Introduction
Human T-cell lymphotropic virus type (HTLV)-1 is a
retrovirus known to cause human infections, and it was first
isolated from a patient with cutaneous T-cell lymphoma (1).
Although the majority of infected individuals remain
lifelong asymptomatic carriers of the virus, ~4% of seropositive
individuals develop adult T-cell leukemia/lymphoma (ATLL) (2). ATLL is highly endemic, particularly in
Southern Japan and in the Caribbean Basin, and exhibits rapid
progression, drug resistance and poor prognosis (3,4). ATLL
patients are in a severely immunocompromised state, which leads to
frequent and severe infectious complications, and the underlying
mechanisms responsible for this remain unclear (5). Recently, several investigators have
reported that forkhead box P3 (Foxp3), which is a master control
gene for the development and function of cluster of differentiation
(CD) 4+CD25+ naturally occurring regulatory T (Treg) cells, is
expressed in the tumor cells of ATLL patients (6). The suppression of the host's normal
effector T-cells [including cytotoxic T lymphocytes (CTLs)] by the
tumor cells could result in a severely immunocompromised state,
which is one of the clinical characteristics of ATLL patients
(7). It was reported that HTLV-1
infection was associated with other infectious diseases such as
Pneumocystis carinii pneumonia and strongyloidiasis
(8). In these diseases, an increase
in the Treg-cell number (9) and a
decrease in the T helper 2 response (10) were observed. In addition, it can be
envisaged that ATLL cells could function as Treg cells and lead to
a profound immunosuppressive environment, enabling them to escape
from the host immune response. The molecular mechanism by which
Treg cells exert their suppressor/regulatory activity was regarded
to require cell-to-cell contact with the cell being suppressed
(11).
Tax, a viral protein, has been reported to be
important in the proliferation of ATLL cells and a target of
Tax-specific CTLs (12). Tax is
encoded by the pX region between envelope and 3′-long terminal
repeat (LTR) (13). Tax is considered
to play a central role in ATL lymphomagenesis by its pleiotropic
actions, including trans-activation of cell proliferation factors
such as nuclear factor-κB, cAMP response element binding and the
serum response factor pathway (14),
and functional inactivation of cell cycle regulators such as p16,
p53 and mitotic arrest deficient like 1 (15). Development of leukemia and lymphoma in
mice transgenic for Tax was reported (16).
Tax is known to be a major target antigen of
HTLV-1-specific CTLs (17). Kannagi
et al (17) indicated that
Tax-specific CTLs in ATLL patients are inactive, and that
Tax-specific CTL response is strongly activated following
hematopoietic stem cell transplantation (HSCT) in certain ATLL
patients in long-term remission. These findings suggest that ATLL
cells escape from the host immune system, and that reactivation of
Tax-specific CTLs may provide promising prophylactic and
therapeutic approaches for HTLV-1 carriers and for ATLL patients
whose ATLL cells retain the ability to express Tax.
In addition, ATLL cells often contain genetic and
epigenetic alterations of the 5′-LTR of the HTLV-1 provirus,
resulting in the loss of Tax expression (18). Takeda et al (18) reported that Tax transcripts were
detected in only 40% of all ATLL cases. Furthermore, human
leukocyte antigen (HLA) class I antigen downregulation or loss has
been detected in numerous malignancies, including melanoma, colon
cancer, prostate cancer and lung cancer (19). This downregulation was not reported in
ATLL, although it may cause ATLL cells to escape from the host
immune system.
There were several reports on whether ATLL cells
function as Treg cells in a peripheral blood autologous setting
(6,7,20,21), but it is not clear how Tax-specific
CTLs behave in lymph nodes of ATLL patients, or whether ATLL cells
in lymph nodes function as Treg cells.
In the present study, Tax-specific CTLs
(HLA-A24-restricted) were stained using MHC Dextramer®
assay. In addition, Tax, interferon (IFN) γ and HLA-A24 were
immunostained in frozen sections, and the association between
Tax-specific CTLs and Tax expression, Foxp3 positivity and HLA-A24
expression was investigated in order to reveal the function of ATLL
cells and Tax-specific CTLs in lymph nodes.
Materials and methods
Case selection
A total of 15 ATLL cases with HLA-A24 (for which Tax
has a high affinity) were selected from the files of the Department
of Pathology, School of Medicine, Kurume University (Kurume,
Japan). The 15 patients were diagnosed between April 2004 and March
2012. All patients were positive for ATLA or amplified HTLV-1 pX
gene. Patients lymph node samples were positive for HLA-A24, as
detected by polymerase chain reaction (PCR).
Clinical information was obtained from clinical
charts and attending physicians. Histologic review was conducted by
three of the current authors (K.O., D.N. and A.I.), and lesions
were classified into three types: i) Pleomorphic cell variant,
large-sized cells predominant (pleomorphic large); ii) pleomorphic
cell variant, medium-sized cells predominant (pleomorphic medium);
and iii) anaplastic large cell variant (ALCL). The diagnosis was
made as previously described (6),
mainly by following the World Health Organization classification
criteria (5).
The present study was approved by the Kurume
University Institutional Review Board (Kurume, Japan), and was
conducted in accordance with the ethical guidelines mandated by the
Declaration of Helsinki.
Polymerase chain reaction (PCR) for
the detection of HLA-A24
DNA samples were extracted using a commercial kit
(Blood & Tissue Genomic Extraction Miniprep System; Viogene
BioTek Corp., Sunnyvale, CA, USA). PCR amplification was performed
with AmpliTaq Gold Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
HLA-A belongs to the HLA class I heavy chain
paralogues. This class I molecule is a heterodimer consisting of a
heavy chain and a light chain (β-2 microglobulin). The heavy chain
is ~45 kDa and its gene contains 8 exons. Polymorphisms within exon
2 and exon 3 are responsible for the peptide invading specificity
of each class one molecule (22).
If exon 2 and exon 3 were detected by PCR, the cases
were regarded as positive for HLA-A24. Exon 2 was amplified by
semi-nested PCR using the sense primers 5′EX2-A (1st FW primer) for
first-round PCR, and A24/EX2-FW1 (2nd FW primer) for second-round
PCR, and anti-sense primer A24/EX2-RV2 (1st 2nd RV primer). Exon 3
was amplified by semi-nested PCR using the sense primer A24/EX3-FW4
(1st 2nd FW primer) and anti-sense primer 3′EX3-A (1st RV primer)
for first-round PCR, and 3′EX3-R4 (2nd RV primer) for second-round
PCR.
The first-round PCR conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 30 cycles of
95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, with a final
extension at 72°C for 10 min. The second-round PCR conditions were
as follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec,
with a final extension at 72°C for 10 min. The primer sequences
were as follows: 5′EX2-A, 5′-CCCCAGGCTCYCACTCCATGAGGTATTTC-3′;
A24/EX2-FW1, 5′-CCACTCCATGAGGTATTTCTC-3′; A24/EX2-RV2,
5′-GTTGTAGTAGCGGAGCGC-3′; A24/EX3-FW4, 5′-CACACCCTCCAGATGATGTT-3′;
3′EX3-A, 5′-TCTCCTTCCCGTTCTCCAGGTATC-3′; and 3′EX3-R4,
5′-CCCGTTCTCCAGGTATCT-3′. The amplified PCR products were
electrophoresed in 3% agarose gels and visualized by ethidium
bromide staining under ultraviolet light. The PCR products were of
the appropriate lengths of ~250 bp.
Immunohistochemistry
Immunohistochemical staining for CD3, CD20, CD4,
CD8, CD15, CD30, T-cell intracellular antigen (TIA)-1 and Foxp3 was
performed using 2.5-µm-thick, formalin-fixed, paraffin-embedded
tissue sections. The slides were deparaffinized with xylene,
followed by ethanol. Following rehydration with water, antigen
retrieval was performed with EDTA or TE buffer in a microwave oven.
Following cooling and rinsing with buffer, endogenous peroxidase
activity was blocked by incubating in 3% hydrogen peroxide for 5
min. Slides were incubated with monoclonal antibodies for 30 min at
room temperature. The slides were incubated with an EnVision+
System horseradish peroxide-labeled anti-rabbit polymer (K4003;
Dako, Glostrup, Denmark) for 30 min for CD4, and an EnVision+
System horseradish peroxide-labeled anti-mouse polymer for CD3,
CD20, CD8, CD15, CD30, TIA-1 and Foxp3.
A portion of each sample was maintained at −80°C.
These 2.5-µm-thick, fixed in cold acetone, frozen sections were
examined using monoclonal antibodies against Tax, INFγ and HLA-24.
Following rehydration with water, endogenous peroxidase activity
was blocked by incubating in 3% hydrogen peroxide for 5 min. Slides
were incubated with monoclonal antibodies for 30 min at room
temperature. The slides were incubated with an EnVision+ System
horseradish peroxide-labeled anti-rabbit polymer for 30 min.
Visualization of immunostaining was performed using
diaminobenzidine for 10 min. Slides were counterstained with
hematoxylin, dehydrated with ethanol and mounted under
coverslips.
The antibodies used included anti-CD3 (clone
F7.2.38; Dako; diluted, 1:50), anti-CD20 (clone L-26; Dako;
diluted, 1:5) anti-CD4 (clone 4H5; Medical & Biological
Laboratories, Co., Ltd., Nagoya, Japan; diluted, 1:30), anti-CD8
(clone 1A5; Leica Microsystems GmbH, Wetzlar, Germany; diluted,
1:50), anti-CD15 (clone MMA; BD Biosciences, Franklin Lakes, NJ,
USA; diluted, 1:50), anti-CD30 (clone Ber-H2; Dako; diluted,
1:100), anti-TIA-1 (clone 2G9A10F5; Immunotech; Beckman Coulter,
Inc., Brea, CA, USA; diluted, 1:200) and anti-Foxp3 (clone FJK-16s;
eBioscience, Inc., San Diego, CA, USA; diluted, 1:50). A portion of
each sample was maintained at −80°C in a deep freezer. These frozen
tissues were examined using monoclonal antibodies against Tax
(kindly donated by Dr M. Matsuoka; Kyoto University, Kyoto, Japan;
diluted 1:200), INFγ (ab9657; Abcam, Cambridge, UK; diluted,
1:1,000) and HLA-A24 (clone 17A10; MBL International Co., Woburn,
MA, USA; diluted, 1:1,000).
Reverse transcription (RT)-PCR
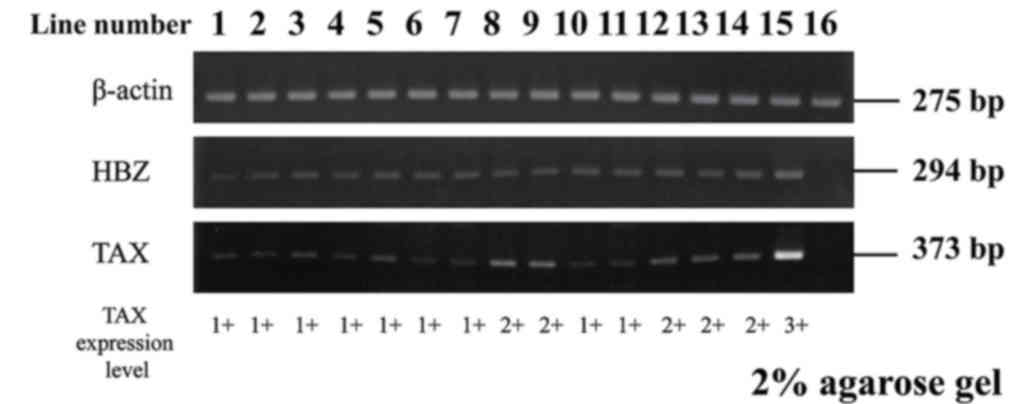
Previous studies have reported that the Tax gene is
expressed in few ATLL cases; however, the HBZ gene is expressed in
all ATLL cases (18,23) We evaluated the messenger RNA
expression of tax and HBZ by RT-PCR in order to ascertain HTLV-1
infection. Complementary (c) DNA was reverse transcribed from ~4.5
µg total RNA using Ready-To-Go You-Prime First-Strand Beads (GE
Healthcare Life Sciences, Chalfont, UK) and primed with an
oligo(dT) oligonucleotide (GE Healthcare Life Sciences). cDNA (3
µl) was subjected to PCR using AmpliTaq Gold DNA Polymerase
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
oligonucleotide primers were as follows: Hemoglobin subunit zeta
(HBZ) 5′-AAACGCATCGTGATCGGCAGCGAC-3′ (sense) and
5′-CTTCCAACTGTCTAGTATAGCCATCA-3′ (antisense); Tax,
5′-CTCTGGGGGACTATGTTCGGCC-3′ (sense) and
5′-GTACATGCAGACAACGGAGCCT-3′ (antisense); and β-actin,
5′-CAAGAGATGGCCACGGCTGCT-3′ (sense) and 5′-TCCTTCTGCATCCTGTCGGCA-3′
(antisense). Product sizes were 294 bp for HBZ, 373 bp for Tax and
275 bp for β-actin. Amplification conditions consisted of
denaturation at 95°C for 30 sec (10 min for the first cycle),
annealing at 60°C for 30 sec and extension at 72°C for 1 min (10
min for the last cycle) for 35 cycles for HBZ and Tax, and 30
cycles for β-actin. The amplified products were evaluated in 2%
agarose gels and visualized by ethidium bromide staining under
ultraviolet light. The quality of cDNA was monitored using RT-PCR
with β-actin primers. cDNAs yielding a 275-bp product for β-actin
messenger RNA without contamination with the 370-bp genomic
amplification product were used for this experiment.
The following cell lines were used: MT-4, HTLV-1
infected cell line, as a positive control and Jurkat, T-cell acute
lymphoblastic leukemia cell line (HTLV-1 uninfected cell line), as
a negative control.
Detection of Tax-specific CTLs
The presence of HLA-A24/Tax reactive CD8+ T
lymphocytes among infiltrating lymphocytes in lymph nodes of ATLL
patients was evaluated by means of HLA/peptide dextramer staining.
Fluorescein isothiocyanate (FITC)-conjugated HLA-A24/Tax dextramers
(Immudex, Copenhagen, Denmark) were used to stain acetone-fixed,
frozen materials as described previously (24,25), and
antigen-specific cells were visualized using a confocal laser
microscope.
For staining with FITC-conjugated multimeric
peptide/major histocompatibility complex (MHC) complexes, sections
were cut (of 2.5-µm thickness) and air-dried for 30 min prior to be
fixed in cold acetone for 5 min. All the incubation steps were
performed at room temperature in the dark, as follows: i) 45 min
with the primary antibody (anti-CD8) (1:100 diluted); ii) cyanine
3-conjugated goat anti-mouse (1:500 diluted; 115-165-100; Jackson
ImmunoResearch Europe, Ltd., Newmarket, UK) for 45 min; and iii)
dextramer for 75 min. Between each step, the slides were washed
three times for 5 min in PBS containing 0.1% bovine serum albumin.
The slides were mounted in VECTASHIELD (Vector Laboratories, Inc.,
Burlingame, CA, USA) and kept in the refrigerator until observed
under a fluorescence microscope (model BZ-9000; Keyence
Corporation, Osaka, Japan).
Statistical analysis
Student's t test and χ2 test were used
for comparisons of clinical and pathological findings among
different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histopathological and
immunohistochemical features
The pathological findings are listed in Table I. The 15 cases enrolled in our study
comprised 8 females and 7 males aged 55–83 years, with a mean age
of 70 years. Of the 15 cases, 8 cases were classified as
pleomorphic large, 6 cases as pleomorphic medium and 1 case as
ALCL.
 | Table I.Pathological findings of 15
cases. |
Table I.
Pathological findings of 15
cases.
|
|
|
|
| Immunostaining |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case | G | Age (y) | Morphology | CD3 (%) | CD4 (%) | CD8 (%) | Foxp3 (%) | HLA.A24 (%) | INFγa |
Tax-CTLa | Tax (%) | Tax (RT-PCR) | HBZ (RT-PCR) | HLA.A24 (PCR) |
|---|
| 1 | F | 69 | Medium | 90 | 100 | 0 | 90 | 95 | 0.0 | 0 | 14 | 1+ | 1+ | + |
| 2 | M | 62 | Medium | 70 | 60 | 70 | 50 | 95 | 1.0 | 0 | 9 | n.d. | n.d. | + |
| 3 | F | 76 | Medium | 90 | 100 | 0 | 40 | 0 | 0.0 | 0 | 18 | 1+ | 1+ | + |
| 4 | M | 75 | Medium | 90 | 100 | 90 | 40 | 10 | 0.5 | 0 | 6 | 1+ | 1+ | + |
| 5 | F | 72 | Medium | 25 | 100 | 0 | 20 | 95 | 0.0 | 6 | 18 | 1+ | 1+ | + |
| 6 | F | 71 | Medium | 50 | 100 | 0 | 5 | 10 | 0.5 | 6 | 6 | 1+ | 2+ | + |
| 7 | M | 76 | Large | 34 | 100 | 0 | 10 | 50 | 0.5 | 4 | 10 | 1+ | 2+ | + |
| 8 | M | 73 | Large | 100 | 100 | 90 | 10 | 80 | 1.0 | 2 | 18 | 1+ | 2+ | + |
| 9 | F | 72 | Large | 100 | 100 | 100 | 10 | 10 | 1.0 | 4 | 2 | 2+ | 2+ | + |
| 10 | M | 61 | Large | 5 | 100 | 0 | 7 | 80 | 0.5 | 7 | 4 | 2+ | 2+ | + |
| 11 | F | 83 | Large | 70 | 100 | 0 | 5 | 80 | 1.0 | n.d. | 8 | 1+ | 2+ | + |
| 12 | M | 55 | Large | 24 | 90 | 80 | 2 | 95 | 0.5 | 4 | 6 | 1+ | 2+ | + |
| 13 | F | 80 | Large | 0 | 100 | 0 | 2 | 90 | 2.5 | 0 | 2 | 2+ | 2+ | + |
| 14 | F | 66 | Large | 50 | 100 | 0 | 0 | 90 | 0.5 | 8 | 3 | 2+ | 2+ | + |
| 15 | M | 69 | Anaplastic | 10 | 100 | 0 | 7 | 5 | 3.5 | 5 | 12 | 2+ | 2+ | + |
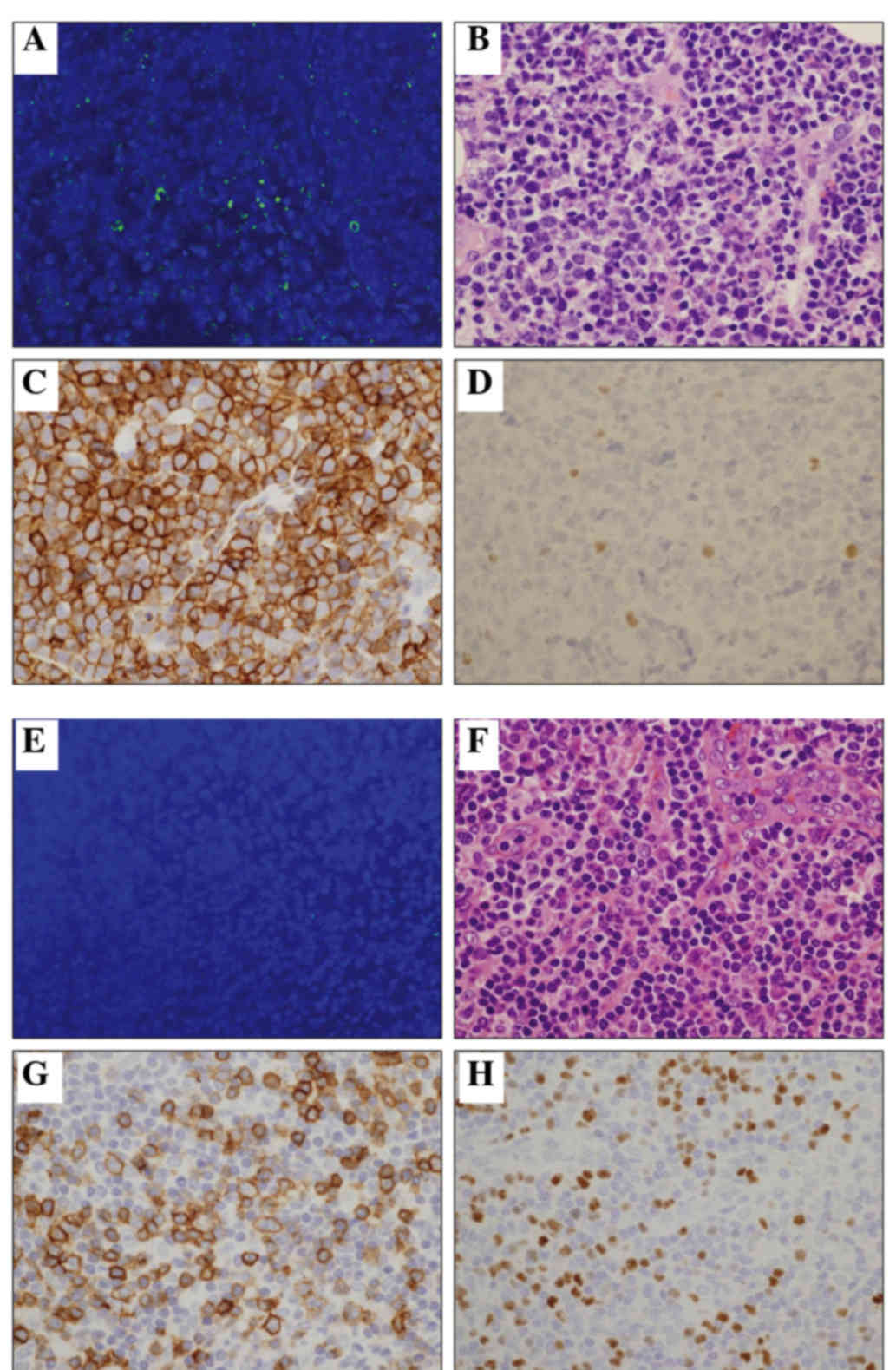
Immunohistochemical staining of CD3 demonstrated
various lymphoma cells to be positive, ranging from 0 to 100%
(median, 70%). The number of CD4-positive lymphoma cells ranged
from 60 to 100% (median, 100%), while the number of CD8-positive
lymphoma cells ranged from 0 to 100% (median, 0%). A certain number
of reactive lymphocytes in the background were also positive for
CD8.
Fluorescent staining of Tax-specific
CTLs and immunostaining of Foxp3
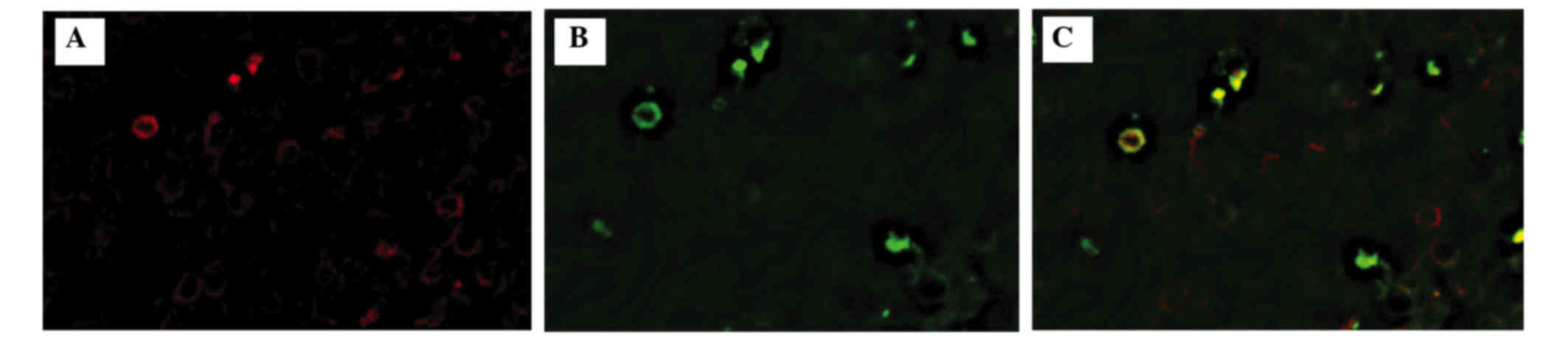
Among the 15 cases, there were positive Tax-specific
CTLs in 9 cases (Fig. 1). The number
of Tax-specific CTLs ranged from 0 to 8 cells/mm2
(Table I). It should be noted that
only CD8/multimer double-positive cells (Fig. 2) were regarded as a positive
signal.
Foxp3 immunostaining was also analyzed. Foxp3
expression >30% was defined as positive, according to previous
reports (6). Among the 15 cases, 5
(33%) were positive for Foxp3 (Fig. 1
and Table I). Of the Foxp3+ cases, 4
were included in the pleomorphic medium type, accounting for 80%, a
significantly higher percentage than that observed for the other
two types.
There was an inverse correlation between
Tax-specific CTL expression and Foxp3 expression (Table II).
 | Table II.Association between Tax-specific CTL
counts and other pathological findings. |
Table II.
Association between Tax-specific CTL
counts and other pathological findings.
|
| Tax-CTL |
|---|
|
|
|
|---|
|
Characteristics | Mean
(cells/mm2) | P-value |
|---|
| Foxp3
expression |
| Foxp3 positive rate
>30% | 0.0 | 0.0006 |
| Foxp3 positive rate
<30% | 4.6 |
|
| HLA-A24
expression |
| HLA-A24 positive
rate >30% | 3.4 | 0.7000 |
| HLA-A24 positive
rate <30% | 3.0 |
|
| Tax expression |
| Tax positive rate
>10% | 2.8 |
|
| Tax positive rate
<10% | 3.6 | 0.6000 |
| Morphology |
| Medium cell
predominant | 2.0 |
|
| Large cell
predominant | 4.0 | 0.1700 |
| Anaplastic
variant | 5.0 |
|
Tax-specific CTL function in lymph
nodes of ATLL patients
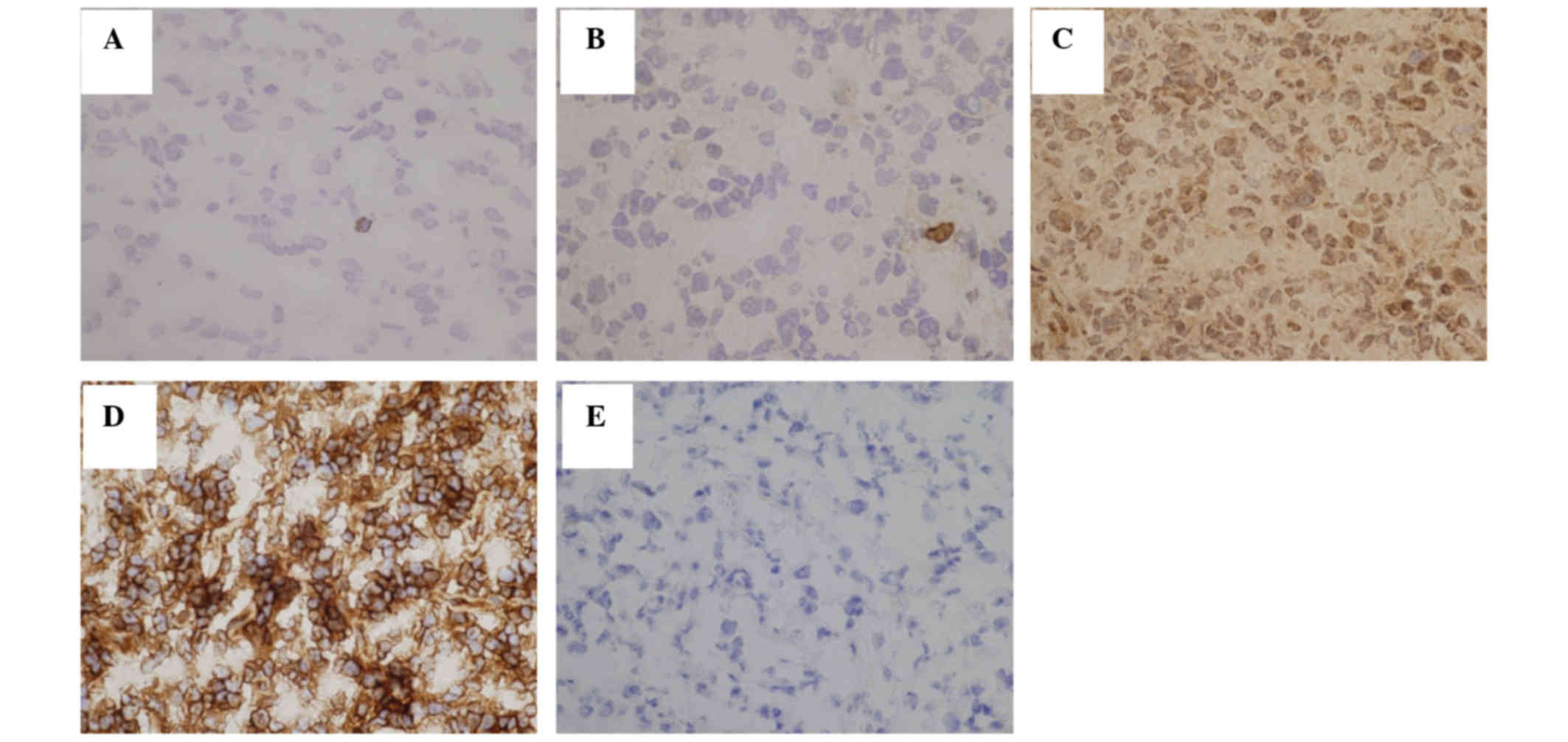
To reveal whether Tax-specific CTLs were activated
or not, immunostaining for INFγ was performed. The number of
INFγ-positive cells ranged from 0.0 to 3.5 cells/mm2,
and was lower than the number of Tax-specific CTLs in the same
area, with certain Tax-specific CTLs being inactive (Fig. 3 and Table
I).
Tax and HLA-A24 expression in ATLL
cells
In the present study, HLA-A24-positive ATLL patients
were selected by a PCR method. RT-PCR products for Tax were
detected in 14/14 patients examined (Fig.
4).
Immunohistochemical staining of Tax protein revealed
that the number of positive lymphoma cells ranged from 5 to 18%,
and the number of HLA-A24-positive lymphoma cells ranged from 1 to
95% (Fig. 3 and Table I). There was no correlation between
the number of Tax-specific CTLs and positivity for Tax or HLA-A24
(Table II).
Discussion
The present study suggested that lymphoma cells in
ATLL patients may evade CTL-mediated immune control by expression
of Foxp3.
In previous reports, it was controversial whether
ATLL cells function as Treg cells (7,20,21). There were three types of opinions
about this argument: First, ATLL cells have a Treg function
(7). In that study, the authors
separated CD4+ C-C motif chemokine receptor (CCR) 4+ ATLL cells
using biotin-conjugated KM2160 (monoclonal antibodies to CCR4) and
Anti-Botin MicroBeads. The proliferation of CD4+CCR4+ ATLL cells
and CD4+ non-ATLL cells, as well as their IFN-γ production in
response to T-cell receptor (TCR) stimulation in the presence of
autologous adenomatous polyposis coli, was assessed. The results
demonstrated that CD4+CCR4+ ATLL cells from a subset of patients
were able to suppress the proliferation and IFNγ production of
autologous CD4+non-ATLL cells in vitro at a ratio of 1:1.
Second, ATLL cells do not have a Treg function (20). In that study, the regulatory function
of ATL cells was evaluated using an alloimmune mixed lymphocyte
reaction system. CD4+CD25+ T-cells and CD8+ T-cells were isolated
from peripheral blood mononuclear cells (PBMCs) of ATL patients and
a normal healthy donor with immunomagnetic beads. In the healthy
control, CD4+CD25+ T-cells inhibited the proliferation of
autologous CD8+ T-cells, thus displaying the ordinary suppressive
activity of Treg cells. However, in the ATL patients tested, the
proliferation of CD8+ T-cells was not suppressed by CD4+CD25+
T-cells. In addition to circulating ATL cells, single-cell
suspensions of skin-infiltrating tumor cells did not inhibit
autologous CD8+ T-cell proliferation at any ratio. Third, not ATLL
cells but only CD4+Foxp3+ cells increasing in peripheral blood of
ATLL patients have a suppressor function (21). In that study, the authors positively
selected CD4+ cells from fresh PBMCs of patients with ATLL, and
then used the respective TCR Vβ-specific monoclonal antibodies to
isolate the expanded T-cell clones. Next, the authors purified the
CD25+ cells from the remaining (TCR Vβ+-depleted) population, and
then added three cell populations (CD4+TCR Vβ+, CD4+CD25+ and
CD4+TCR Vβ-CD25-) to CD4+CD25- cells labeled with
carboxyfluorescein succinimidyl ester (CFSE) from a healthy
individual. The authors investigated the frequency of CFSE
expression in CD4+ cells after incubation for 4 days in order to
assess the inhibition of proliferation of CD4+CD25- cells. It was
observed that only the CD4+CD25+ population caused strong
inhibition of proliferation, and that this CD25+ population
expressed high frequency of Foxp3.
In these studies, peripheral blood cells were
separated using the autoMACS® magnetic separation
system. In order to separate ATLL cells, the authors used Tax,
Foxp3, CD4, CD25 and CCR4 as markers. However, it remained
difficult to distinguish ATLL cells from normal Treg cells
phenotypically and to separate the population physically, since
both cell types express cell-surface markers characteristic of Treg
cells (6).
In our study, it was concluded that ATLL cells,
which were positive for Foxp3, had a Treg-like function, since
morphologically atypical T-cells were positive for Foxp3, and that
there was an inverse correlation between Foxp3 expression and
Tax-specific CTL counts.
Karube et al reported that ATLL cells were
positive for Foxp3, and that there was a correlation between Foxp3
expression and Epstein-Barr virus (EBV)-positive cell counts
(6). This phenomenon may occur by the
same mechanism, since Foxp3+ ATLL cells may suppress EBV-specific
CTLs.
Treg cells are known to have numerous suppressive
mechanisms, including suppressive cytokines, metabolic disruption
and targeting of dendritic cells (11). Treg cells secrete perforin and
granzyme B, and induce effector T-cell apoptosis, being CTLs one of
their targets (11). In addition,
Treg cells secrete transforming growth factor-β1 and change active
CTLs to inactive ones (11). In our
study, the number of INFγ-positive cells was lower than the number
of Tax-specific CTLs in the same area, and it was concluded that
several Tax-specific CTLs may be inactive due to the T-reg-like
function of ATLL cells. Harashima et al (26) investigated cellular immune responses
of ATL patients who obtained complete remission following
non-myeloablative allogenic peripheral blood HSCT using cultured
PBMCs from patients and healthy donors. The authors measured the
cytotoxic activity of CTLs by 6-h 51Cr release assay at
various effector-to-target cell ratios (27). INF-γ production of effector cells was
also measured, and the results indicated that Tax-specific CTLs in
ATLL patients are inactive, and that the Tax-specific CTL response
is strongly activated following HSCT in certain ATLL patients in
long-term remission, by using peripheral blood of ATLL
patients.
It has been reported that the level of Tax
expression in HTLV-1-infected cells decreases during disease
progression, and Tax transcripts have been detected only in ~40% of
established ATLL cases in peripheral blood (18).
In our study, immunohistochemical staining of Tax
protein revealed the number of positive cells to range from 5 to
18%, and RT-PCR products for Tax were detected in all patients.
Since the results of RT-PCR may be influenced not only by the
presence of ATLL cells, but also by the presence of HTLV-1-infected
cells, immunohistochemistry was performed. The results of
immunostaining demonstrated that few ATLL cells and few
HTLV-1-infected lymphocytes were positive for Tax in certain cases.
Thus, even if Tax expression is decreased, Tax protein can be a
target of Tax-specific CTLs.
As aforementioned, Tax-specific CTLs were considered
to be important in controlling ATLL. Recently, a humanized
anti-CCR4 antibody, mogamulizumab, yielded a good response in
CCR4-positive ATLL patients (28).
While mogamulizumab attacks CCR4-positive tumor cells, it also
attacks Treg cells (29). Therefore,
CTLs are activated (29).
Furthermore, immunotherapy with anti programmed-death (PDL) 1
antibody and anti CTL antigen (CTLA)-4 antibody has become an
increasingly appealing therapeutic strategy for patients with
cancer, with numerous late-stage clinical trials demonstrating
overall survival advantages in melanoma and castration-resistant
prostate cancer (30,31). CTLA-4 and PDL1 have distinct roles in
regulating immunity, and blocking these pathways resulted in the
removal of inhibitory signals and activation of CTLs (32,33). These
immunotherapies may become a promising strategy for ATLL in the
future.
In humans, CTLs are important in controlling
virus-infected cells and malignant cell growth (34). The interactions between CTLs and their
target cells are mediated by HLA class I antigens loaded with viral
and tumor antigen-derived peptides, along with co-stimulatory
receptor/ligand stimuli (34).
The present finding that HLA-A24-positive lymphoma
cells ranged from 1 to 95% is in agreement with the findings of
previous studies on ATLL, which reported that the expression of
surface MHC-I was significantly downregulated in the resistant
cells (27). In order to escape from
CTL recognition, viruses and tumor cells have developed strategies
to inhibit the expression and/or function of HLA class I antigens
(19). For example, adenovirus, EBV,
human papillomavirus, human immunodeficiency virus and human herpes
virus have such strategies (35).
HTLV-1 also has such a strategy. HTLV-1 inhibits the association of
MHC class I heavy chain with β2 microglobulin and inhibits the
expression of HLA class I antigens (36). In malignant tumors such as squamous
cell carcinoma, small cell carcinoma, colon cancer, cutaneous
melanoma, prostate cancer and Hodgkin lymphoma, HLA class I antigen
downregulation or loss has been detected (19). The region of chromosome 6, which
carries the human MHC (37), is
unstable during malignant transformation of cells (38). Mutations may lead to downregulation or
loss of the HLA class I antigen-tumor associated-derived peptide
complex (38), and the same mechanism
may be present in ATLL patients.
In conclusion, our study demonstrated that lymphoma
cells in ATLL patients evaded CTL-mediated immune control by
expression of Foxp3 and downregulation of Tax and HLA-A24. To the
best of our knowledge, this is the first experiment that revealed
how Tax-specific CTLs behave in lymph nodes of ATLL patients, and
supported previous cell culture experiments that reported that the
Tax-specific CTL response was important to control HTLV-1
infection. This finding suggest that reactivation of the
Tax-specific CTL response may provide prophylactic and therapeutic
approaches for HTLV-1 carriers and for ATLL patients whose ATLL
cells retain the ability to express Tax.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research of The Ministry of Education, Culture,
Sports, Science and Technology (Tokyo, Japan; grant no.,
26460446).
References
|
1
|
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn
PA, Minna JD and Gallo RC: Detection and isolation of type C
retrovirus particles from fresh and cultured lymphocytes of a
patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA.
77:pp. 7415–7419. 1980; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdonck K, González E, van Dooren S,
Vandamme AM, Vanham G and Gotuzzo E: Human T-lymphotropic virus 1:
Recent knowledge about an ancient infection. Lancet Infect Dis.
7:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gessain A: Epidemiology of HTLV-1 and
associated diseaseHuman T-cell Lymphotropic Virus Type-1. Hollsberg
P and Hafler DA: Wiley; Chichister: pp. 33–64. 1996
|
|
4
|
Tobinai K: Current management of adult
T-cell leukemia/lymphoma. Oncology (Williston Park). 23:1250–1256.
2009.PubMed/NCBI
|
|
5
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 2. 4th. IARC Press;
Lyon, France: 2008
|
|
6
|
Karube K, Aoki R, Sugita Y, Yoshida S,
Nomura Y, Shimizu K, Kimura Y, Hashikawa K, Takeshita M, Suzumiya
J, et al: The relationship of Foxp3 expression and
clinicopathological characteristics in adult T-cell
leukemia/lymphoma. Mod Pathol. 21:617–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yano H, Ishida T, Inagaki A, Ishii T,
Kusumoto S, Komatsu H, Iida S, Utsunomiya A and Ueda R: Regulatory
T-cell function of adult T-cell leukemia/lymphoma cells. Int J
Cancer. 120:2052–2057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh BJ: Infectious complication of Human
T cell leukemia/lymphoma virus type I infection. Clin Infect Dis.
23:138–145. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montes M, Sanchez C, Verdonck K, Lake JE,
Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E and
White AC Jr: Regulatory T cell expansion in HTLV-1 and
strongyloidiasis co-infection is associated with reduced IL-5
responses to strongyloides stercoralis antigen. PLoS Negl Trop Dis.
3:e4562009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marcos LA, Terashima A, Dupont HL and
Gotuzzo E: Strongyloides hyperinfection syndrome: An emerging
global infectious disease. Trans R Soc Trop Med Hyg. 2:314–318.
2008. View Article : Google Scholar
|
|
11
|
Sakaguchi S, Wing K, Onishi Y,
Prieto-Martin P and Yamaguchi T: Regulatory T cells: How do they
suppress immune responses? Int Immunol. 21:1105–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki S, Masaki A, Ishida T, Ito A, Mori
F, Sato F, Narita T, Ri M, Kusumoto S, Komatsu H, et al: Tax is a
potential molecular target for immunotherapy of adult T-cell
leukemia/lymphoma. Cancer Sci. 3:1764–1773. 2012. View Article : Google Scholar
|
|
13
|
Seiki M, Hattori S, Hirayama Y and Yoshida
M: Human adult T-cell leukemia virus: Complete nucleotide sequence
of the provirus genome integrated in leukemia cell DNA. Proc Natl
Acad Sci USA. 80:pp. 3618–3622. 1983; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franchini G: Molecular mechanisms of human
T-cell leukemia/lymphotropic virus type I infection. Blood.
86:3619–3639. 1995.PubMed/NCBI
|
|
15
|
Jin DY, Spencer F and Jeang KT: Human T
cell leukemia virus type 1 oncoprotein Tax targets the human
mitotic checkpoint protein MAD1. Cell. 93:81–91. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossman WJ, Kimata JT, Wong FH, Zutter M,
Ley TJ and Ratner L: Development of leukemia in mice transgenic for
the Tax gene of human T-cell leukemia virus type I. Proc Natl Acad
Sci USA. 92:pp. 1057–1061. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kannagi M: Immunologic control of human
T-Cell leukemia virus type I and adult T-cell leukemia. Int J
Hematol. 86:113–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeda S, Maeda M, Morikawa S, Taniguchi
Y, Yasunaga J, Nosaka K, Tanaka Y and Matsuoka M: Genetic and
epigenetic inactivation of Tax gene in adult T-cell leukemia cells.
Int J Cancer. 109:559–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seliger B, Cabrera T, Garrido F and
Ferrone S: HLA class I antigen abnormalities and immune escape by
malignant cells. Semin Cancer Biol. 12:3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimauchi T, Kabashima K and Tokura Y:
Adult T-cell leukemia/lymphoma cells from blood and skin tumors
express cytotoxic T lymphocyte-associated antigen-4 and Foxp3 but
lack suppressor activity toward autologous CD8+ T cells. Cancer
Sci. 9:98–106. 2008.
|
|
21
|
Toulza F, Nosaka K, Takiguchi M, Pagliuca
T, Mitsuya H, Tanaka Y, Taylor GP and Bangham CR: Foxp3+ regulatory
T cells are distinct from leukemia cells in HTLV-1-associated adult
T-cell leukemia. Int J Cancer. 5:2375–2382. 2009. View Article : Google Scholar
|
|
22
|
Little AM and Parham P: Polymorphism and
evolution of HLA class I and II genes and molecules. Rev
Immunogenet. 1:105–123. 1999.PubMed/NCBI
|
|
23
|
Satou Y, Yasunaga J, Yoshida M and
Matsuoka M: HTLV-1 basic leucine zipper factor gene mRNA supports
proliferation of adult T cell leukemia cells. Proc Natl Acad Sci
USA. 103:pp. 720–725. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen MH, Pedersen LO, Capeller B,
Bröcker EB, Becker JC and Straten P thor: Spontaneous cytotoxic
T-cell responses against survivin-derived MHC class I-restricted
T-cell epitopes in situ as well as ex vivo in cancer patients.
Cancer Res. 16:5964–5968. 2001.
|
|
25
|
Schrama D, Pedersen LØ, Keikavoussi P,
Andersen MH, Straten Pt, Bröcker EB, Kämpgen E and Becker JC:
Aggregation of antigen-specific T cells at the inoculation site of
mature dendritic cells. J Invest Dermatol. 9:1443–1448. 2002.
View Article : Google Scholar
|
|
26
|
Harashima N, Kurihara K, Utsunomiya A,
Tanosaki R, Hanabuchi S, Masuda M, Ohashi T, Fukui F, Hasegawa A,
Masuda T, et al: Graft-versus-Tax response in adult T-cell leukemia
patients after hematopoietic stem cell transplantation. Cancer Res.
64:391–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohashi T, Hanabuchi S, Suzuki R, Kato H,
Masuda T and Kannagi M: Correlation of major histocompatibility
complex class I downregulation with resistance of human T-cell
leukemia virus type 1-infected T cells to cytotoxic T-lymphocyte
killing in a rat model. J Virol. 76:7010–7019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamamoto K, Utsunomiya A, Tobinai K,
Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S,
Tamura K, et al: Phase I study of KW-0761, a defucosylated
humanized anti-CCR4 antibody, in relapsed patients with adult
T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin
Oncol. 28:1591–1598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tobinai K, Takahashi T and Akinaga S:
Targeting chemokine receptor CCR4 in adult T-cell leukemia/lymphoma
and other T-cell lymphomas. Curr Hematol Malig Rep. 7:235–240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kono K: Current status of cancer
immunotherapy. J Stem Cells Regen Med. 30:8–13. 2014.
|
|
31
|
Tosti G, Cocorocchio E and Pennacchioli E:
Anti-cytotoxic T lymphocyte antigen-4 antibodies in melanoma. Clin
Cosmet Investig Dermatol. 6:245–256. 2013.PubMed/NCBI
|
|
32
|
Chambers CA, Kuhns MS, Egen JG and Allison
JP: CTLA-4-mediated inhibition in regulation of T cell responses:
mechanisms and manipulation in tumor immunotherapy. Annu Rev
Immunol. 19:565–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parekh VV, Lalani S, Kim S, Halder R,
Azuma M, Yagita H, Kumar V, Wu L and Kaer LV: PD-1/PD-L blockade
prevents anergy induction and enhances the anti-tumor activities of
glycolipid-activated invariant NKT cells. J Immunol. 182:2816–2826.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andersen MH, Schrama D, Straten P Thor and
Becker JC: Cytotoxic T cells. J Invest Dermatol. 126:32–41. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seliger B, Ritz U and Ferrone S: Molecular
mechanisms of HLA class I antigen abnormalities following viral
infection and transformation. Int J Cancer. 118:129–138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson JM, Nicot C, Fullen J, Ciminale V,
Casareto L, Mulloy JC, Jacobson S and Franchini G: Free major
histocompatibility complex class I heavy chain is preferentially
targeted for degradation by human T-cell leukemia/lymphotropic
virus type 1 p12I protein. J Virol. 75:6086–6094. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Francke U and Pellegrino MA: Assignment of
the major histocompatibility complex to aregion of the short arm of
human chromosome 6. Proc Natl Acad Sci USA. 74:pp. 1147–1151. 1977;
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Richardson JH, Edwards AJ, Cruickshank JK,
Rudge P and Dalgleish AG: In vivo cellular tropism of human T-cell
leukemia virus type 1. J Virol. 64:5682–5687. 1990.PubMed/NCBI
|