Introduction
Prostate cancer is the most prevalent malignancy in
men and the second most frequent cause of cancer-associated
mortality in the United States (1).
The incidence of prostate cancer in Korea increased by 12.8%
annually between 1999 and 2010 (2).
Testing for prostate-specific antigen (PSA) has led to increased
diagnoses of localized prostate cancer; however, despite this
downward stage migration, a number of patients are still diagnosed
with metastatic prostate cancer (2).
According to the Korea Central Cancer Registry, between 2006 and
2010 ~73.3% of patients with prostate cancer were diagnosed with
loco-regional disease, whereas 9.0% presented with distant disease
(extension to organs other than the prostate, or metastases to
distant lymph nodes or organs) (2).
Androgen deprivation therapy (ADT), including
bilateral orchiectomy and treatment with luteinizing
hormone-releasing hormone (LHRH) agonists with or without
anti-androgens, is a well-established initial treatment for
metastatic prostate cancer (3).
Despite the initial positive response, a number of patients may
experience significant adverse effects (4–6).
Castration-resistant prostate cancer (CRPC) is inevitable following
a variable period of response to ADT (7–10).
Strategies to delay CRPC and minimize the adverse effects of ADT,
including intermittent androgen deprivation therapy (IADT) or novel
agents, remains an active area of investigation (11).
During the management of advanced prostate cancer,
previous studies have demonstrated that IADT is at least as
effective as continuous treatment with regard to disease
progression and overall survival (12–14).
However, IADT should be considered as an alternative therapeutic
approach to CADT, accounting for the caveats and cautions suggested
by meta-analysis of eight randomized controlled trials of
intermittent vs. continuous ADT (12–14).
In high-volume metastatic prostatic cancer,
including various lymph node or bone metastases, initial bulky
tumors and high baseline PSA levels, IADT is not a suitable
treatment modality (15–17). In these patients, continuous ADT must
remain the standard treatment approach (14). Continuous ADT comprises surgical
castration, LHRH agonist with/without anti-androgen and LHRH
antagonist (14).
Therefore, the present study compared the efficacy
of continuous maximal androgen blockade (MAB) and conversion to
monotherapy, with an LHRH agonist or orchiectomy, after reaching
PSA nadir following initial MAB therapy in patients with metastatic
prostate cancer at initial presentation. Additionally, various
factors affecting disease progression were also investigated.
Materials and methods
A retrospective review of the medical records of
male patients diagnosed with prostate cancer with bone metastasis
between 1996 and 2011 at the Asan Medical Center (Seoul, Korea) was
performed. A total of 354 patients diagnosed with prostate cancer
with bone metastasis received MAB as a first-line therapy. Patients
with no recorded follow up for ≥1 year, or who had incomplete data,
were excluded, and the remaining 293 patients formed the study
cohort. Bone metastasis was assessed using a radionuclide bone
scan. The median age at diagnosis was 70.2 years (range, 47–89
years).
MAB consisted of an oral anti-androgen (750 mg/day
flutamide or 50 mg/day bicalutamide) with orchiectomy (89/293,
30.4%) or an LHRH agonist (3.75 mg/month leuprolide or 3.6 mg/month
goserelin acetate; 204/293, 69.6%). Following attainment of the PSA
nadir with initial MAB treatment (at a median of 8.5 months),
patients received maintenance therapy with MAB (227/293, 77.5%),
monotherapy (66/293, 22.5%) with an LHRH agonist or no treatment
following bilateral orchiectomy.
PSA was recorded every 3 months, and a bone scan was
performed every 6–12 months. However, if clinical progression was
suspected due to the elevation of PSA levels at two consecutive
readings or the development of skeletal symptoms, an immediate
image study, including an X-ray, computed tomography or bone scan,
was conducted.
The efficacy of ADT was assessed via serum PSA
levels and bone lesion responses (18). Disease progression was defined as
progression to CRPC (biochemical progression and/or radiological
progression). CRPC was defined by using the Prostate Cancer Working
Group criteria as a continuous rise in PSA levels (biochemical
progression and/or a radiological progression) even when the serum
testosterone level remained in the castrate range (<50 ng/dl)
due to surgical orchiectomy or medical therapy (18). Biochemical progression was defined as
three consecutive rises in PSA levels one week apart, resulting in
two 50% increases over the nadir with PSA concentrations of >2
ng/ml. Radiological progression was defined as the appearance of ≥2
novel lesions on a bone scan.
The propensity score was determined from a
non-parsimonious logistic regression model for treatment with
continuous MAB vs. conversion to monotherapy. The variables
included in this model were age, Gleason score, pretreatment PSA
and pretreatment extent of bone metastasis. Patients receiving
continuous MAB were matched at a ratio of 2:1 (105:53) with
patients converting to monotherapy, by using the closest available
pair matching method (19). Model
discrimination was assessed with c-statistics (P=0.724) and model
calibration was assessed with Hosmer-Lemeshow statistics (χ2=4.662;
P=0.793).
To evaluate disease progression and compare
treatment methods, χ2 and Student's t-tests were used as
appropriate. Survival was estimated using the Kaplan-Meier method
and compared using the log-rank test. A Cox proportional hazards
model was used for multivariate analysis. All statistical analyses
were performed with SPSS 18.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
The median follow-up period was 39.1 months (range,
12–170 months). Following initial MAB treatment, serum PSA had
decreased to an undetectable level in 37.4% of patients, and to a
detectable nadir in 62.6%. The median time to a PSA nadir was 8.5
months; following this nadir, 77.5% of the patients stayed on MAB
and the remaining 22.5% were switched to monotherapy.
The clinical characteristics of the 293 patients
based on post-nadir treatment status are presented in Table I. Patients on maintenance monotherapy
had more favorable clinicopathological characteristics, including a
lower pretreatment PSA level (P=0.005) and a lower metastatic
burden (P=0.018). Following propensity score matching, the baseline
clinicopathological characteristics remained similar between the
groups (Table II).
 | Table I.Baseline characteristics of patients
who received continuing MAB, and those who converted to monotherapy
once the PSA nadir was reached following initial MAB. |
Table I.
Baseline characteristics of patients
who received continuing MAB, and those who converted to monotherapy
once the PSA nadir was reached following initial MAB.
| Characteristics | Continuing MAB
(n=227) | Conversion to
monotherapy (n=66) | P-value |
|---|
| Median age ± SD | 68.74±7.48 | 70.94±7.60 | 0.906 |
| LN pretreatment
PSA | 6.41±1.42 | 5.78±1.29 | 0.005a |
| Gleason score |
| ≤6 | 6 (2.6%) | 5 (7.6%) | 0.171 |
|
7 | 17 (7.5%) | 4 (6.0%) |
|
| ≥8 | 204 (89.9%) | 57 (8.64%) |
|
| Pretreatment extent
of bone metastasis |
|
Focal | 53 (23.3%) | 17 (25.8%) | 0.018a |
|
Multiple | 104 (45.8%) | 40 (60.6%) |
|
|
Disseminated | 70 (30.9%) | 9 (13.6%) |
|
 | Table II.Baseline characteristics of
propensity-matched patients. |
Table II.
Baseline characteristics of
propensity-matched patients.
| Characteristics | Continuing MAB
(n=102) | Conversion to
monotherapy (n=53) | P-value |
|---|
| Median age ± SD | 69.38±8.16 | 70.38±8.50 | 0.068 |
| LN pretreatment
PSA | 4.82±1.48 | 4.98±1.21 | 0.683 |
| Gleason score |
| ≤6 | 2 (2.0%) | 1 (1.9%) | 0.495 |
|
7 | 4 (3.9%) | 1 (1.9%) |
|
| ≥8 | 96 (94.1%) | 51 (96.2%) |
|
| Pretreatment extent
of bone metastasis |
|
Focal | 24 (23.5%) | 12 (22.6%) | 0.942 |
|
Multiple | 63 (61.8%) | 34 (64.2%) |
|
|
Disseminated | 17 (14.7%) | 7 (13.2%) |
|
After a median treatment period of 22.7 months, 110
(70.9%) patients exhibited evidence of progression to CRPC, of
which 95 (86.4%) demonstrated biochemical progression and 15
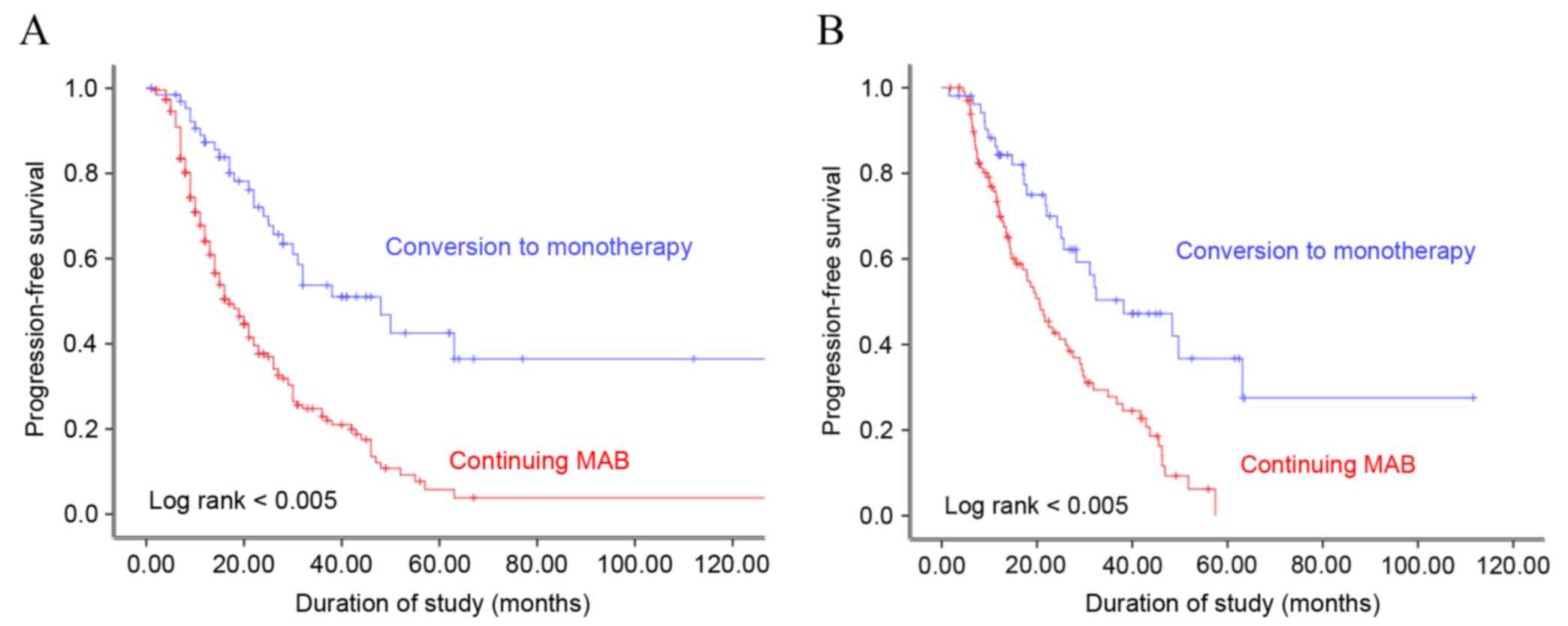
(13.6%) had radiological progression. Kaplan-Meier analysis
demonstrated that progression-free survival (PFS) was significantly
associated with the type of maintenance ADT administered to the
patients, prior to and following propensity score matching (log
rank, P<0.005; Fig. 1). The median
PFS was 19.5 and 28.8 months following continuous MAB and
conversion to monotherapy, respectively (P=0.008). Multivariate
analysis using the Cox proportional hazard model revealed that the
type of maintenance ADT and the pretreatment extent of bone
metastasis were independent predictive factors of disease
progression (Table III).
 | Table III.Cox proportional hazards model
multivariate analysis of predictive factors of progression-free
survival. |
Table III.
Cox proportional hazards model
multivariate analysis of predictive factors of progression-free
survival.
| Characteristics | Hazard ratio | 95% CI | P-value |
|---|
| ADT method |
| Continuing MAB | 2.293 | 1.536–3.424 |
<0.001a |
| Conversion to
monotherapy | 1 |
|
|
| Age | 0.992 | 0.975–1.009 | 0.364 |
| Gleason score |
| ≤6 | 1 |
| 0.450 |
|
7 | 2.090 | 0.600–7.279 | 0.247 |
| ≥8 | 2.110 | 0.663–6.720 | 0.206 |
| LN pretreatment
PSA | 0.997 | 0.908–1.095 | 0.950 |
| Pretreatment extent
of bone metastasis |
|
Focal | 1 |
|
<0.001a |
|
Multiple | 1.666 | 1.090–2.524 | 0.016a |
|
Disseminated | 2.547 | 1.614–4.022 |
<0.001a |
Discussion
Even with the availability of PSA screening and the
resulting reduction in cancer-stage progression, numerous patients
with prostate cancer are initially diagnosed with metastatic
disease, with 2.6% of patients in the CaPSURE™ trial possessing
metastatic disease at the time of diagnosis (20). Of the patients in the present study,
16.4% were initially diagnosed with metastatic prostate cancer.
Among these patients, ADT was the primary treatment for metastatic
disease, with its use increasing throughout the study period.
Advanced prostate cancer typically becomes
androgen-independent following castration (8). The duration of response to ADT in
metastatic prostate cancer is ~14–20 months (7–10). To
maximize the effect of ADT, numerous strategies were used for the
treatment of patients (continuous ADT vs. intermittent ADT; MAB vs.
mono-ADT) (21).
As aforementioned, the relative efficacy of IADT has
yet to be established in metastatic prostate cancer. Previous
studies have demonstrated that IADT was associated with a poorer
prognosis and PFS for patients with metastatic prostate cancer
(8,20,22). In
the Southwest Oncology Group (SWOG) 9,346 trial, the median overall
survival exhibited an absolute difference moderately >6 months
in favor of continuous therapy; 5.1 years in the
intermittent-therapy group compared with 5.8 years in the
continuous-therapy group (22).
Therefore, continuous ADT remains the standard
treatment for metastatic prostate cancer; however, which continuous
ADT methods (MAB vs. mono-ADT) are beneficial in metastatic
settings remain to be established. Systematic reviews have
demonstrated that MAB using non-steroidal anti-androgens appears to
yield a small survival advantage (<5%) beyond five years, as
compared with mono-ADT (surgical castration or LHRH agonists)
(23–25).
The European Association of Urology and The European
Society of Medical Oncology recommend short-term administration of
anti-androgen for between 1 and 4 weeks only to minimize the risk
of the ‘flare-up’ phenomenon in patients with advanced metastatic
prostate cancer (26,27). In the present study, maintenance of
the anti-androgen treatment was required until the PSA nadir was
reached at ~8.5 months.
In the present study, conversion to monotherapy
prolonged PFS for 9.3 months, as compared with continuing MAB. In
addition, a marked difference was observed in PFS in patients with
metastatic prostate cancer who received ADT. Patients who were
treated with a continuous LHRH agonist, or observation following
orchiectomy had improved PFS, as compared with those who received
continuous MAB following attainment of the PSA nadir (28.8 vs. 19.5
months). Due to variations in the baseline characteristics,
including pretreatment PSA and the extent of bone metastasis,
propensity score matched analysis was performed. Prior to and
following matching, conversion to monotherapy was observed to be
superior to continuous MAB with respect to PFS in patients with
hormone-sensitive prostate cancer. Continuous MAB was associated
with a 2.3-fold higher risk of prostate cancer progression,
compared with conversion to monotherapy.
The inappropriate use of hormonal therapy, including
short-term, intermittent, incomplete ADT or anti-androgen only, may
lead to disease progression and an increased proportion of cancer
cells with a higher malignant potential (28). A patient's progression-free survival
may be affected by a delay in the appearance of
androgen-insensitive clones, following conversion to monotherapy.
It is hypothesized that continuous MAB treatment may have
facilitated the earlier development of androgen-insensitive clones;
by contrast, conversion to monotherapy may have delayed the
development of androgen-insensitive clones. MAB is associated with
treatment-associated costs and increased adverse effects, including
increased risk of cardiovascular disease, diabetes and metabolic
abnormalities (29). Therefore,
maintenance therapy with an LHRH agonist, or observation following
orchiectomy, may be associated with a reduction in adverse effects
and treatment-associated costs, in addition to yielding improved
PFS, as compared with the continuation of MAB therapy.
One limitation of retrospective studies is the
potential for selection bias. To mitigate this possibility during
the present study, propensity score matching analysis was
performed. Furthermore, the quality of life associated with
specific treatments was not evaluated, and an investigation of
treatment-associated adverse events was not performed. Due to the
intermediate duration of follow-up (39.1 months), the impact of ADT
on cancer-specific or overall survival was not analyzed in the
present study.
In conclusion, conversion to monotherapy following
attainment of the PSA nadir with initial MAB therapy prolonged
progression-free survival in patients with prostate cancer. The
results suggest that monotherapy maintenance following initial MAB
therapy may benefit patients by reducing the incidence of side
effects without decreasing treatment efficacy.
Glossary
Abbreviations
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
PFS
|
progression-free survival
|
|
MAB
|
maximal androgen blockade
|
|
LHRH
|
luteinizing-hormone releasing
hormone
|
|
PSA
|
prostate specific antigen
|
|
CRPC
|
castration-resistant prostate
cancer
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Shin A
and Lee JS: Survival of Korean adult cancer patients by stage at
diagnosis, 2006–2010: National cancer registry study. Cancer Res
Treat. 45:162–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salonen AJ, Taari K, Ala-Opas M, Viitanen
J, Lundstedt S and Tammela TL; FinnProstate Group, : The
FinnProstate study VII: intermittent versus continuous androgen
deprivation in patients with advanced prostate cancer. J Urol.
187:2074–2081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor LG, Canfield SE and Du XL: Review
of major adverse effects of androgen-deprivation therapy in men
with prostate cancer. Cancer. 115:2388–2399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwandt A and Garcia JA: Complications of
androgen deprivation therapy in prostate cancer. Curr Opin Urol.
19:322–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crawford ED, Eisenberger MA, McLeod DG,
Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA and
Goodman PJ: A controlled trial of leuprolide with and without
flutamide in prostatic carcinoma. N Engl J Med. 321:419–424. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharifi N, Gulley JL and Dahut WL:
Androgen deprivation therapy for prostate cancer. JAMA.
294:238–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mottet N, van Damme J, Loulidi S, Russel
C, Leitenberger A and Wolff JM; TAP22 Investigators Group, :
Intermittent hormonal therapy in the treatment of metastatic
prostate cancer: A randomized trial. BJU Int. 110:1262–1269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seidenfeld J, Samson DJ, Hasselblad V,
Aronson N, Albertsen PC, Bennett CL and Wilt TJ: Single-therapy
androgen suppression in men with advanced prostate cancer: A
systematic review and meta-analysis. Ann Intern Med. 132:566–577.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alva AS and Hussain M: Initial management
of metastatic prostate cancer. In: Comprehensive Textbook of
Genitourinary OncologyScardino PT, Linehan WM, Zelefsky MJ and
Vogelzang NJ: Lippincott Williams & Wilkins; Philadelphia: pp.
251–261. 2011
|
|
12
|
da Silva F Calais, da Silva FM Calais,
Gonçalves F, Santos A, Kliment J, Whelan P, Oliver T, Antoniou N,
Pastidis S, Queimadelos A Marques and Robertson C: Locally advanced
and metastatic prostate cancer treated with intermittent androgen
monotherapy or maximal androgen blockade: Results from a randomised
phase 3 study by the South European Uroncological Group. Eur Urol.
66:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Silva FE Calais, Bono AV, Whelan P,
Brausi M, Queimadelos A Marques, Martin JA, Kirkali Z, da Silva FM
Calais and Robertson C: Intermittent androgen deprivation for
locally advanced and metastatic prostate cancer: Results from a
randomised phase 3 study of the South European Uroncological Group.
Eur Urol. 55:1269–1277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai HT, Penson DF, Makambi KH, Lynch JH,
Van Den Eeden SK and Potosky AL: Efficacy of intermittent androgen
deprivation therapy vs conventional continuous androgen deprivation
therapy for advanced prostate cancer: A meta-analysis. Urology.
82:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prapotnich D, Fizazi K, Escudier B, Mombet
A, Cathala N and Vallancien G: A 10-year clinical experience with
intermittent hormonal therapy for prostate cancer. Eur Urol.
43:233–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salonen AJ, Viitanen J, Lundstedt S,
Ala-Opas M, Taari K and Tammela TL; FinnProstate Group, : Finnish
multicenter study comparing intermittent to continuous androgen
deprivation for advanced prostate cancer: Interim analysis of
prognostic markers affecting initial response to androgen
deprivation. J Urol. 180:915–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grossfeld GD, Small EJ and Carroll PR:
Intermittent androgen deprivation for clinically localized prostate
cancer: Initial experience. Urology. 51:137–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the Prostate Cancer Clinical
Trials Working Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryan CJ, Elkin EP, Small EJ, Duchane J and
Carroll P: Reduced incidence of bony metastasis at initial prostate
cancer diagnosis: Data from CaPSURE. Urol Oncol. 24:396–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiota M and Eto M: Current status of
primary pharmacotherapy and future perspectives toward upfront
therapy for metastatic hormone-sensitive prostate cancer. Int J
Urol. 23:360–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussain M, Tangen CM, Berry DL, Higano CS,
Crawford ED, Liu G, Wilding G, Prescott S, Sundaram S Kanaga, Small
EJ, et al: Intermittent versus continuous androgen deprivation in
prostate cancer. N Engl J Med. 368:1314–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maximum androgen blockade in advanced
prostate cancer: An overview of the randomised trials with 3283
deaths in 5710 patients. Prostate Cancer Trialists' Collaborative
Group. Lancet. 346:265–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akaza H, Hinotsu S, Usami M, Arai Y,
Kanetake H, Naito S and Hirao Y; Study Group for the Combined
Androgen Blockade Therapy of Prostate Cancer, : Combined androgen
blockade with bicalutamide for advanced prostate cancer: Long-term
follow-up of a phase 3, double-blind, randomized study for
survival. Cancer. 115:3437–3445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitt B, Bennett C, Seidenfeld J, Samson
D and Wilt T: Maximal androgen blockade for advanced prostate
cancer. Cochrane Database Syst Rev: CD001526. 2000.
|
|
26
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horwich A, Parker C, de Reijke T and
Kataja V; ESMO Guidelines Working Group, : Prostate cancer: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi106–vi114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akaza H, Labrie F and Namiki M: A way of
thinking of a MAB therapy for local/locally advanced prostate
cancer: The theory and recent evaluation. Gan To Kagaku Ryoho.
34:657–669. 2007.(In Japanese). PubMed/NCBI
|
|
29
|
Sharifi N, Gulley JL and Dahut WL: An
update on androgen deprivation therapy for prostate cancer. Endocr
Relat Cancer. 17:R305–R315. 2010. View Article : Google Scholar : PubMed/NCBI
|