Introduction
The most common type of pancreatic tumor is
pancreatic ductal adenocarcinoma (PDA), which arises from the
exocrine component of the pancreas (1,2). Although
surgical resection is the only curative treatment for PDA, <20%
of patients with PDA are able to undergo surgery (3). Despite improved surgical techniques,
chemotherapeutic agents and/or radiation therapy, patients with PDA
have an overall 5-year survival rate of only 5% (4). Notably, a significant population of
patients with PDA demonstrate early local recurrence and/or
metastases following surgical resection (5). Therefore, improvement of early mortality
following surgical resection for patients with PDA is urgently
needed. One major focus of researchers has been to identify
prognostic factors associated with early recurrence in patients
with PDA following surgical resection.
Several previous studies have demonstrated the
potential functions of chondroitin sulfate (CS), which is an
important component of the extracellular matrix (ECM) in normal and
tumor tissues (6–9). CS is a unique, highly sulfated sugar,
and is abundantly expressed in the ECM of tumor cells, resulting in
tumor development and progression (10,11). CS
has been classified into five different types due to the presence
of differently sulfated disaccharide units: CS-A (4-sulfated),
CS-B, (dermatan sulfate), CS-C (6-sulfated), CS-D (2,6-disulfated),
and CS-E (4,6-disulfated) (10). The
sulfation pattern is involved in cancer progression (12). In particular, CS-E, a highly sulfated
glycosaminoglycan, is expressed in the ECM of adenocarcinoma
(12) and is involved in tumor
proliferation, metastasis and angiogenesis (13,14). The
biosynthesis of CS-E is mediated by N-acetylgalactosamine 4-sulfate
6-O-sulfotransferase [GalNAc4S-6ST, also known as carbohydrate
sulfotransferase 15 (CHST15)], which transfers sulfate from
3′-phosphoadenosine-5′-phosphosulfate to the 6-O of GalNAc4S on
CS-A (15). A previous report
indicated a direct association between CHST15 and the proliferation
of human PDA cell lines in vivo and in vitro
(16). Therefore, CHST15 levels in
human PDA tissue may represent a potential prognostic biomarker.
Furthermore, the products of CS-E degradation in certain
tumor-associated ECM promote cell adhesion and migration by
cleaving CD44 in PDA tissue (17).
CD44 is a cell surface receptor for several ECM components and is
also associated with tumor cell migration and metastasis (18,19).
Notably, the ectodomain of CD44 on tumor cells is cleaved by
multiple stimulations (20). The
level of CD44 cleavage and circulating soluble CD44 is involved in
tumor migration and invasion (21).
In addition, the cleaved intracellular domain of CD44 activates
stemness factors, including Nanog homeobox, sex determining region
Y-box 2, and POU class 5 homeobox 1, and contributes to
tumorigenesis (22). Overexpression
of CD44 in the presence of CS-E may be associated with enhanced
amounts of CD44 cleavage and result in early recurrence in patients
with PDA following surgical resection (17,20). These
reports indicated the potential of CHST15 and CD44 as prognostic
factors in patients with PDA following surgical resection. Previous
reports have indicated that low neutrophil-to-lymphocyte ratios
(NLRs) and low carbohydrate antigen 19–9 (CA19-9) levels may be
associated with significantly improved prognoses in patients with
PDA (23,24). Thus, the present study analyzed NLRs
and CA19-9 levels in the peripheral blood and the expression of
CHST15 and CD44 in PDA tissue from surgical specimens as prognostic
factors for patients with PDA following surgical resection. The
major aim of the present study was to identify prognostic factors
associated with early recurrence in patients with PDA following
surgical resection. Overexpression of CHST15 in PDA tissue was
demonstrated to be significantly associated with shorter
disease-free survival (DFS) and overall survival (OS) in patients
with PDA following surgical resection.
Materials and methods
PDA samples from patients
The present study included patients with PDA who
underwent macroscopically curative resection by total
pancreatectomy, pancreaticoduodenectomy or pylorus-preserving
pancreaticoduodenectomy with lymph node dissection at Jikei
University Kashiwa Hospital (Kashiwa, Japan) between January 2008
and December 2014. Tumor samples from 36 consecutive patients with
PDA were collected. Information regarding clinical features of the
patients, including age, sex, tumor location, tumor differentiation
and tumor recurrence, was obtained from medical records. All
laboratory data were obtained around the time of surgery. OS was
defined as the time from diagnosis to mortality from any cause. DFS
was defined as the time from the date of surgery to the first
radiological evidence of recurrence or mortality without evidence
of recurrence or a second primary cancer. Pathological data,
including tumor stage, grade, and size were obtained from surgical
reports and assessed using the pancreatic cancer TNM staging system
set forth by the American Joint Committee on Cancer (AJCC)
(25). The baseline characteristics
of patients with PDA are presented in Table I. The present study was reviewed and
approved by the Ethics Committee of Jikei University School of
Medicine (Tokyo, Japan) and by the clinical study committee of
Jikei University Kashiwa Hospital (grant no. 26-370, 7876). The
review board approved the present investigation and waived the need
for written informed consent from study participants due to the
retrospective, non-interventional nature of this study. Study
procedures were conducted in accordance with the Helsinki
Declaration.
 | Table I.Baseline characteristics of patients
with pancreatic cancer. |
Table I.
Baseline characteristics of patients
with pancreatic cancer.
| Clinical
characteristic | n | (%) |
|---|
| Age at surgery |
| <65
years | 12 | 33.3 |
| ≥65
years | 24 | 66.7 |
| Sex |
| Male | 20 | 55.6 |
|
Female | 16 | 44.4 |
| Tumor location |
| Head | 30 | 83.3 |
|
Body-to-tail | 6 | 16.7 |
| Pathology |
|
Well-to-moderate | 28 | 77.8 |
| Poor | 8 | 22.2 |
| Tumor stage |
| I/II | 33 | 91.7 |
|
III/IV | 3 | 8.3 |
| Tumor size |
| <3
cm | 10 | 27.8 |
| ≥3
cm | 26 | 72.2 |
Laboratory data
All laboratory data, including levels of CA19-9,
neutrophils, lymphocytes, leukocytes, platelets (PLTs), hemoglobin
(Hb), albumin (Alb), and C-reactive protein (CRP), were obtained
around the time of surgery. NLR was calculated by dividing the
neutrophil count by the lymphocyte count. All data were assessed to
determine the prognostic impact of the examined metrics on patients
with PDA following surgical resection.
Immunohistochemical staining
Staining for CHST15 and human CD44 was performed on
primary tumor samples from patients with PDA. Formalin-fixed,
paraffin-embedded sections (thickness, 6 µm) were stained with
hematoxylin and eosin for histopathological evaluation. All
immunohistochemical staining of tumor sections (size, 20×30 mm) was
performed on formalin-fixed and paraffin-embedded sections.
Briefly, the sections were incubated with goat anti-human CHST15
antibody (1:200; cat. no. AF3365; R&D Systems, Inc.,
Minneapolis, MN, USA) for 1 h at room temperature or rabbit
anti-human CD44 antibody (1:500; cat. no. ab41478, Abcam,
Cambridge, MA, USA) for 1 h at room temperature, and washed with
phosphate-buffered solution (PBS). For CHST15 staining, the
sections were incubated for 30 min at room temperature with rabbit
anti-goat IgG-biotin (67 µg/ml; cat. no. ab6741, Abcam). For CD44
staining, the sections were incubated for 30 min at room
temperature with goat anti-rabbit IgG-biotin (26.7 µg/ml; cat. no.
111-036-045; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA). The IgG labeled-sections were incubated for 30 min at
room temperature, with horseradish peroxidase-conjugated
streptavidin, and developed with 3,3′-diaminobenzidine (Nichirei
Biosciences Inc., Tokyo, Japan). The nuclei were subsequently
counterstained with hematoxylin. The assessment of CHST15 or CD44
expression was performed in five random fields within the tumor
under a light microscope (magnification, ×100). CHST15 and CD44
staining statuses were graded as follows: 1, Negative, no staining
or little staining in ≤20% of PDA tissue; 2, Weak, weak staining in
>80% of PDA tissue; 3, Moderate, moderate staining in >80% of
PDA tissue; and 4, Strong, strong or complete staining in >80%
of PDA tissue (26). CHST15 and CD44
expression levels were independently interpreted by three
investigators who were blinded to the clinical information of the
patients. The negative control was stained using an identical
procedure but with the primary antibody omitted.
Statistical analysis
All statistical analyses were performed using SAS
software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Categorical variables were compared using the χ2 test.
Continuous variables were compared using the Mann-Whitney U test.
DFS and OS were estimated using the Kaplan-Meier method, and
differences were measured using log-rank tests. A multivariate Cox
hazard regression model was used to examine independent predictors
of DFS and OS. P<0.05 was considered to indicate a statistically
significant difference.
Results
PDA patient characteristics
A total of 36 consecutive patients with PDA were
enrolled in the present study, and their clinical characteristics
are summarized in Table I. The
numbers of male and female patients were 20 (55.6%) and 16 (44.4%),
respectively. The median patient age at diagnosis was 65.6 years
(range, 44–79 years). There were 3, 30, and 3 patients with PDA
classified into AJCC stages I, II, and III, respectively. The
median DFS of the patients was 310 days (range, 62–2050 days), and
the median OS was 512.5 days (range, 216–2296 days). The majority
of tumors (n=30, 83.3%) were located in the head of the pancreas.
In addition, all tumors were confirmed to be associated with
invasive ductal adenocarcinoma.
Laboratory data
All laboratory data were obtained by exploration
around the time of surgery and assessed for prognostic impact on
patients with PDA following surgical resection. As expected,
pathology, tumor stage, and low NLRs in patients with PDA were
significantly associated with improved DFS (Table II). In addition, pathology, low NLRs
and high Alb levels in patients with PDA were significantly
associated with longer OS in the present study. In contrast,
laboratory data, including leukocyte and PLT counts and Hb and CRP
levels, were not associated with DFS or OS (Tables II and III).
 | Table II.Multivariate analysis of disease-free
survival. |
Table II.
Multivariate analysis of disease-free
survival.
| Characteristic | HR | 95% CI | P-value |
|---|
| Age at surgery (≥65
years vs. <65 years) | 0.615 | 0.213–1.773 | 0.368 |
| Tumor location
(body-to-tail vs. head) | 0.237 | 0.056–1.001 | 0.050 |
| Pathology (poor vs.
well-to-moderate) | 2.952 | 1.033–8.435 | 0.043 |
| Tumor stage (III/IV
vs. I/II) | 0.179 | 0.035–0.909 | 0.038 |
| Leukocytes | 1.000 | 1.000–1.000 | 0.891 |
| NLR | 1.385 | 1.098–1.748 | 0.006 |
| CRP levels | 0.783 | 0.445–1.380 | 0.398 |
| Hemoglobin
levels | 1.096 | 0.700–1.714 | 0.689 |
| Platelet
levels | 1.021 | 0.972–1.072 | 0.412 |
| Albumin levels | 0.657 | 0.267–1.619 | 0.362 |
| CA19-9 levels | 1.000 | 1.000–1.000 | 0.914 |
| CHST15 (strong vs.
negative-to-moderate) | 9.456 | 2.644–33.815 | <0.001 |
 | Table III.Multivariate analysis of overall
survival. |
Table III.
Multivariate analysis of overall
survival.
| Characteristic | HR | 95% CI | P-value |
|---|
| Age at surgery (≥65
years vs. <65 years) | 0.380 | 0.125–1.160 | 0.089 |
| Tumor location
(body-to-tail vs. head) | 1.783 | 0.472–6.746 | 0.394 |
| Pathology (poor vs.
well-to-moderate) | 3.169 | 1.074–9.349 | 0.037 |
| Tumor stage (III/IV
vs. I/II) | 0.588 | 0.187–1.848 | 0.364 |
| Leukocytes | 1.000 | 1.000–1.000 | 0.790 |
| NLR | 1.279 | 1.014–1.615 | 0.038 |
| CRP levels | 0.690 | 0.347–1.374 | 0.291 |
| Hemoglobin
levels | 0.750 | 0.482–1.165 | 0.201 |
| Platelet
levels | 1.040 | 0.987–1.096 | 0.140 |
| Albumin levels | 0.420 | 0.178–0.995 | 0.049 |
| CA19-9 levels | 1.000 | 1.000–1.001 | 0.210 |
| CHST15 (strong vs.
negative-to-moderate) | 3.690 | 1.331–10.231 | 0.012 |
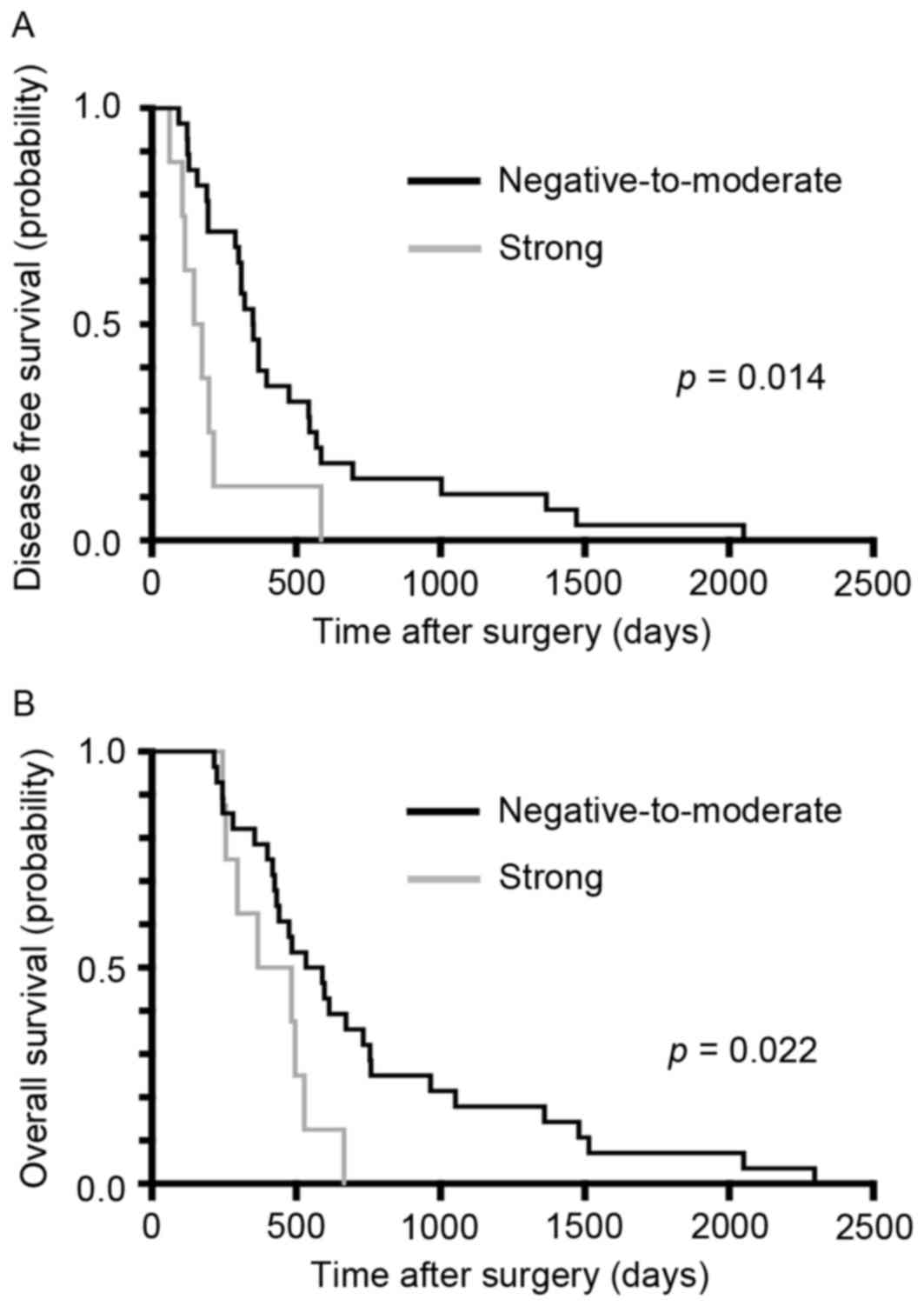
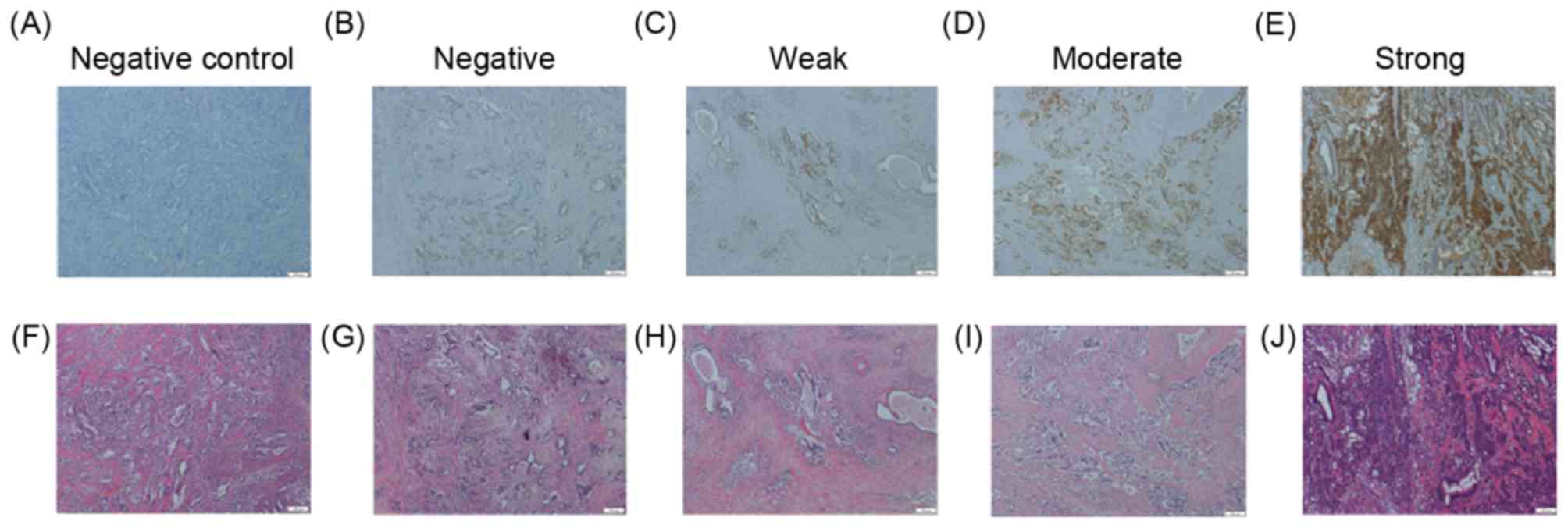
CHST15 expression in PDA tissue
To assess the significance of CHST15 expression on
PDA tissue as a prognostic factor in patients with PDA following
surgical resection, surgical specimens were examined by
immunohistochemical staining (Fig.
1). CHST15 expression was detected in the cytoplasm and
membrane of PDA cells. All patients with PDA were divided into two
groups according to CHST15 expression in the PDA tissue as follows:
A strong CHST15 expression group and a negative-to-moderate CHST15
expression group. The 36 patients with PDA included 8 patients
(22.2%) with strong CHST15 expression and 28 patients (77.8%) with
negative-to-moderate CHST15 expression. Of the 36 patients, 4
patients with PDA (11.1%) demonstrated negative CHST15 expression
in the present study. There were no significant differences in sex,
age at surgery, tumor location, tumor stage, tumor size or tumor
pathology between the strong and negative-to-moderate CHST15
expression groups (Table IV).
Kaplan-Meier curves for PDA DFS and OS in patients following
surgery are presented in Fig. 2A.
Notably, the recurrence period was significantly shorter in the
strong CHST15 expression group compared with the
negative-to-moderate expression group (P=0.014). Furthermore, OS
was significantly shorter in the strong CHST15 expression group
compared with the negative-to-moderate expression group (P=0.022).
The multivariate analysis also indicated a significant association
of DFS with CHST15 expression [P<0.001; hazard ratio (HR),
9.456; 95% confidence interval (CI), 2.644–33.815] and of OS with
CHST15 expression (P=0.012; HR, 3.690; 95% CI, 1.331–10.231). The
median DFS was 160.5 days in the strong CHST15 expression group and
351 days in the negative-to-moderate CHST15 expression group
(Fig. 2A). In contrast, the median OS
was 426 days in the strong CHST15 expression group and 594.5 days
in the negative-to-moderate CHST15 expression group (Fig. 2B). In addition, no CHST15 staining
positivity was detected in cells when omitting the first antibody,
supporting the specificity of the staining. These results indicated
that strong CHST15 expression may be a potential prognostic factor
for patients with PDA following surgery.
 | Figure 1.CHST15 intensity in PDA tissue.
Representative images of PDA tissue stained with CHST15 antibody
(upper panel) and H&E (lower panel) are depicted. The negative
control was stained using an identical procedure but with the
primary antibody omitted. CHST15 staining status was assessed as
(A) control, (B) negative, (C) weak, (D) moderate or (E) strong.
H&E staining of the same areas of (F) control, (G) negative,
(H) weak, (I) moderate and (J) strong tissue. Scale bars, 200 µm;
magnification, ×100. CHST15, carbohydrate sulfotransferase 15; PDA,
pancreatic ductal adenocarcinoma; H&E, hematoxylin and
eosin. |
 | Table IV.Characteristics of patients with
strong and negative-to-moderate CHST15 expression. |
Table IV.
Characteristics of patients with
strong and negative-to-moderate CHST15 expression.
| CHST15
intensity | Strong |
Negative-to-moderate | P-value |
|---|
| Age at surgery
(years) |
|
| N.S. |
|
≥65 | 7 (87.5%) | 17 (60.7%) |
|
|
<65 | 1 (12.5%) | 11 (39.3%) |
|
| Sex |
|
| N.S. |
|
Male | 3 (37.5%) | 17 (60.7%) |
|
|
Female | 5 (62.5%) | 11 (39.3%) |
|
| Tumor location |
|
| N.S. |
|
Head | 7 (87.5%) | 23 (82.1%) |
|
|
Body-to-tail | 1 (12.5%) | 5 (17.9%) |
|
| Pathology |
|
| N.S. |
|
Well-to-moderate | 6 (75%) | 22 (78.6%) |
|
|
Poor | 2 (25%) | 6 (21.4%) |
|
| Tumor stage |
|
| N.S. |
|
I/II | 8 (100%) | 25 (89.3%) |
|
|
III/IV | 0 (0%) | 3 (10.7%) |
|
| Tumor size |
|
| N.S. |
| <3
cm | 2 (25%) | 8 (28.6%) |
|
| ≥3
cm | 6 (75%) | 20 (71.4%) |
|
| Leukocytes
(counts/µl) | 5,200
(4375–7275) | 5,850
(4725–8275) | N.S. |
| Neutrophils
(counts/µl) | 2,850
(2525–5175) | 3,950
(2950–6625) | N.S. |
| Lymphocytes
(counts/µl) | 1,400
(1300–1900) | 1,200
(925–1400) | N.S. |
| NLR (%) | 1.75
(1.25–3.94) | 4.21
(2.23–5.28) | N.S. |
| CRP (mg/dl) | 0.10
(0.10–0.25) | 0.2
(0.10–1.15) | N.S. |
| Hemoglobin
(g/dl) | 12.35
(11.10–13.13) | 12.50
(11.53–13.98) | N.S. |
| Platelet (x104
counts/µl) | 20.85
(15.95–24.83) | 24.25
(19.10–30.05) | N.S. |
| Albumin (g/dl) | 3.75
(3.63–3.80) | 3.95
(3.60–4.30) | N.S. |
| CA19-9 (U/ml) | 70.50
(14.0–171.75) | 103.0
(45.75–382.50) | N.S. |
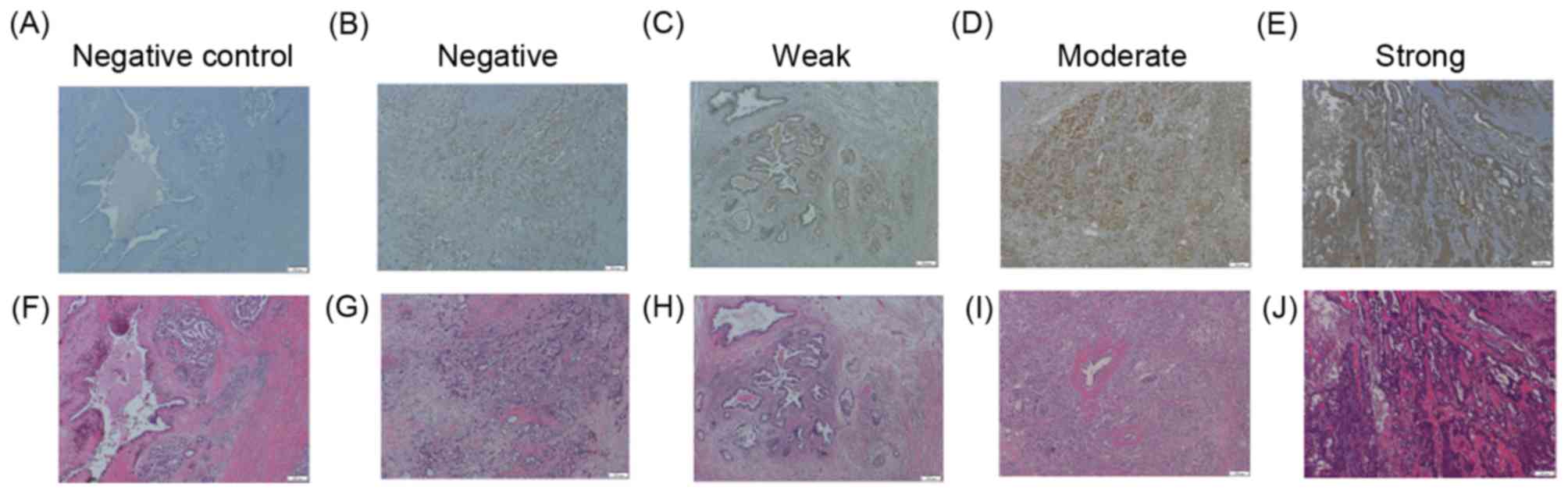
CD44 expression on PDA tissue
CS-E degradation products that are mediated by
CHST15 (15) have previously been
reported to promote tumor cell adhesion and migration by cleaving
CD44 in PDA tissue (17). The CD44
antibody used in the present study was generated from an immunogen
sequence, DHTKQNQDWTQWNPSHSN, located within exon 8 of CD44 isoform
1. This epitope is present in the CD44 epican of tumor cells, but
not in CD44H or CD44E (27,28). CD44 expression was detected in the
cytoplasm and membrane of PDA cells (Fig.
3). CD44 expression status was also assessed as negative, weak,
moderate, or strong. Of the 36 patients, 17 (47.2%), 5 (13.9%), and
9 (25.0%) had surgical specimens exhibiting weak, moderate, and
strong CD44 expression, respectively (Fig. 3). Of the 36 patients, 5 patients with
PDA (13.9%) were negative for CD44 expression in the present study.
No association was observed between CD44 expression levels in PDA
tissue and progression, defined as DFS and OS (P=0.598 and 0.326,
respectively). Furthermore, to evaluate the relationship between
CHST15 and CD44, the same immunohistochemical stained areas were
compared in PDA tissue. PDA cells expressing high levels of CHST15
did not necessarily exhibit high CD44 expression levels. In
addition, no CD44 staining positivity in cells was detected when
omitting the first antibody, supporting the specificity of the
staining.
Discussion
In the present study, CHST15 and CD44 intensity was
analyzed in PDA surgical specimens and associations with the
prognosis, defined as DFS and OS, were assessed in patients
following surgical resection. Furthermore, the laboratory data
concerning the peripheral blood, obtained around the time of
surgery, were assessed as prognostic markers. Significantly
prolonged DFS and OS were observed in patients with PDA without
strong expression of CHST15. Furthermore, low NLRs were
significantly associated with prolonged DFS and OS in the present
study. Thus, the data presented herein suggested that high CHST15
expression in PDA tissue and high NLRs may be recurrence markers in
patients with PDA following surgery.
It has previously been demonstrated that tumor
cell-derived CS-E is mediated by CHST15 and is involved in the
various stages of tumor progression and invasion (14). Human PDA tissue has been successfully
treated in nude mice using small interfering RNA-based selective
silencing of the CHST15 gene to selectively inhibit CS-E expression
(16). Therefore, CHST15/CS-E
axis-mediated PDA cell proliferation may be a therapeutic target
for the early progression of PDA in humans. As CHST15 expression is
significantly associated with prognosis in patients with PDA
following surgical resection, CHST15 may be a novel therapeutic
target. These reports led to the assessment of CHST15 expression in
surgical specimens as a prognostic marker in patients with PDA
following surgery in the present study. A total of 36 consecutive
patients with PDA were classified into two types based on CHST15
expression: Strong overexpression in PDA tissue, and
negative-to-moderate expression in PDA tissue.
The results of the present study demonstrated that
CHST15 overexpression was significantly associated with DFS and OS.
Patients with PDA with overexpressed CHST15 had significantly worse
DFS and OS rates than patients with negative-to-moderate CHST15
expression. In addition, there were no differences in sex, age at
surgery, tumor location, tumor stage, tumor size, or tumor
pathology between the strong and negative-to-moderate CHST15
expression groups. Therefore, CHST15 may be a recurrence and
prognostic marker and a candidate therapeutic target in patients
with PDA following surgical resection. In a previous study, CHST15
double stranded RNA that selectively inhibits CHST15 genes was
injected in 4 patients with non-resectable PDA through endoscopic
ultrasonography (29). In the
clinical trial, reduction of tumor size was detected. Notably,
CHST15 protein and partial necrosis were also detectable in biopsy
specimens. Furthermore, the serum CD44 variant and CA19-9 levels
decreased. These results suggested that CHST15 may be a novel
target for inoperable patients and patients following surgical
resection to prevent early recurrences.
CS-E is known to promote tumor invasion by cleaving
CD44 in PDA tissue (17,20). In the present study, serum samples
were not available to assess soluble CD44. Therefore, the
association of CD44 expression with DFS and OS was analyzed in PDA
tissue obtained from surgical specimens. Unexpectedly, it was not
possible to identify high expression of CD44 as a prognostic factor
for patients with PDA following surgical resection. The association
of CD44 expression levels with cancer prognosis and the utility of
CD44 as a cancer stem cell marker have been debated. In the present
study, a significant association between CD44 and CHST15 expression
levels in PDA tissue was detected. However, certain PDA cells that
expressed high CHST15 levels did not exhibit CD44 expression. This
may be a reason, at least in part, why it was not possible to
detect high CD44 expression as a prognostic marker for patients
with PDA following surgical resection. As CD44 cleavage is enhanced
by the CS-E fragment, which is synthesized by CHST15 (17), the levels of soluble CD44 in serum may
be more closely associated with CHST15 expression intensity. The
CS-E fragment modulates tumor cell adhesion and migration by
interacting with CD44 on tumor cells, leading to CD44-mediated
tumor progression (17). Therefore,
CD44 cleavage levels in patients with PDA may be a prognostic
factor following surgical treatment. The exact mechanism of CS-E
fragment production in vivo and the association with CD44
remain unclear. Further studies are required to evaluate the
clinical significance of CHST15 and CD44 as prognostic factors in a
large sample of patients with PDA.
Several previous reports have indicated that low
NLRs are also associated with significantly improved prognoses in
patients with PDA. Therefore, NLR may be a novel marker for
survival (23,24). As expected, NLR was also identified as
a prognostic factor in patients with PDA following surgical
resection. In addition, a previous report indicated that a high NLR
was significantly associated with tumor metastasis, poor tumor
differentiation and high CA19-9, low Alb and high CRP levels
(23). In the present study, low Alb
levels and pathology were also associated with OS. However, neither
CA19-9 nor CRP levels were associated with DFS or OS in the present
study.
The results of the present study reinforced the
importance of CHST15 as a prognostic factor in patients with PDA
following surgical resection. Further investigation of CHST15 and
its interaction with CD44 may provide novel insights into the
manner in which tumor recurrence is regulated. PDA remains a lethal
disease with a high early mortality rate following surgical
therapy. To overcome early mortality following surgical resection,
the analysis of mechanisms of CHST15 in tumor progression may lead
to the development of alternative treatments that are able to
control early recurrence.
Acknowledgements
The present study was supported, in part, by
Grants-in-Aid for Scientific Research (C) from the Ministry of
Education, Cultures, Sports, Science and Technology of Japan (grant
no. 15K09050).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chari ST, Kelly K, Hollingsworth MA,
Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto
M, Cleeter DF, et al: Early detection of sporadic pancreatic
cancer: Summative review. Pancreas. 44:693–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arslan C and Yalcin S: Current and future
systemic treatment options in metastatic pancreatic cancer. J
Gastrointest Oncol. 5:280–295. 2014.PubMed/NCBI
|
|
5
|
Fischer R, Breidert M, Keck T, Makowiec F,
Lohrmann C and Harder J: Early recurrence of pancreatic cancer
after resection and during adjuvant chemotherapy. Saudi J
Gastroenterol. 18:118–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pothacharoen P, Siriaunkgul S, Ong-Chai S,
Supabandhu J, Kumja P, Wanaphirak C, Sugahara K, Hardingham T and
Kongtawelert P: Raised serum chondroitin sulfate epitope level in
ovarian epithelial cancer. J Biochem. 140:517–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vallen MJ, Schmidt S, Oosterhof A, Bulten
J, Massuger LF and van Kuppevelt TH: Primary ovarian carcinomas and
abdominal metastasis contain 4,6-disulfated chondroitin sulfate
rich regions, which provide adhesive properties to tumour cells.
PLoS One. 9:e1118062014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricciardelli C, Mayne K, Sykes PJ, Raymond
WA, McCaul K, Marshall VR, Tilley WD, Skinner JM and Horsfall DJ:
Elevated stromal chondroitin sulfate glycosaminoglycan predicts
progression in early-stage prostate cancer. Clin Cancer Res.
3:983–992. 1997.PubMed/NCBI
|
|
9
|
Ricciardelli C, Quinn DI, Raymond WA,
McCaul K, Sutherland PD, Stricker PD, Grygiel JJ, Sutherland RL,
Marshall VR, Tilley WD and Horsfall DJ: Elevated levels of
peritumoral chondroitin sulfate are predictive of poor prognosis in
patients treated by radical prostatectomy for early-stage prostate
cancer. Cancer Res. 59:2324–2328. 1999.PubMed/NCBI
|
|
10
|
Vallen MJ, van der Steen SC, van Tilborg
AA, Massuger LF and van Kuppevelt TH: Sulfated sugars in the
extracellular matrix orchestrate ovarian cancer development: ‘when
sweet turns sour’. Gynecol Oncol. 135:371–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Steen SC, van Tilborg AA, Vallen
MJ, Bulten J, van Kuppevelt TH and Massuger LF: Prognostic
significance of highly sulfated chondroitin sulfates in ovarian
cancer defined by the single chain antibody GD3A11. Gynecol Oncol.
140:527–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
ten Dam GB, van de Westerlo EM,
Purushothaman A, Stan RV, Bulten J, Sweep FC, Massuger LF, Sugahara
K and van Kuppevelt TH: Antibody GD3G7 selected against embryonic
glycosaminoglycans defines chondroitin sulfate-E domains highly
up-regulated in ovarian cancer and involved in vascular endothelial
growth factor binding. Am J Pathol. 171:1324–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wegrowski Y and Maquart F: Chondroitin
sulfate proteoglycans in tumor progression. Adv Pharmacol.
53:297–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizumoto S, Watanabe M, Yamada S and
Sugahara K: Expression of N-acetylgalactosamine 4-sulfate
6-O-sulfotransferase involved in chondroitin sulfate synthesis is
responsible for pulmonary metastasis. Biomed Res Int.
2013:6563192013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohtake S, Ito Y, Fukuta M and Habuchi O:
Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is
related to human B cell recombination activating gene-associated
gene. J Biol Chem. 276:43894–43900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takakura K, Shibazaki Y, Yoneyama H, Fujii
M, Hashiguchi T, Ito Z, Kajihara M, Misawa T, Homma S, Ohkusa T and
Koido S: Inhibition of cell proliferation and growth of pancreatic
cancer by silencing of carbohydrate sulfotransferase 15 in vitro
and in a Xenograft model. PLoS One. 10:e01429812015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugahara KN, Hirata T, Tanaka T, Ogino S,
Takeda M, Terasawa H, Shimada I, Tamura J, ten Dam GB, van
Kuppevelt TH and Miyasaka M: Chondroitin sulfate E fragments
enhance CD44 cleavage and CD44-dependent motility in tumor cells.
Cancer Res. 68:7191–7199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas L, Byers HR, Vink J and Stamenkovic
I: CD44H regulates tumor cell migration on hyaluronate-coated
substrate. J Cell Biol. 118:971–977. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Günthert AR, Sträter J, von Reyher U,
Henne C, Joos S, Koretz K, Moldenhauer G, Krammer PH and Möller P:
Early detachment of colon carcinoma cells during CD95
(APO-1/Fas)-mediated apoptosis. I. De-adhesion from hyaluronate by
shedding of CD44. J Cell Biol. 134:1089–1096. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goebeler M, Kaufmann D, Bröcker EB and
Klein CE: Migration of highly aggressive melanoma cells on
hyaluronic acid is associated with functional changes, increased
turnover and shedding of CD44 receptors. J Cell Sci. 109:1957–1964.
1996.PubMed/NCBI
|
|
21
|
Hirata K, Suzuki H, Imaeda H, Matsuzaki J,
Tsugawa H, Nagano O, Asakura K, Saya H and Hibi T: CD44 variant 9
expression in primary early gastric cancer as a predictive marker
for recurrence. Br J Cancer. 109:379–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho Y, Lee HW, Kang HG, Kim HY, Kim SJ and
Chun KH: Cleaved CD44 intracellular domain supports activation of
stemness factors and promotes tumorigenesis of breast cancer.
Oncotarget. 6:8709–8721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ and
Yuan SG: Prognostic significance of neutrophil to lymphocyte ratio
in pancreatic cancer: A meta-analysis. World J Gastroenterol.
21:2807–2815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takakura K, Ito Z, Suka M, Kanai T,
Matsumoto Y, Odahara S, Matsudaira H, Haruki K, Fujiwara Y, Saito
R, et al: Comprehensive assessment of the prognosis of pancreatic
cancer: Peripheral blood neutrophil-lymphocyte ratio and
immunohistochemical analyses of the tumour site. Scand J
Gastroenterol. 51:610–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surgi Oncol. 17:1471–1474. 2010.
View Article : Google Scholar
|
|
26
|
Metindir J, Dilek GB and Pak I: Staining
characterization by immunohistochemistry of tumor cancer antigen in
patients with endometrial cancer. Eur J Gynaecol Oncol. 29:489–492.
2008.PubMed/NCBI
|
|
27
|
Lara MF, González-González E, Speaker TJ,
Hickerson RP, Leake D, Milstone LM, Contag CH and Kaspar RL:
Inhibition of CD44 gene expression in human skin models, using
self-delivery short interfering RNA administered by dissolvable
microneedle arrays. Hum Gene Ther. 23:816–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kugelman LC, Ganguly S, Haggerty JG,
Weissman SM and Milstone LM: The core protein of epican, a heparan
sulfate proteoglycan on keratinocytes, is an alternative form of
CD44. J Invest Dermatol. 99:886–891. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishimura M, Yahagi N, Itoi T, Ochiai Y
and Matsuda Y: A translational study to investigate the role of
carbohydrate sulfotransferase 15 for pancreatic cancer biology from
in vitro to first-in-human clinical research. J Clin Oncol.
33:e222012015.
|