-FU/LV+CPT-11) for ≥6 months in stage III (25,31–36). To
detect metastasis/recurrence, ultrasound scan (US) and computed
tomography (CT) were performed 3–4 times annually, and patients in
whom US and CT simultaneously identified metastatic/recurrent
disease were classified as positive for metastasis/recurrence
(25,31–36). The
5-year relapse-free survival (5Y-RFS) and 5-year overall survival
(5Y-OS) were calculated for each group and the results were
compared.
Statistical analysis
The Kaplan-Meier method was used to estimate 5Y-RFS
and 5Y-OS, while the log-rank test and hazard ratio [95% confidence
interval (CI)] were used for comparison between the two groups. The
χ2 test and Mann-Whitney U test were employed for any
other parameters. SPSS statistics 21.1 software (IBM Corp., Armonk,
NY, USA) was used. P<0.05 was considered to indicate a
significant difference in all the analyses.
Results
Prognosis
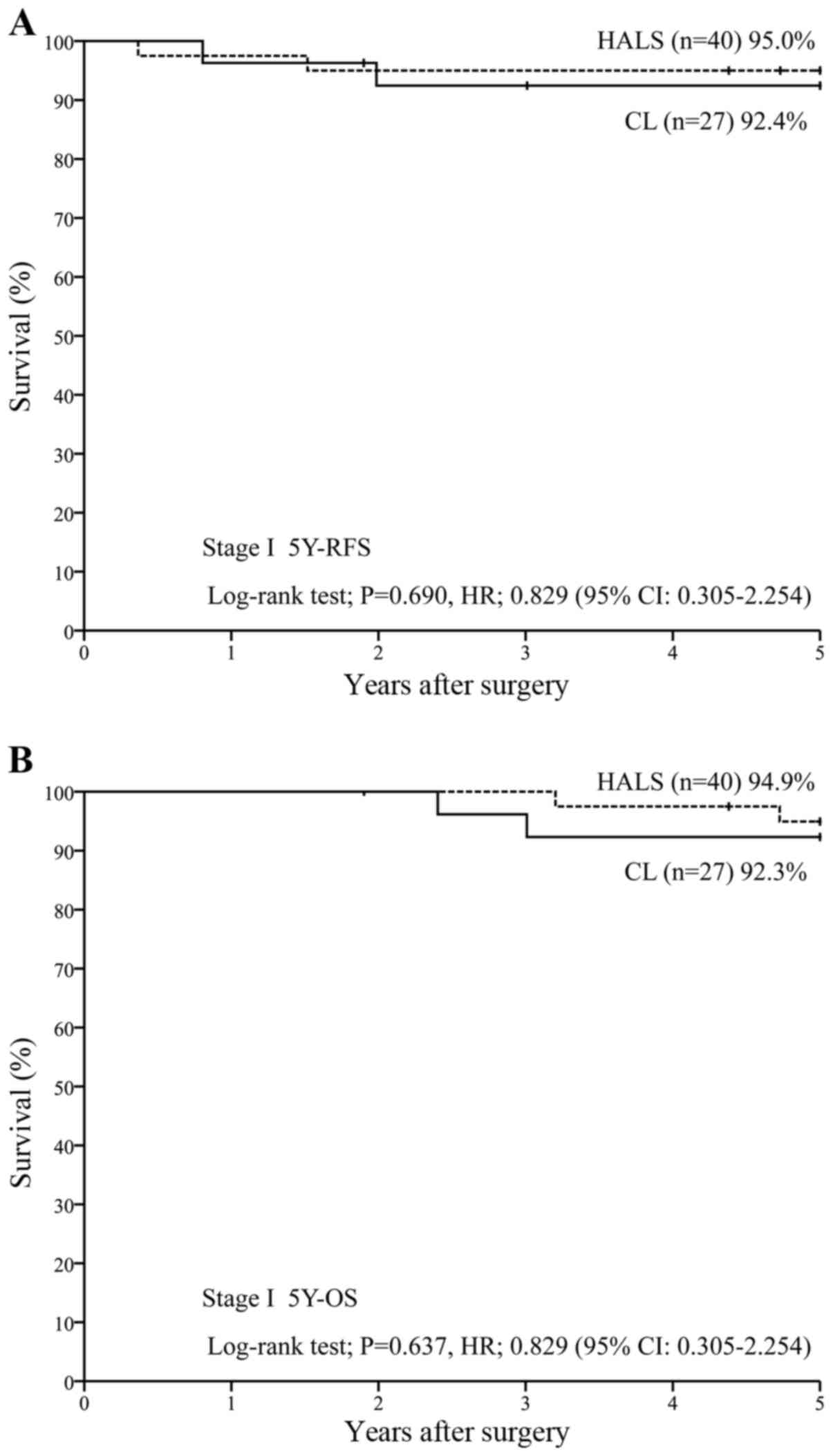
For patients in stage I (n=67), 5Y-RFS was 95.0%
with HALS (n=40) vs. 92.4% with CL (n=27) [P=0.690; hazard ratio
(HR), 0.829 (95% CI, 0.305–2.254)] (Fig.
1A), while 5Y-OS was 94.9% with HALS (n=40) vs. 92.3% with CL
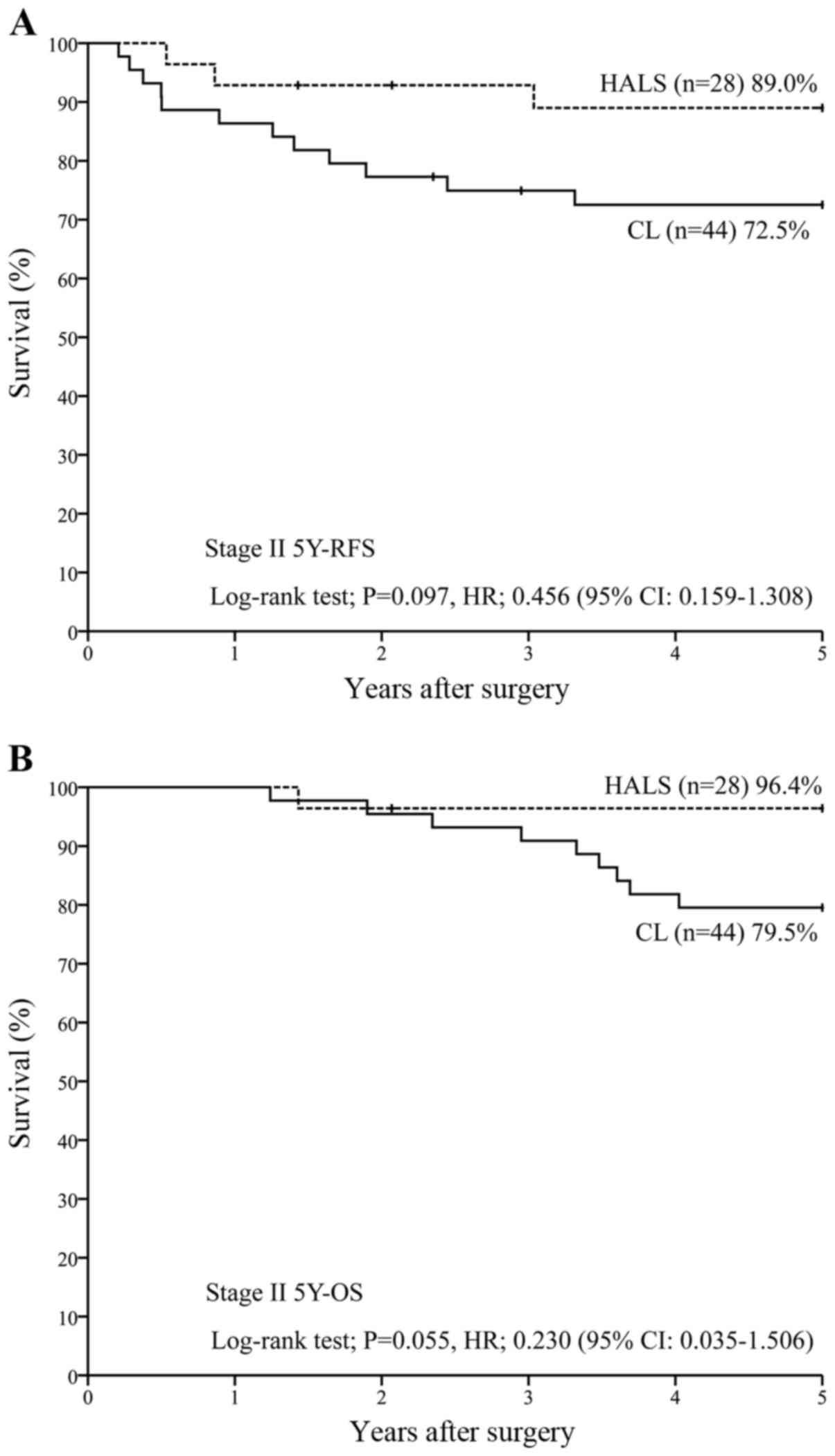
(n=27) [P=0.637; HR, 0.829 (95% CI, 0.305–2.254)] (Fig. 1B). For patients in stage II (n=72),
5Y-RFS was 89.0% with HALS (n=28) vs. 72.5% with CL (n=44)
[P=0.097; HR, 0.456 (95% CI, 0.159–1.308)] (Fig. 2A), while 5Y-OS was 96.4% with HALS
(n=28) vs. 79.5% with CL (n=44) [P=0.055; HR, 0.230 (95% CI,
0.035–1.506)] (Fig. 2B). For patients
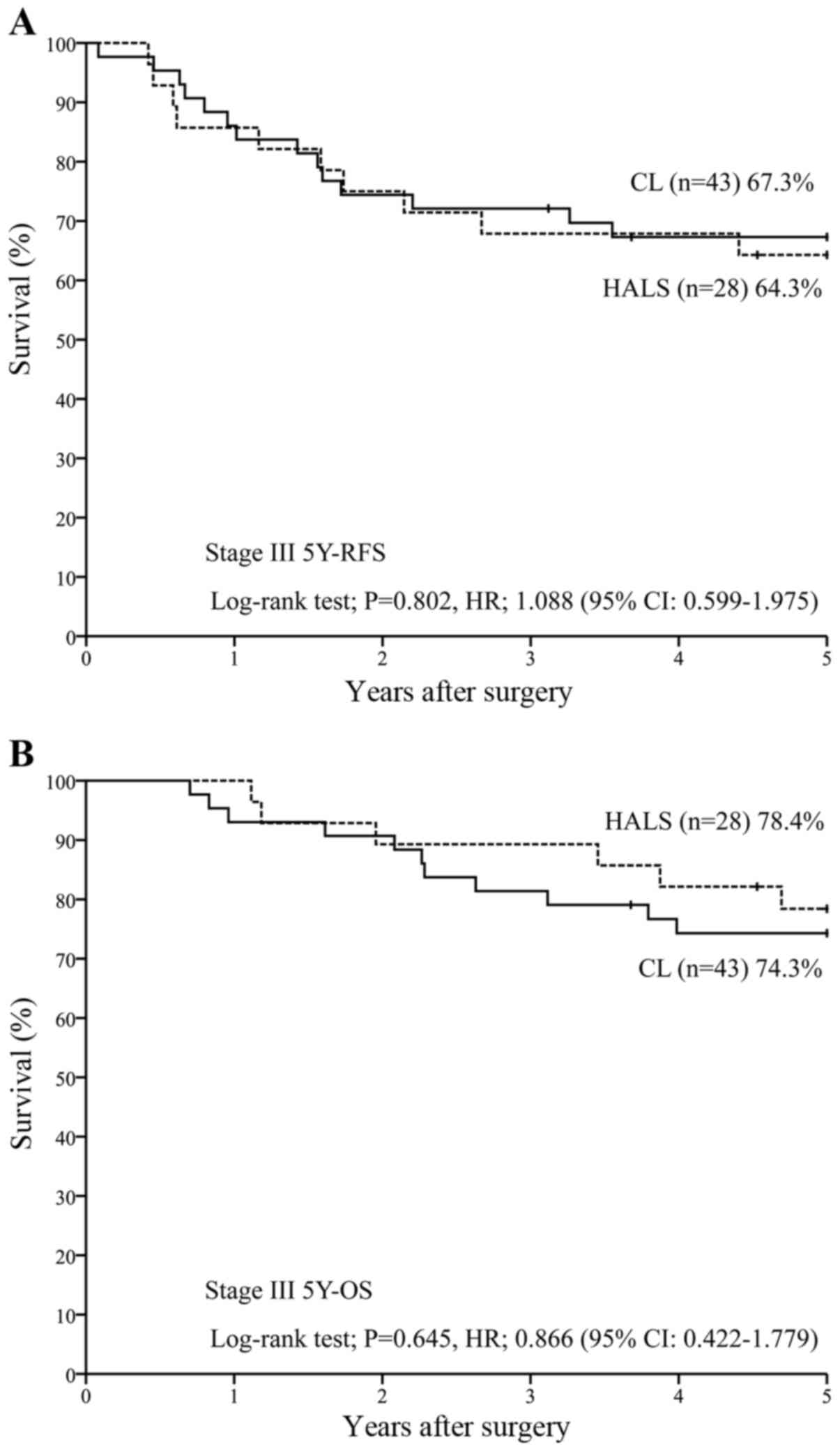
in stage III (n=71), 5Y-RFS was 64.3% with HALS (n=28) vs. 67.3%
with CL (n=43) [P=0.802; HR, 1.088 (95% CI, 0.599–1.975)] (Fig. 3A), while 5Y-OS was 78.4% with HALS
(n=28) vs. 74.3% with CL (n=43) [P=0.645; HR, 0.866 (95% CI,
0.422–1.779)] (Fig. 3B).
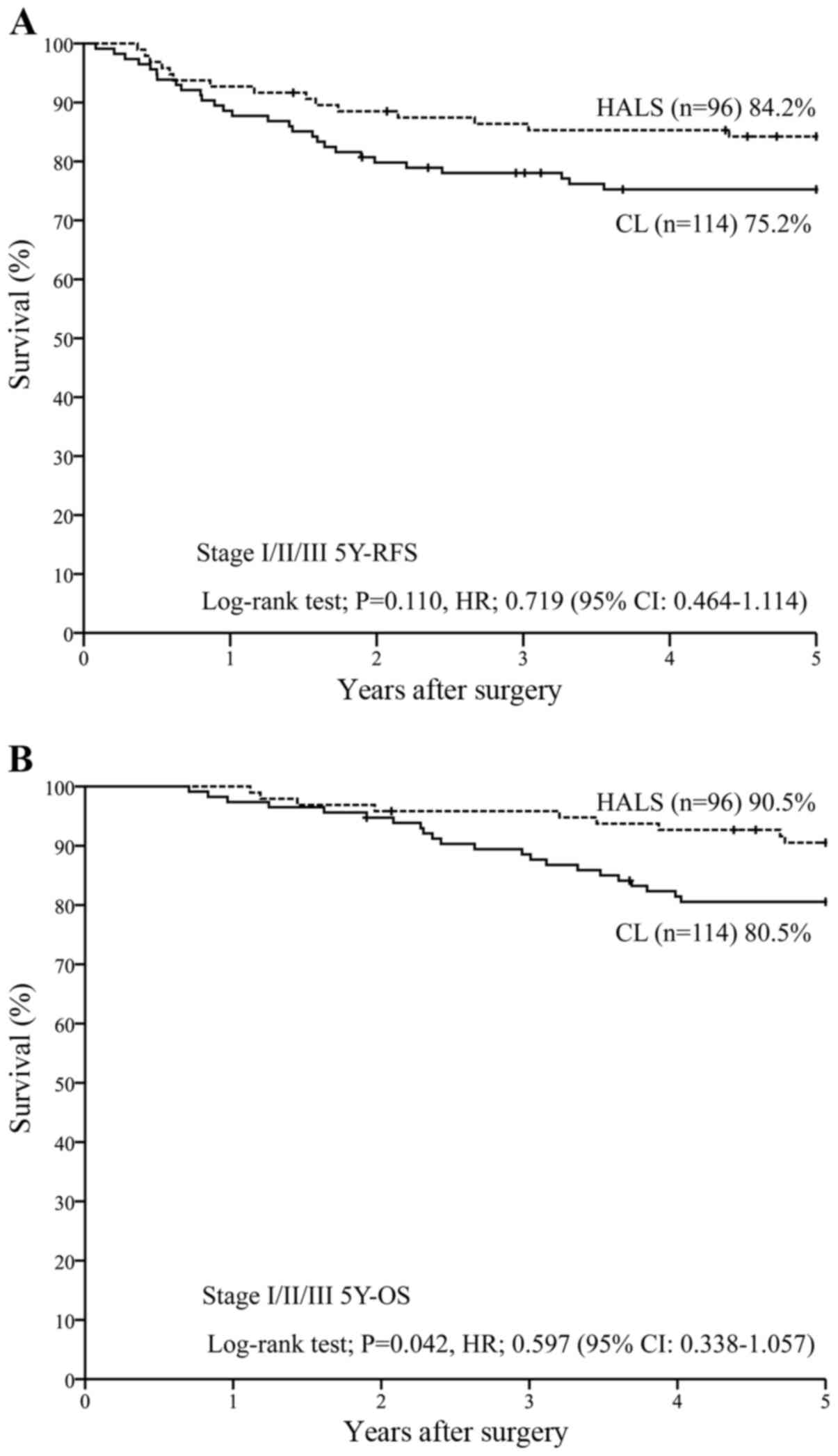
For all the patients in stages I–III (n=210), 5Y-RFS
was 84.2% with HALS (n=96) vs. 75.2% with CL (n=114) [P=0.110; HR,
0.719 (95% CI, 0.464–1.114)] (Fig.
4A), while 5Y-OS was 90.5% with HALS (n=96) vs. 80.5% with CL
(n=114) [P=0.042; HR, 0.597 (95% CI, 0.338–1.057)] (Fig. 4B).
Patient characteristics
There were 67 patients with stage I disease. Forty
stage I patients underwent HALS, accounting for 41.7% of the HALS
group, while 27 stage I patients underwent CL, accounting for only
23.7% of the CL group, indicating a significant difference
(P=0.005). Of the 72 patients in stage II, 28 patients underwent
HALS (29.2% of the HALS group) and 44 patients were treated by CL
(38.6% of the CL group), showing no significant difference
(P=0.152). Of the 71 stage III patients, 28 patients underwent HALS
(29.1% of the HALS group) and 43 patients received CL (37.7% of the
CL group), also showing no significant difference (P=0.192;
Table I). These results indicated
that HALS was performed significantly more often than CL for stage
I disease (Table I).
 | Table I.Comparison of stage between the HALS
group (n=96) and the CL group (n=114). |
Table I.
Comparison of stage between the HALS
group (n=96) and the CL group (n=114).
| Stage | HALS | CL | P-value
(χ2) |
|---|
| 1 | 41.7% (40/96) | 23.7% (27/114) | 0.005 |
| 2 | 29.2% (28/96) | 38.6% (44/114) | 0.152 |
| 3 | 29.1% (28/96) | 37.7% (43/114) | 0.192 |
Regarding tumor site, the tumor was located in the
colon in 53 patients undergoing HALS (55.2% of the HALS group) and
77 patients undergoing CL (67.5% of the CL group), while the tumor
was located in the rectum in 43 patients undergoing HALS (44.8% of
the HALS group) and 37 patients undergoing CL (32.5% of the CL
group), and no significant difference was observed (P=0.067)
(Table IIA).
 | Table II.Tumor site and stage distribution of
patients. |
Table II.
Tumor site and stage distribution of
patients.
| A, Comparison of
tumor site between the HALS group (n=96) and the CL group
(n=114) |
|---|
|
|---|
| Tumor site | HALS | CL | P-value
(χ2) |
|---|
| Colon | 55.2% (53/96) | 67.5% (77/114) | 0.067 |
| Rectum | 44.8% (43/96) | 32.5% (37/114) |
|
|
| B, Stage
distribution of patients with colon cancer (n=130) and rectal
cancer (n=80) |
|
| Stage | Colon | Rectum | P-value
(χ2) |
|
| 1 | 30.8% (40/130) | 33.8% (27/80) | 0.653 |
| 2 | 38.5% (50/130) | 27.5% (22/80) | 0.104 |
| 3 | 30.8% (40/130) | 38.8% (31/80) | 0.235 |
Patients in stage I (n=40) accounted for 30.8% of
all the colon cancer patients, while stage I rectal cancer patients
(n=27) comprised 33.8% of all rectal cancer patients (P=0.653).
There were 50 patients with stage II colon cancer, accounting for
38.5% of all colon cancer patients, and there were 22 patients with
stage II rectal cancer, accounting for 27.5% of all rectal cancer
patients (P=0.104). There were 40 patients with stage III colon
cancer, accounting for 30.8% of all colon cancer patients, and
there were 31 patients with stage III rectal cancer, accounting for
38.8% of all rectal cancer patients (P=0.235). There were no
significant differences in stages I–III (Table IIB).
Regarding the age distribution, the mean age was
62.4 (median, 62) years in the HALS group and 65.6 (median, 66)
years in the CL group (Table
IIIA).
 | Table III.Comparison of age between the HALS
group (n=96) and the CL group (n=114). |
Table III.
Comparison of age between the HALS
group (n=96) and the CL group (n=114).
| A, All patients,
n=210 |
|---|
|
|---|
|
Characteristics | HALS, n=96 | CL, n=114 | P-value,
Mann-Whitney U test |
|---|
| Age, years |
|
| 0.010 |
|
Average | 62.4 | 65.6 |
|
|
Median | 62 (36–81) | 66 (42–87) |
|
|
| B, Stage I
patients, n=67 |
|
| Colon and
rectum | HALS, n=40 | CL, n=27 | P-value,
Mann-Whitney U test |
|
| Age, years |
|
| 0.090 |
|
Average | 64.5 | 68.3 |
|
|
Median | 64.5 (42–81) | 71.0 (42–87) |
|
|
| C, Stage II
patients, n=72 |
|
| Colon and
rectum | HALS, n=28 | CL, n=44 | P-value,
Mann-Whitney U test |
|
| Age, years |
|
| 0.017 |
|
Average | 60.7 | 66.1 |
|
|
Median | 61.0 (40–75) | 66.5 (45–81) |
|
|
| D, Stage III
patients, n=71 |
|
| Colon and
rectum | HALS, n=28 | CL, n=43 | P-value,
Mann-Whitney U test |
|
| Age, years |
|
| 0.207 |
|
Average | 60.9 | 63.5 |
|
|
Median | 60.0 (36–72) | 64.0 (45–76) |
|
The mean age of the stage I patients was 64.5 years
(median, 64.5) years and 68.3 (median, 71.0) years in the HALS and
CL groups, respectively (P=0.090) (Table IIIB). In addition, the mean age of
stage II patients was 60.7 (median, 61.0) years and 66.1 (median,
66.5) years in the HALS and laparotomy groups, respectively
(P=0.017) (Table IIIC), while that
of stage III patients was 60.9 (median, 60.0) years and 63.5
(median, 64.0) years, respectively (P=0.207; Table IIID).
Discussion
Due to its rapid utilization in recent years, a
number of studies have reported on pure LACS in comparison with CL
and HALS (8,9,19–23). When surgical procedures are reviewed
at a single center, CL is often selected as the control. However,
it is difficult to exclude bias from the clinical background of the
control group in relation to both pure LACS and HALS. In addition,
problems with the standardization of subsequent treatment are
likely to occur, such as the postoperative chemotherapy or
radiotherapy regimens and the methods of handling recurrence. In
the present study, we used historical controls treated prior to the
utilization of HALS. The controls were matched for stage and for
the postoperative adjuvant chemotherapy regimen, and were compared
(4–10). The HALS and CL procedures were
performed by Mukai et al (4,27); thus,
the management of stage I/II/III colorectal cancer was standardized
in the study population. Patients in stages II and III from the two
groups received standardized chemotherapy and the ≥6-month
completion rate was >80% in the two groups (data not shown)
(25). The results of the 5-year
follow up were also analyzed in the present study.
A comparison of pure LACS with CL has identified
problems with human resources, surgical skill, a prolonged
operating time, and higher cost in relation to LACS, although there
have also been reports of a shorter hospital stay and a decrease in
the total analgesic dose (7–10). Previous findings showed that the
conversion rate of HALS was much lower than that of pure LACS
(15). The rate for HALS in the
present study was 4.2% (4/98 patients) in our study. There were
significant differences of blood loss for stages I and II and in
the length of hospital stay for stage III (25). However, stage III patients with
multiple organ infiltration accounted for 18.6% (8/43 patients) in
the CL group vs. 3.6% in the HALS group (1/28, P=0.063, data not
shown) (25). There were no
significant differences between the two groups with respect to
complications.
Blood loss was obviously lower in stage I and II
patients from the HALS group, suggesting that this method is safe
when based on strict indications (25).
The present study also involved rectal cancer
patients. Findings of studies conducted in Europe and America
suggest that HALS does not show non-inferiority versus pure LACS
and CL for the resection of rectal cancer (37,38).
However, results of those studies, which included rectal cancer
patients showed no significant difference between CL and HALS.
Based on those findings, HALS is a safe and reliable technique for
patients with colorectal cancer that achieves the same 5Y-RFS and
5Y-OS as CL, suggesting that it is a reasonable procedure to employ
and is positioned between pure LACS and CL. Since HALS is easy to
perform and is a cost-effective method, it is considered to be a
superior technique that deserves to be reconsidered in the current
medical environment where availability of surgeons and
anesthesiologists is on the decrease at small and medium-sized
hospitals in Japan.
Acknowledgements
The present study was supported by grants from the
Hand-Assisted Laparoscopic Surgery Research Group (no. 2010–5007;
Tokai University Hachioji Hospital, Hachioji, Tokyo, Japan) and the
Research and Study Program of Tokai University Educational System
General Research Organization (Tokai University Hospital, Isehara,
Kanagawa, Japan).
Glossary
Abbreviations
Abbreviations:
|
HALS
|
hand-assisted laparoscopic surgery
|
|
CRC
|
colorectal cancer
|
|
CL
|
conventional laparotomy
|
|
LACS
|
laparoscopy-assisted colorectal
surgery
|
References
|
1
|
Franklin ME Jr, Rosenthal D, Abrego-Medina
D, Dorman JP, Glass JL, Norem R and Diaz A: Prospective comparison
of open vs. laparoscopic colon surgery for carcinoma. Five-year
results. Dis Colon Rectum. 39 Suppl 10:S35–S46. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yano H, Ohnishi T, Kanoh T and Monden T:
Hand-assisted laparoscopic low anterior resection for rectal
carcinoma. J Laparoendosc Adv Surg Tech A. 15:611–614. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukai M, Tanaka A, Tajima T, Fukasawa M,
Yamagiwa T, Okada K, Sato K, Tobita K, Oida Y and Makuuchi H:
Two-port hand-assisted laparoscopic surgery for the 2-stage
treatment of a complete bowel obstruction by left colon cancer: A
case report. Oncol Rep. 19:875–879. 2008.PubMed/NCBI
|
|
4
|
Mukai M, Kishima K, Tajima T, Hoshikawa T,
Yazawa N, Fukumitsu H, Okada K, Ogoshi K and Makuuchi H: Efficacy
of hybrid 2-port hand-assisted laparoscopic surgery (Mukai's
operation) for patients with primary colorectal cancer. Oncol Rep.
22:893–899. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koh DC, Law CW, Kristian I, Cheong WK and
Tsang CB: Hand-assisted laparoscopic abdomino-perineal resection
utilizing the planned end colostomy site. Tech Coloproctol.
14:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerrieri M, Campagnacci R, de Sanctis A,
Lezoche G, Massucco P, Summa M, Gesuita R, Capussotti L, Spinoglio
G and Lezoche E: Laparoscopic versus open colectomy for TNM stage
III colon cancer: Results of a prospective multicenter study in
Italy. Surg Today. 42:1071–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung CC, Ng DC, Tsang WW, Tang WL, Yau
KK, Cheung HY, Wong JC and Li MK: Hand-assisted laparoscopic versus
open right colectomy: A randomized controlled trial. Ann Surg.
246:728–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin WY, Wei CK, Tseng KC, Lin SP, Lin CH,
Chang CM and Hsu TW: Open colectomy versus laparoscopic-assisted
colectomy supported by hand-assisted laparoscopic colectomy for
resectable colorectal cancer: A comparative study with minimum
follow-up of three years. Hepatogastroenterology. 56:998–1006.
2009.PubMed/NCBI
|
|
9
|
Pendlimari R, Holubar SD, Pattan-Arun J,
Larson DW, Dozois EJ, Pemberton JH and Cima RR: Hand-assisted
laparoscopic colon and rectal cancer surgery: Feasibility,
short-term, and oncological outcomes. Surgery. 148:378–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aimaq R, Akopian G and Kaufman HS:
Surgical site infection rates in laparoscopic versus open
colorectal surgery. Am Surg. 77:1290–1294. 2011.PubMed/NCBI
|
|
11
|
Nakajima K, Lee SW, Cocilovo C, Foglia C,
Sonoda T and Milsom JW: Laparoscopic total colectomy: Hand-assisted
vs standard technique. Surg Endosc. 18:582–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JC, Chung MH, Chao PC, Yeh CC, Hsiao
CW, Lee TY and Jao SW: Hand-assisted laparoscopic colectomy vs open
colectomy: A prospective randomized study. Surg Endosc. 18:577–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ringley C, Lee YK, Iqbal A, Bocharev V,
Sasson A, McBride CL, Thompson JS, Vitamvas ML and Oleynikov D:
Comparison of conventional laparoscopic and hand-assisted oncologic
segmental colonic resection. Surg Endosc. 21:2137–2141. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young-Fadok TM: Colon cancer: Trials,
results, techniques (LAP and HALS), future. J Surg Oncol.
96:651–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tjandra JJ, Chan MK and Yeh CH:
Laparoscopic- vs. hand-assisted ultralow anterior resection: A
prospective study. Dis Colon Rectum. 51:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pattana-arun J, Sahakitrungruang C,
Atithansakul P, Tantiphlachiva K, Khomvilai S and Rojanasakul A:
Multimedia article. Hand-assisted laparoscopic total mesorectal
excision: A stepwise approach. Dis Colon Rectum. 52:17872009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oncel M, Akin T, Gezen FC, Alici A and
Okkabaz N: Left inferior quadrant oblique incision: A new access
for hand-assisted device during laparoscopic low anterior resection
of rectal cancer. J Laparoendosc Adv Surg Tech A. 19:663–666. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitano S, Yamashita N, Shiraishi N, et al:
11th Nationwide survey of endoscopic surgery in Japan. J Jpn Soc
Endoscopic Surg. 17:595–611. 2012.(In Japanese).
|
|
19
|
Cima R and Pemberton JH: How a hand-assist
can help in lap colectomy. Contemp Surg. 63:19–23. 2007.
|
|
20
|
Cima RR, Pattana-arun J, Larson DW, Dozois
EJ, Wolff BG and Pemberton JH: Experience with 969 minimal access
colectomies: The role of hand-assisted laparoscopy in expanding
minimally invasive surgery for complex colectomies. J Am Coll Surg.
206:946–950, discussion 950–952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng QS, Lin JJ, Chen WB, Liu FL, Xu XM,
Lin CZ, Wang JH and Li YD: Hand-assisted laparoscopic versus open
right hemicolectomy: Short-term outcomes in a single institution
from China. Surg Laparosc Endosc Percutan Tech. 22:267–271. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng LW, Tung LM, Cheung HY, Wong JC, Chung
CC and Li MK: Hand-assisted laparoscopic versus total laparoscopic
right colectomy: A randomized controlled trial. Colorectal Dis.
14:e612–e617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sim JH, Jung EJ, Ryu CG, Paik JH, Kim G,
Kim SR and Hwang DY: Short-term outcomes of hand-assisted
laparoscopic surgery vs. open surgery on right colon cancer: A
case-controlled study. Ann Coloproctol. 29:72–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meshikhes AW, El Tair M and Al Ghazal T:
Hand-assisted laparoscopic colorectal surgery: Initial experience
of a single surgeon. Saudi J Gastroenterol. 17:16–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tajima T, Mukai M, Yamazaki M, Higami S,
Yamamoto S, Hasegawa S, Nomura E, Sadahiro S, Yasuda S and Makuuchi
H: Comparison of hand-assisted laparoscopic surgery and
conventional laparotomy for colorectal cancer: Interim results from
a single institution. Oncol Lett. 8:627–632. 2014.PubMed/NCBI
|
|
26
|
Mukai M, Fukasawa M, Kishima K, Iizuka S,
Fukumitsu H, Yazawa N, Tajima T, Nakamura M and Makuuchi H:
Trans-anal reinforcing sutures after double stapling for lower
rectal cancer: Report of two cases. Oncol Rep. 21:335–339.
2009.PubMed/NCBI
|
|
27
|
Mukai M, Sekido Y, Hoshikawa T, Yazawa N,
Fukumitsu H, Okada K, Tajima T, Nakamura M and Ogoshi K: Two-stage
treatment (Mukai's method) with hybrid 2-port HALS (Mukai's
operation) for complete bowel obstruction by left colon cancer or
rectal cancer. Oncol Rep. 24:25–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukai M, Ito I, Mukoyama S, Tajima T,
Saito Y, Nakasaki H, Sato S and Makuuchi H: Improvement of 10-year
survival by Japanese radical lymph node dissection in patients with
Dukes' B and C colorectal cancer: A 17-year retrospective study.
Oncol Rep. 10:927–934. 2003.PubMed/NCBI
|
|
29
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR), . General rules for clinical and pathological
studies on cancer of the colon, rectum and anus. 7th. Kanehara
& Co., Ltd.; Tokyo: pp. 10–13. 2009
|
|
30
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR), . JSCCR Guidelines 2010 for the treatment of
colorectal cancer. Kanehara & Co., Ltd.; Tokyo: pp. 10–15.
2010
|
|
31
|
Mukai M, Tajima T, Nakasaki H, Sato S,
Ogoshi K and Makuuchi H: Efficacy of postoperative adjuvant oral
immunochemotherapy in patients with Dukes' B colorectal cancer. Ann
Cancer Res Therap. 11:201–214. 2003. View Article : Google Scholar
|
|
32
|
Mukai M, Tajima T, Nakasaki H, Sato S,
Ogoshi K and Makuuchi H: Efficacy of postoperative adjuvant oral
immunochemotherapy in patients with Dukes' C colorectal cancer. Ann
Cancer Res Therap. 11:215–229. 2003. View Article : Google Scholar
|
|
33
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Oida Y, Nakamura M and Makuuchi H: Comparison
between intravenous and oral postoperative adjuvant
immunochemotherapy in patients with stage II colorectal cancer.
Oncol Rep. 20:1189–1194. 2008.PubMed/NCBI
|
|
34
|
Ito I, Mukai M, Ninomiya H, Kishima K,
Tsuchiya K, Tajima T, Nakamura M and Makuuchi H: Comparison between
intravenous and oral postoperative adjuvant immunochemotherapy in
patients with stage III colorectal cancer. Oncol Rep. 20:1521–1526.
2008.PubMed/NCBI
|
|
35
|
Mukai M, Okada K, Fukumitsu H, Yazawa N,
Hoshikawa T, Tajima T, Hirakawa H, Ogoshi K and Makuuchi H:
Efficacy of 5-FU/LV plus CPT-11 as first-line adjuvant chemotherapy
for stage IIIa colorectal cancer. Oncol Rep. 22:621–629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mukai M, Kishima K, Uchiumi F, Ishibashi
E, Fukasawa M, Tajima T, Nakamura M and Makuuchi H: Clinical
comparison of QOL and adverse events during postoperative adjuvant
chemotherapy in outpatients with node-positive colorectal cancer or
gastric cancer. Oncol Rep. 21:1061–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stevenson ARL, Solomon MJ, Lumley JW,
Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W and
Simes J: ALaCaRT Investigators: Effect of laparoscopic-assisted
resection vs open resection on pathological outcomes in rectal
cancer: The ALaCaRT randomized clinical trial. JAMA. 314:1356–1363.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fleshman J, Branda M, Sargent DJ, Boller
AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, et
al: Effect of laparoscopic-assisted resection vs open resection of
stage II or III rectal cancer on pathologic outcomes: The ACOSOG
Z6051 randomized clinical trial. JAMA. 314:1346–1355. 2015.
View Article : Google Scholar : PubMed/NCBI
|