Introduction
The small molecule multi-kinase inhibitor sorafenib,
a Food & Drug Administration-approved oral agent for the
treatment of hepatocellular carcinoma (HCC) and renal cell
carcinoma, was originally developed as an inhibitor of Raf kinases,
including c-Raf kinase, wild-type and mutant B-Raf, and the
essential serine/threonine kinase constituents of the
Ras/Raf/mitogen-activated protein kinase pathway (1). In addition to its effect on Raf
proteins, sorafenib also potently inhibits receptor tyrosine
kinases, including vascular endothelial growth factor receptors −2
and −3 and the platelet-derived growth factor receptor β (2). Sorafenib has been shown to exert
antitumor activity through inhibiting tumor cell proliferation and
tumor angiogenesis, which is likely due to its effects on these
multiple targets (2). Sorafenib
treatment resulted in ~3-month extension of survival in advanced
HCC patients in two placebo-controlled phase III studies (3,4). Another
phase III study in advanced HCC also demonstrated a significant
improvement in progression-free survival relative to placebo (167
vs. 84 days, respectively) (5).
However, considerable unresponsiveness or acquired resistance to
sorafenib are commonly observed in HCC patients (6,7), the
precise mechanism of which is largely elusive. Thus, there is an
increasing interest in defining the molecular mechanisms underlying
sorafenib resistance in order to increase sorafenibs efficacy and
overcome resistance in patients.
Autophagy is an important cellular process in
response to cellular stresses to maintain proper cell function and
survival (8). Numerous studies have
shed light on the importance of autophagy in tumorigenesis, tumor
proliferation and response to chemotherapy (9). Depending on the cellular context and the
strength and duration of the stimulus, autophagy can either promote
or inhibit cancer cell survival (10). Consistently, autophagy can promote or
suppress apoptosis. As regard to response to chemotherapy,
autophagy may mediate apoptosis to kill cancer cells or suppress
apoptosis to contribute to chemoresistance in different
circumstances. Thus, the manipulation of autophagy could be a
useful strategy to improve the anticancer activity of therapeutics
(11,12). Previous studies revealed that
sorafenib is able to activate autophagy, which may confer a
survival advantage to cancer cells and lead to sorafenib
resistance. By contrast, inhibiting autophagy was shown to promote
sorafenib-induced cell death and increase its anticancer effect
(13–15). A cell death-promoting role of
sorafenib-induced autophagy has also been reported by several
groups (16–18). Therefore, the precise role of
autophagy in sorafenib-induced cell death requires to be
elucidated.
In an effort to increase sorafenibs effects, one
promising strategy is to combine sorafenib with other anticancer
reagents (19,20). However, the outcomes of several of
these studies were not desirable due to safety concerns or not
achieving the expected end point such as improved overall survival
(21,22). Thus, it is important to identify
agents that can be used in combination with sorafenib. Multiple
naturally occurring compounds from diets or medicinal plants can
modulate different cellular survival pathways, thus potentiating
the anticancer activity of drugs used in anticancer therapy
(23,24). One of such compounds is wogonin
(5,7-dihydroxy-8-methoxyflavone), a flavonoid derived from the root
of the medicinal herb Scutellaria baicalensis Georgi
(25). Wogonin has been shown to have
antioxidant, antiviral, antithrombotic and anti-inflammatory
activities in both in vitro and in vivo studies
(26–29). The anticancer activity of wogonin is
demonstrated by inducing apoptosis in cancer cells and suppressing
growth of human cancer xenografts in vivo (30,31).
Wogonin has also been tested to kill cancer cells in combination
with other chemotherapeutics (32,33).
However, the effect of wogonin in combination with sorafenib has
never been reported. Therefore, the present study was designed to
investigate whether the combination of sorafenib and wogonin is
able to increase the anticancer activity of sorafenib in HCC cells
and to elucidate the underlying mechanism. To the best of our
knowledge, the present study is the first report showing that the
combination of wogonin and sorafenib results in a synergistic
cytotoxicity in HCC cells, which occurs through enhancing apoptosis
and inhibiting autophagy.
Materials and methods
Reagents
Sorafenib (Nexavar) and wogonin were purchased from
Bayer AG (Leverkusen, Germany) and Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany), respectively. Antibodies against poly
(ADP-ribose) polymerase (PARP) (catalog no. 556494) and p62
(catalog no. 610832) were acquired from BD Biosciences (Franklin
Lakes, NJ, USA). Anti-β-actin (catalog no. 60008–1-Ig) and
anti-light chain 3 (LC3)B antibodies (catalog no. L7543) were
obtained from ProteinTech Group, Inc. (Chicago, IL, USA) and
Sigma-Aldrich (Merck Millipore), respectively. Chloroquine (CQ) and
3-methyladenine (3-MA) were purchased from Sigma-Aldrich (Merck
Millipore). The pan-caspase inhibitor
carbobenzoxy-valyl-alanyl-aspartyl (Z-VAD) was obtained from
Calbiochem (Merck Millipore).
Cell lines and cell culture
The human HCC cell lines Hep3B, Bel-7402, HepG2 and
SMMC-7721 were purchased from the Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). These cells were
cultured in Dulbeccos modified Eagles medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Sigma-Aldrich; Merck Millipore), 100 U/ml
penicillin and 100 µg/ml streptomycin under standard incubation
conditions (37°C and 5% CO2).
Cytotoxicity assay based on the
release of lactate dehydrogenase (LDH) and apoptosis analysis by
flow cytometry
Following treatment, cell death was quantitatively
detected by a cytotoxicity assay based on the release of LDH using
a cytotoxicity detection kit (Promega Corporation, Madison, WI,
USA) as described previously (34).
All the experiments were repeated 3–5 times and data were expressed
as the mean ± standard deviation (SD). Flow cytometry was applied
to detect apoptosis in cultured cells by using an Annexin V-FITC
Apoptosis Detection kit purchased from Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China). Cells were double stained with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI).
Apoptosis was then analyzed by flow cytometry (BD Biosciences).
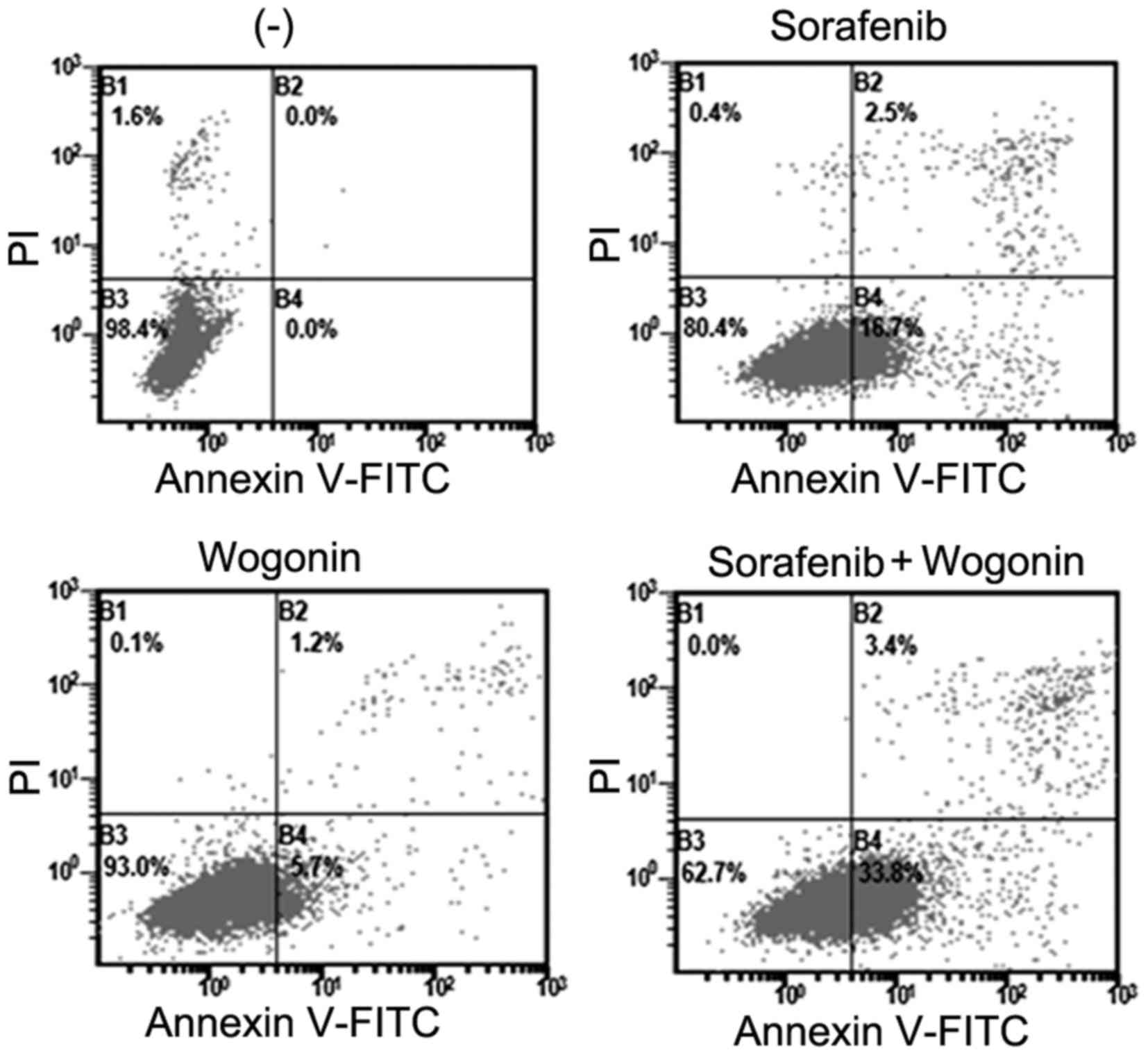
Early apoptotic cells with exposed phosphatidylserine but intact
cell membranes bind Annexin V-FITC but exclude PI, and will be
reported in the lower right-hand quadrant (B4). Necrotic or
apoptotic cells in terminal stages will be both Annexin V-FITC and
PI positive, and will be reported in the upper right-hand quadrant
(B2).
Western blotting
Whole cell lysates were prepared by lysing cells in
M2 buffer [20 mmol/l Tris-HCl (pH 7.6), 0.5% NP40, 250 mmol/l NaCl,
3 mmol/l EDTA, 3 mmol/l ethylene glycol-bis(β-aminoethyl
ether)-N,N,N’,N’-tetraacetic acid, 2 mmol/l dithiothreitol, 0.5
mmol/l phenylmethylsulfonyl fluoride, 20 mmol/l β-glycerophosphate,
1 mmol/l sodium vanadate and 1 µg/ml leupeptin]. The concentration
of proteins in the cell lysates was quantified by Bio-Rad Protein
Assay (Bio Rad Laboratories, Inc., Hercules, CA, USA). Cell lysates
(~50 µg) were resolved by SDS-PAGE ((8% for detecting PARP, 10% for
detecting p62 and β-actin, and 15% for detecting LC3B), transferred
to a polyvinylidene fluoride membrane and detected with various
antibodies: Anti-PARP (1:500; overnight at 4°C); anti-p62 (1:1,000;
overnight at 4°C); anti-β-actin (1:5,000; 1 h at room temperature);
and anti-LC3B (1:1,000; overnight at 4°C). Subsequently, the
membrane was washed three times for 5 min each with TBS containing
Tween-20. Next, peroxidase-conjugated goat anti-mouse
immunoglobulin (Ig)G (catalog no. ZB 2305; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) and
peroxidase-conjugated goat anti-rabbit IgG (catalog no. ZB 2301;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) were added
at a 1:5,000 dilution and incubated with the membrane at room
temperature for 30 min. The specific proteins were visualized by
enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA)
using Image Lab™ station (Bio-Rad Laboratories, Inc.). Each
experiment was repeated at ≥3 times and representative results are
shown.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
significance was examined by paired Students t test using
SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Combined treatment of sorafenib and
wogonin induces synergistic cytotoxicity in human HCC cells
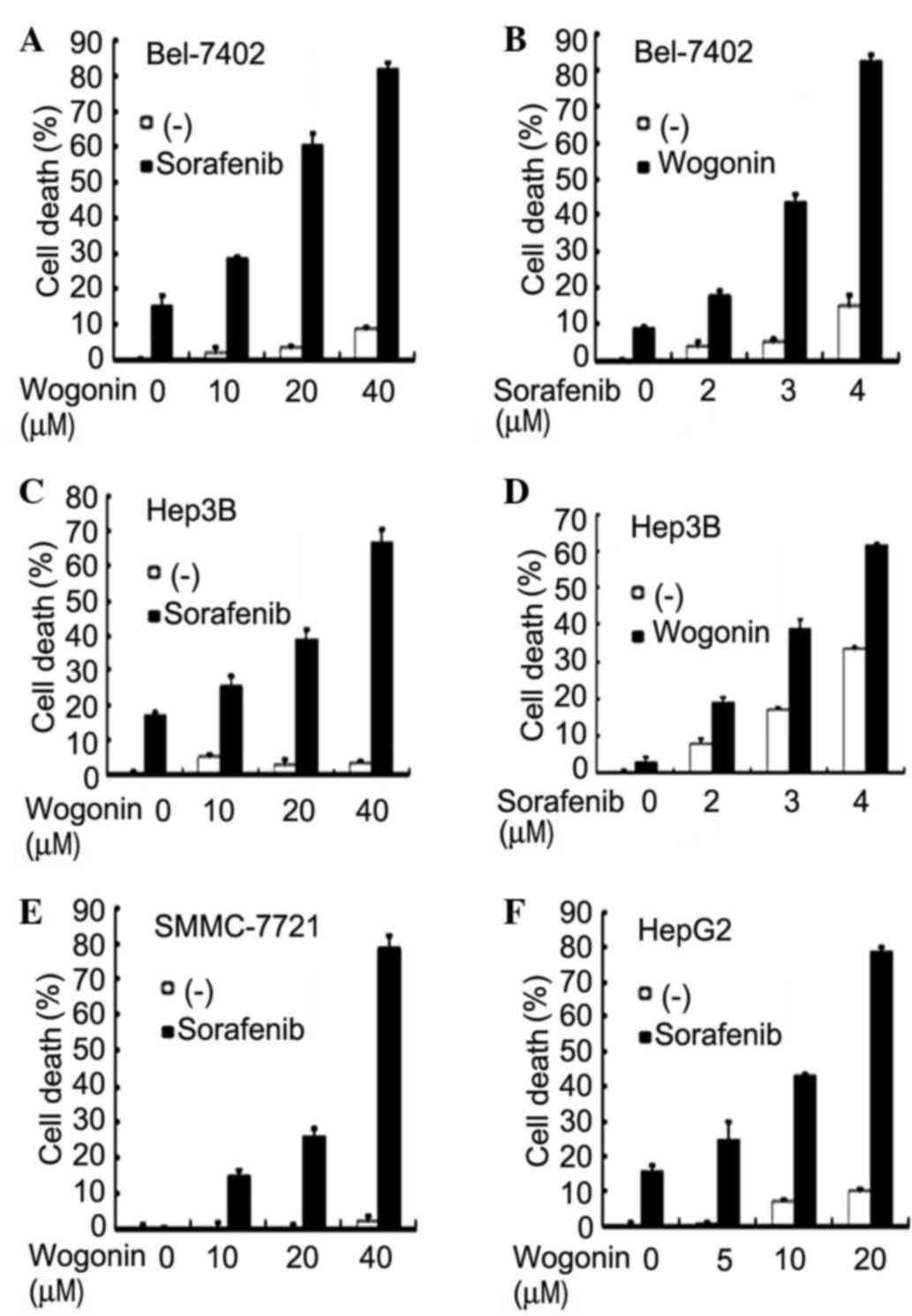
To investigate whether wogonin is able to enhance
the anticancer activity of sorafenib, Bel-7402 cells were first
treated with increasing concentrations of wogonin (10–40 µM) and a
fixed concentration of sorafenib (4 µM), and cell death was
measured by LDH release assay. The results indicated that, while
sorafenib alone caused <20% cell death and wogonin had little
cytotoxicity, wogonin sensitized Bel-7402 cells to
sorafenib-induced cell death in a dose-dependent manner (Fig. 1A). Approximately 80% of the cells were
killed at the highest dose of wogonin used (40 µM), a concentration
at which wogonin alone caused little cell death (<10%). The
combined cytotoxic effect of wogonin and sorafenib was synergistic,
as evaluated by combination index analysis as described previously
(35) (Table I). A similar dose-dependent
potentiation of cytotoxicity was detected when increasing
concentrations of sorafenib (2–4 µM) with a fixed wogonin dose (40
µM) were used (Fig. 1B). The
sensitization of sorafenibs anticancer activity by wogonin was
validated in other human HCC cell lines. In Hep3B cells, a similar
dose-dependent synergism with fixed concentration of either
sorafenib or wogonin was observed (Fig.
1C and D). Consistently, wogonin sensitized HepG2 and SMMC-7721
cells to sorafenib-induced cell death (Fig. 1E and F). These results suggest that
the combination of wogonin and sorafenib is effective in
sensitizing HCC cells to sorafenib-induced cytotoxicity.
 | Table I.Synergistic interaction of sorafenib
and wogonin in human hepatocellular carcinoma cells. |
Table I.
Synergistic interaction of sorafenib
and wogonin in human hepatocellular carcinoma cells.
| A, Bel-7402 |
|---|
|
|---|
| Sorafenib
(µmol/l) | Wogonin (µmol/l) | CI |
|---|
| 2 | 40 | 0.781 |
| 3 | 40 | 0.457 |
| 4 | 40 | 0.225 |
| 4 | 10 | 0.643 |
| 4 | 20 | 0.351 |
|
| B, Hep3B |
|
| Sorafenib
(µmol/l) | Wogonin
(µmol/l) | CI |
|
| 2 | 20 | 0.691 |
| 3 | 20 | 0.663 |
| 4 | 20 | 0.640 |
| 3 | 10 | 0.873 |
| 3 | 40 | 0.448 |
|
| C, SMMC-7721 |
|
| Sorafenib
(µmol/l) | Wogonin
(µmol/l) | CI |
|
| 2 | 20 | 0.223 |
| 4 | 20 | 0.057 |
| 6 | 20 | 0.003 |
| 4 | 10 | 0.153 |
|
| D, HepG2 |
|
| Sorafenib
(µmol/l) | Wogonin
(µmol/l) | CI |
|
| 4 | 5 | 0.985 |
| 4 | 10 | 0.895 |
| 4 | 20 | 0.683 |
The potentiated cytotoxicity induced
by sorafenib and wogonin combination is achieved through apoptosis
potentiation
As both wogonin and sorafenib can induce apoptosis,
it was hypothesized that the enhanced cell death observed in
wogonin and sorafenib co-treatment was achieved through
potentiation of apoptosis. Hep3B cells were treated with sorafenib
in the absence or presence of wogonin, and apoptosis was analyzed
by Annexin V-FITC and PI staining followed by flow cytometric
assay. Both early (B4) and late (B2) apoptotic cells were
significantly increased upon sorafenib and wogonin co-treatment
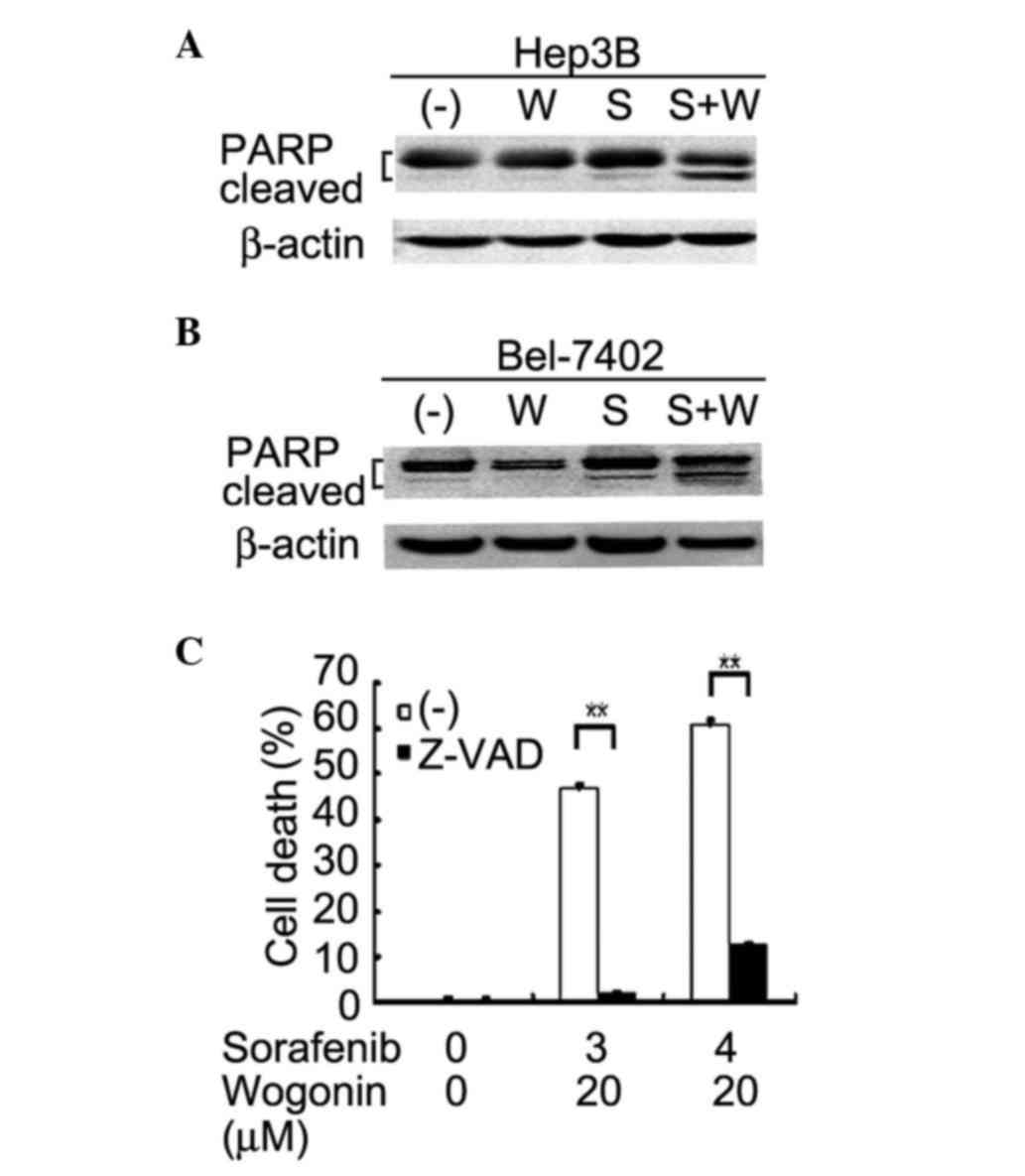
(Fig. 2). The cleavage of the
caspase-3 substrate PARP, which is a marker of apoptotic pathway
activation, was increased in co-treated Hep3B and Bel-7402 cells,
as detected by western blotting (Fig. 3A
and B). Additionally, the pan-caspase inhibitor Z-VAD
significantly suppressed the enhanced cytotoxicity induced by
co-treatment with sorafenib and wogonin in Hep3B and Bel-7402 cells
(Fig. 3C and data not shown,
respectively). These results suggest that the enhanced cytotoxicity
induced by sorafenib and wogonin combination was due to
potentiation of apoptosis.
Wogonin inhibits sorafenib-induced
cytoprotective autophagy and sensitizes cells to cytotoxicity
As sorafenib activates autophagy and the latter can
either activate or suppress apoptosis, it was further investigated
if autophagy is involved in the synergistic cytotoxity of sorafenib
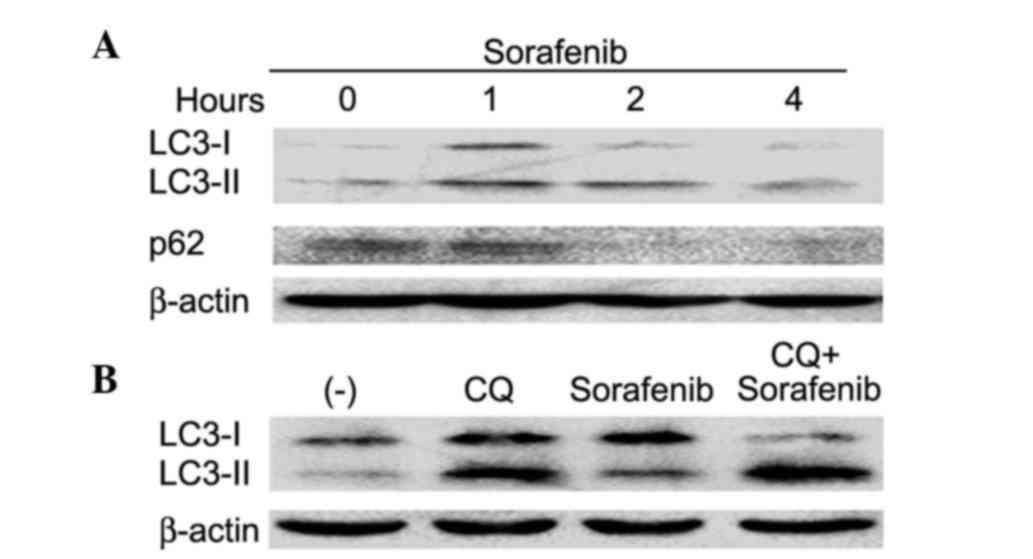
and wogonin. Sorafenib induced autophagy, which was shown as
increased expression of LC3-II and decreased expression of p62, two
autophagy hallmarks (Fig. 4A), in
addition to autophagic flux (Fig.
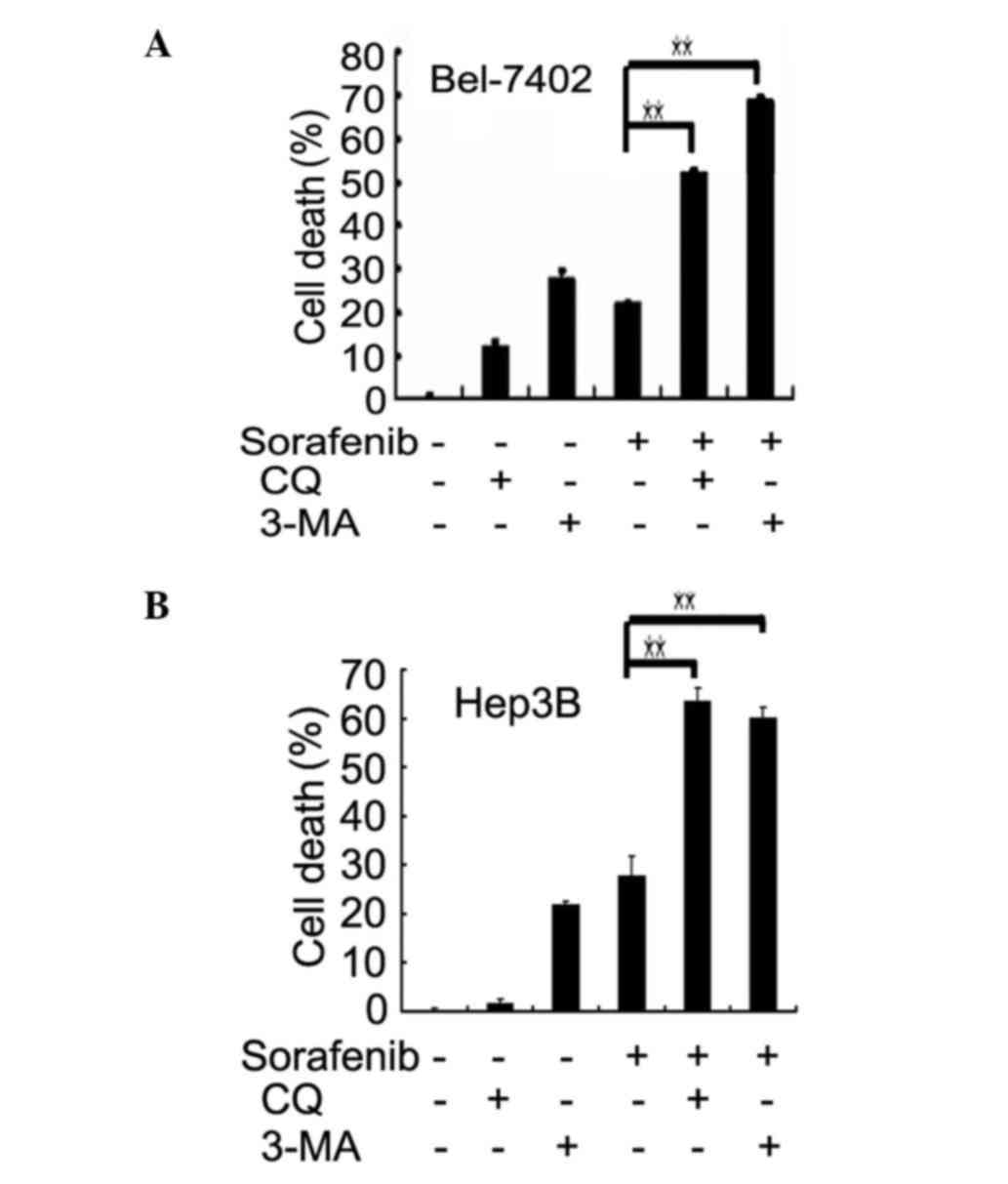
4B). The role of sorafenib-induced autophagy was determined to
be cytoprotective, since suppression of autophagy with two
different autophagy inhibitors, CQ and 3-MA, remarkably increased
sorafenib-induced cell death in both Bel-7402 and Hep3B cells
(Fig. 5A and B). Next, the effect of
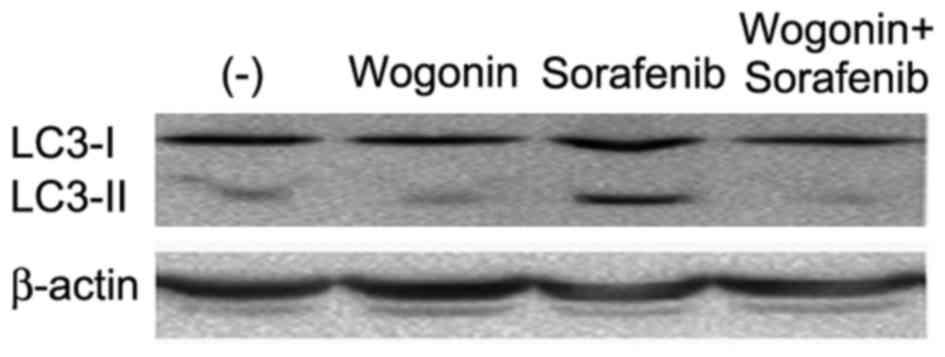
wogonin on sorafenib-induced autophagy was examined. Wogonin
effectively inhibited sorafenib-induced autophagy, which was shown
as suppression of sorafenib-induced LC3-II expression (Fig. 6). Therefore, these data demonstrate
that wogonin likely potentiated sorafenib-induced cell death
through inhibiting autophagy.
Discussion
The present study first determined that sorafenib
and wogonin combination is effective in killing multiple human HCC
cell types. Enhanced cell death in co-treated cells was accompanied
by potentiation of apoptosis. Furthermore, it was confirmed that
sorafenib induced cytoprotective autophagy in HCC cells. Notably,
wogonin also inhibited sorafenib-induced autophagy. Thus,
potentiation of apoptosis and inhibition of sorafenib-induced
protective autophagy may contribute to the enhanced cancer cell
death caused by sorafenib and wogonin combination.
Wogonin is a candidate anticancer agent, which was
shown to exert an apoptotic effect in tumor cells but has no
obvious toxicity in normal cells (36,37).
Besides combination with classic DNA damaging agents for cancer
therapy, it is of great interest to investigate if wogonin can be
combined with targeted-therapy agents. The present study
demonstrated for the first time that the combination of wogonin and
sorafenib effectively kills human HCC cells, suggesting that
wogonin could be an ideal candidate for increasing sorafenibs
activity in HCC therapy, which warrants further in vivo
investigation.
Apoptosis activation is one of the major mechanism
underlying the anticancer activity of chemotherapeutics. The
present study clearly demonstrated that the combination of
sorafenib and wogonin cooperatively promoted apoptosis, which is at
least one of the mechanism for enhanced anticancer activity. The
mechanism by which sorafenib-induced apoptosis is promoted by
wogonin deserves further study.
Previous studies have shown that sorafenib induces
autophagy in multiple cancer cell types, which could be
cytoprotective or cytotoxic, thus suppressing or promoting the
anticancer activity of sorafenib (13–18). The
autophagy induced by sorafenib in our experimental system was
clearly determined to be cytoprotective in both Bel-7402 and Hep3B
cells. As wogonin effectively blocked sorafenib-induced autophagy,
it is likely that blocking cytoprotective autophagy underlies
another mechanism of the enhanced cancer cell death caused by
sorafenib and wogonin combination.
Taken together, the present study is the first to
report that wogonin sensitizes sorafenibs anti-HCC activity, which
is associated with apoptosis potentiation and autophagy inhibition.
Sorafenib and wogonin combination may be an ideal approach for
increasing sorafenibs anticancer activity, which warrants further
in vivo investigation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81172111
and 81372377) and the Science & Technology Department of
Sichuan Province, China (grant no. 2015JY0096).
References
|
1
|
Wilhelm S, Carter C, Lynch M, Lowinger T,
Dumas J, Smith RA, Schwartz B, Simantov R and Kelley S: Discovery
and development of sorafenib: A multikinase inhibitor for treating
cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C.: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Grou: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kane RC, Farrell AT, Saber H, Tang S,
Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et
al: Sorafenib for the treatment of advanced renal cell carcinoma.
Clin Cancer Res. 12:7271–7278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keating GM and Santoro A: Sorafenib: A
review of its use in advanced hepatocellular carcinoma. Drugs.
69:223–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czaja MJ, Ding WX, Donohue TM Jr, Friedman
SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, et
al: Functions of autophagy in normal and diseased liver. Autophagy.
9:1131–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding ZB, Hui B, Shi YH, Zhou J, Peng YF,
Gu CY, Yang H, Shi GM, Ke AW, Wang XY, et al: Autophagy activation
in hepatocellular carcinoma contributes to the tolerance of
oxaliplatin via reactive oxygen species modulation. Clin Cancer
Res. 17:6229–6238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo T, Fu J, Xu A, Su B, Ren Y, Li N, Zhu
J, Zhao X, Dai R, Cao J, et al: PSMD10/gankyrin induces autophagy
to promote tumor progression through cytoplasmic interaction with
ATG7 and nuclear transactivation of ATG7 expression. Autophagy.
12:1355–1371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizu S, Takehara T, Hikita H, Kodama T,
Tsunematsu H, Miyagi T, Hosui A, Ishida H, Tatsumi T, Kanto T, et
al: Inhibition of autophagy potentiates the antitumor effect of the
multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J
Cancer. 131:548–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke
AW, Wang XY, Dai Z, Peng YF, Gu CY, et al: Targeting autophagy
enhances sorafenib lethality for hepatocellular carcinoma via ER
stress-related apoptosis. Autophagy. 7:1159–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park MA, Zhang G, Martin AP, Hamed H,
Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al:
Vorinostat and sorafenib increase ER stress, autophagy and
apoptosis via ceramide-dependent CD95 and PERK activation. Cancer
Biol Ther. 7:1648–1662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai WT, Shiau CW, Chen HL, Liu CY, Lin CS,
Cheng AL, Chen PJ and Chen KF: Mcl-1-dependent activation of Beclin
1 mediates autophagic cell death induced by sorafenib and SC-59 in
hepatocellular carcinoma cells. Cell Death Dis. 4:e4852013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CI, Whang EE, Lorch JH and Ruan DT:
Autophagic activation potentiates the antiproliferative effects of
tyrosine kinase inhibitors in medullary thyroid cancer. Surgery.
152:1142–1149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bareford MD, Hamed HA, Tang Y,
Cruickshanks N, Burow ME, Fisher PB, Moran RG, Nephew KP, Grant S
and Dent P: Sorafenib enhances pemetrexed cytotoxicity through an
autophagy-dependent mechanism in cancer cells. Autophagy.
7:1261–1262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sajithlal GB, Hamed HA, Cruickshanks N,
Booth L, Tavallai S, Syed J, Grant S, Poklepovic A and Dent P:
Sorafenib/regorafenib and phosphatidyl inositol 3 kinase/thymoma
viral proto-oncogene inhibition interact to kill tumor cells. Mol
Pharmacol. 84:562–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grignani G, Palmerini E, Ferraresi V,
DAmbrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Italian Sarcoma Group: Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flaherty KT, Lee SJ, Zhao F, Schuchter LM,
Flaherty L, Kefford R, Atkins MB, Leming P and Kirkwood JM: Phase
III trial of carboplatin and paclitaxel with or without sorafenib
in metastatic melanoma. J Clin Oncol. 31:373–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paz-Ares LG, Biesma B, Heigener D, von
Pawel J, Eisen T, Bennouna J, Zhang L, Liao M, Sun Y, Gans S, et al
NSCLC [non-small-cell lung cancer] Research Experience Utilizing
Sorafenib (NExUS) Investigators Study Group: Phase III, randomized,
double-blind, placebo-controlled trial of gemcitabine/cisplatin
alone or with sorafenib for the first-line treatment of advanced,
nonsquamous non-small-cell lung cancer. J Clin Oncol. 30:3084–3092.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banerjee S, Wang Z, Kong D and Sarkar FH:
3,3′-Diindolylmethane enhances chemosensitivity of multiple
chemotherapeutic agents in pancreatic cancer. Cancer Res.
69:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turrini E, Ferruzzi L and Fimognari C:
Natural compounds to overcome cancer chemoresistance: Toxicological
and clinical issues. Expert Opin Drug Metab Toxicol. 10:1677–1690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fas SC, Baumann S, Zhu JY, Giasi M,
Treiber MK, Mahlknecht U, Krammer PH and Li-Weber M: Wogonin
sensitizes resistant malignant cells to TNF- and TRAIL-induced
apoptosis. Blood. 108:3700–3706. 2007. View Article : Google Scholar
|
|
26
|
Zhao Y, Li H, Gao Z, Gong Y and Xu H:
Effects of flavonoids extracted from Scutellaria baicalensis Georgi
on hemin-nitrite-H2O2 induced liver injury.
Eur J Pharmacol. 536:192–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma SC, Du J, But PP, Deng XL, Zhang YW,
Ooi VE, Xu HX, Lee SH and Lee SF: Antiviral Chinese medicinal herbs
against respiratory syncytial virus. J Ethnopharmacol. 79:205–211.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura Y, Okuda H and Ogita Z: Effects of
flavonoids isolated from Scutellariae radix on fibrinolytic system
induced by trypsin in human umbilical vein endothelial cells. J Nat
Prod. 60:598–601. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chi YS, Lim H, Park H and Kim HP: Effects
of wogonin, a plant flavone from Scutellaria radix, on skin
inflammation: In vivo regulation of inflammation-associated gene
expression. Biochem Pharmacol. 66:1271–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Polier G, Ding J, Konkimalla BV, Eick D,
Ribeiro N, Köhler R, Giaisi M, Efferth T, Desaubry L, Krammer PH
and Li-Weber M: Wogonin and related natural flavones are inhibitors
of CDK9 that induce apoptosis in cancer cells by transcriptional
suppression of Mcl-1. Cell Death Dis. 2:e1822011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung H, Jung YM, Shin DH, Lee JY, Oh MY,
Kim HJ, Jang KS, Jeon SJ, Son KH and Kong G: Anticancer effects of
wogonin in both estrogen receptor-positive and -negative human
breast cancer cell lines in vitro and in nude mice xenografts. Int
J Cancer. 122:816–822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He F, Wang Q, Zheng XL, Yan JQ, Yang L,
Sun H, Hu LN, Lin Y and Wang X: Wogonin potentiates
cisplatin-induced cancer cell apoptosis through accumulation of
intracellular reactive oxygen species. Oncol Rep. 28:601–605.
2012.PubMed/NCBI
|
|
33
|
Lee E, Enomoto R, Koshiba C and Hirano H:
Inhibition of P-glycoprotein by wogonin is involved with the
potentiation of etoposide-induced apoptosis in cancer cells. Ann N
Y Acad Sci. 1171:132–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Ju W, Renouard J, Aden J, Belinsky
SA and Lin Y: 17-Allylamino-17-demethoxygeldanamycin
synergistically potentiates tumor necrosis factor-induced lung
cancer cell death by blocking the nuclear factor-kappaB pathway.
Cancer Res. 66:1089–1095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, Wientjes MG and Au JL: Evaluation
of combination chemotherapy: Integration of nonlinear regression,
curve shift, isobologram, and combination index analyses. Clin
Cancer Res. 10:7994–8004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baumann S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCgamma1- and Ca2+-dependent
apoptosis. Blood. 111:2354–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|