Introduction
In the second half of the 20th century, the low
sensitivity of medical examination techniques was only able to
detect large tumors and advanced-stage cancer, leading to high
mortality (1). With the progress of
examination techniques, early-stage cancer can now be detected and
treated with the development of multidisciplinary therapies.
Surgery and radiotherapy are used for local control, whereas
chemotherapy is used for systemic control (2–8). The most
recent examination techniques occasionally enable the detection of
early-stage cancer, oligometastases and oligo-recurrence (9–11). For
these forms of cancer, patients may recover completely with
multidisciplinary therapy (12–15).
However, the majority of cancer types contain numerous hypoxic
cells and/or large amounts of antioxidative enzymes and are,
therefore, resistant to radiotherapy (16–26). For
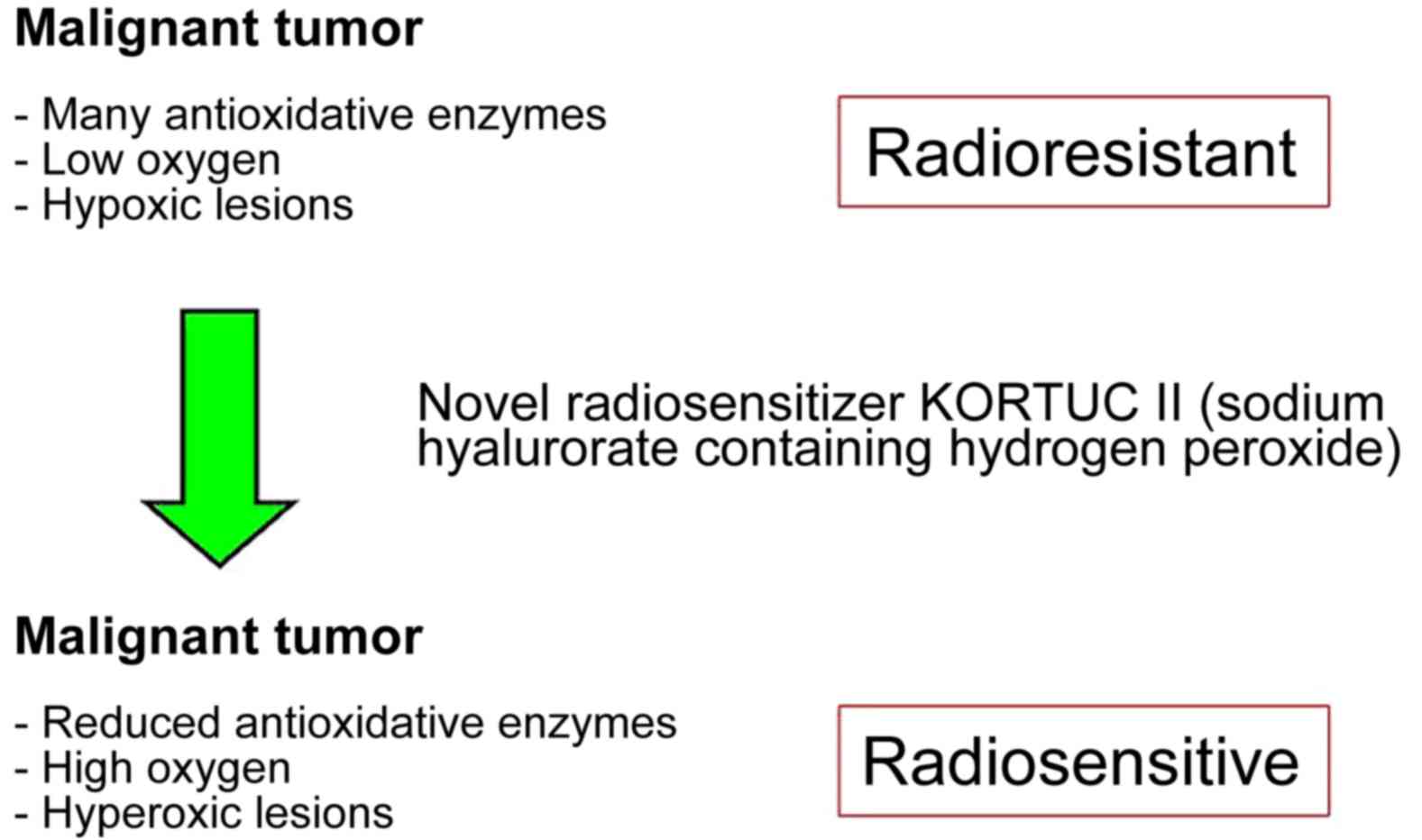
these types of cancer, the novel radiosensitizer Kochi
oxydol-radiation therapy for unresectable carcinomas (KORTUC) II
has been developed, the concept of which is presented in Fig. 1 (18–20).
Through the use of KORTUC II, hypoxic and radioresistant cancer
cells become hyperoxic and radiosensitive, with demonstrable
therapeutic effects (16–26). In our previous studies, the
effectiveness of KORTUC II for the treatment of supraclavicular
lymph node metastases, recurrent breast cancer and stage IV primary
breast cancer was demonstrated (18–20).
According to the aforementioned strategy, the purpose of this study
was to evaluate the safety and efficacy of KORTUC II in patients
with stage I primary breast cancer that was not treated with
surgery.
Materials and methods
Patient selection
The present study was performed between January 2008
and April 2013 at the Kochi Medical School Hospital. The
Institutional Ethics Committee of the Kochi Medical School
(Nankoku, Japan) approved KORTUC II for the radiation therapy of
stage I primary breast cancer, and 15 female patients were enrolled
in the study following the provision of written, informed consent.
The inclusion criteria were as follows: The patient requested
KORTUC II treatment; the tumor size was ≤20 mm; there were no
clinical metastases; the patient presented with stage I breast
cancer; the presence of contraindications to general anesthesia due
to a significant comorbidity, or the patient declined surgical
treatment. Patient ages ranged between 40 and 76 years (mean age,
58 years).
To evaluate whether adjuvant therapy was necessary,
hormone receptors (estrogen and progesterone), human epidermal
growth factor receptor-2, cluster of differentiation 44 and Ki-67
expression status of the tumor were examined using
immunohistochemistry in a needle biopsy tissue specimen obtained
prior to treatment. In addition, a risk category was assigned to
each patient according to the pathological results of the needle
biopsy using the updated St. Gallen consensus based on the tumor
size (27).
Treatments
Radiotherapy consisted of 44 Gray (Gy) of X-ray
irradiation and 9 Gy of electron boost irradiation. X-ray
irradiation consisted of 5 fractions/week at 2.75 Gy/fraction for a
total of 16 fractions, and the X-ray energy level was 4 MeV.
Electron boost irradiation consisted of 3 fractions of electron
beam irradiation at 3 Gy/fraction, and the electron beam energy
level, from 4 to 12 MeV, was individually selected for each
patient. Tangential irradiation was administered at the target
region. KORTUC II consists of 2.5 ml of 1% w/v sodium hyaluronate
and 0.5 ml of 3% w/v solution of hydrogen peroxide. Injection of
1.5–3 ml of KORTUC II was initiated at the sixth radiation fraction
and was performed twice a week (Monday and Thursday) under
ultrasonographic guidance to maintain a high oxygen concentration
in the tumor tissue. The combined use of ~0.5 ml of 1% lidocaine in
conjunction with the KORTUC injection was initiated in March 2008,
and the amount of KORTUC II administered was adjusted according to
the tumor size. The total number of injections was 4–6. Patient
data and therapeutic effects are presented in Table I. Following KORTUC II treatment,
hormone therapy [leuprorelin acetate (Takeda Pharmaceutical
Company, Ltd., Tokyo, Japan): Subcutaneous injection, 3.75 mg every
4 weeks; letrozole (Novartis International AG, Basel, Switzerland):
Oral administration, 2.5 mg daily; tamoxifen (AstraZeneca PLC,
Cambridge, UK): oral administration, 20 mg daily; or anastrozole
(AstraZeneca PLC): oral administration, 1 mg daily] was
administered for five years to prevent recurrence, according to the
St. Gallen consensus.
 | Table I.Patient data and therapeutic effects
of kochi oxydol-radiation therapy for unresectable carcinomas. |
Table I.
Patient data and therapeutic effects
of kochi oxydol-radiation therapy for unresectable carcinomas.
|
|
| Hormone receptor |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Pt. | Age, years | ER | PgR | HER2 | Treatment region (RT
field) | Exposure dose,
Gy | Evaluation
method | FDG accumulation | Tumor size, mm | Therapeutic
effect |
|---|
| 1 | 54 | (+) | (+) | (1+) | Left breast | X 44+E9 | PET-CT | 3.9→D | 14→D | CR |
| 2 | 67 | (+) | (+) | (−) | Right breast | X 44+E9 | PET-CT | 3.2→1.6 | 15→D | CR |
| 3 | 46 | (+) | (+) | (1+) | Left breast | X 44+E9 | PET-CT | No data→D | 10→D | CR |
| 4 | 70 | (+) | (+) | (−) | Right breast | X 44+E9 | PET-CT | No data→D | 7→D | CR |
| 5 | 48 | (+) | (+) | (1+) | Right breast | X 44+E9 | PET-CT | 5.4→D | 12→D | CR |
| 6 | 40 | (+) | (+) | (−) | Right breast | X 44+E9 | PET-CT | 2.7→D | 9→D | CR |
| 7 | 62 | (+) | (+) | (1+) | Right breast | X 44+E9 | PET-CT | No data | 11→D | CR |
| 8 | 63 | (+) | (+) | (1+) | Right breast | X 44+E9 | PET-CT | 1.9→1.0 | 15→D | CR |
| 9 | 76 | (+) | (+) | (−) | Left breast | X 44+E9 | PET-CT | No data→D |
7→D | CR |
| 10 | 43 | (+) | (+) | (1+) | Right breast | X 44+E9 | PET-CT | No data→D |
9→D | CR |
| 11 | 50 | (+) | (+) | (2+) | Right breast | X 44+E9 | PET-CT | 4.5→D | 20→D | CR |
| 12 | 60 | (−) | (−) | (2+) | Right breast | X 44+E9 | MRI | No data | 18→D | CR |
| 13 | 61 | (+) | (+) | (−) | Right breast | X 44+E9 | MRI | No data | 23→D | CR |
| 14 | 63 | (+) | (+) | (1+) | Right breast | X 44+E 9 | MRI | No data | 13→D | CR |
| 15 | 73 | (+) | (+) | (2+) | Left breast | X 44+E9 | PET-CT | 1.6→no data | 10→D | CR |
Assessment of therapeutic
response
The majority of the 15 patients underwent positron
emission tomography-computed tomography (PET-CT) and/or dynamic
magnetic resonance imaging (MRI) examinations prior to, and 1–7
months following, KORTUC II treatment, and every 6 months
thereafter where possible; 12 patients remain available for
follow-up at the time of writing. Therapeutic effects were
evaluated by comparing the results of pre-treatment and
post-treatment PET-CT and MRI examination. The final therapeutic
response of the lesion was assessed according to the Revised
Response Evaluation Criteria In Solid Tumors (RECIST) guidelines
(version 1.1), and patient monitoring and tumor assessment were
performed monthly (19).
Treatment-associated complications were assessed in detail in order
to evaluate the feasibility of this treatment approach by assigning
patients a toxicity grade according to the Common Terminology
Criteria for Adverse Events (CTCAE criteria; version 4.0) (19). The patient follow-up duration ranged
from 25–92 months.
Formulation of KORTUC II
A syringe (2.5 ml) of hyaluronic acid preparation
with a 1% w/v concentration of sodium hyaluronate was used for each
patient dose. This preparation contained 25 mg of sodium
hyaluronate with isotonizing agent (ARTZ Dispo®;
Seikagaku Corporation, Tokyo, Japan) and 2.5 mg each of
L-methionine, sodium chloride, potassium phosphate and crystalline
sodium dihydrogen phosphate. The preparation was a colorless,
transparent, viscous and aqueous solution, with a pH of 6.8–7.8, a
specific osmotic pressure of 1.0–1.2 (relative to physiological
saline) and an average molecular weight of 600,000–1,200,000 g/mol.
A solution (0.5 ml, 3% w/v) of hydrogen peroxide (Oxydol; Ken-ei
Pharmaceutical Co., Ltd., Osaka, Japan) was added immediately prior
to use. The solution was mixed well to prepare the radiosensitizer,
with a final sodium hyaluronate concentration of 0.83% and a
hydrogen peroxide concentration of ~0.5%. The constituents of the
radiosensitizer were the same as those used in previous studies for
the treatment of chemotherapy-resistant supraclavicular lymph node
metastases, recurrent breast cancer and stage IV primary breast
cancer (18–20).
Results
The KORTUC II treatment was well tolerated with
minimal adverse effects. All patients experienced low inflammation
at the treatment site, and all were cured by local treatment. The
patients were determined to exhibit grade I complications (CTCAE
criteria Version 4.0). Although four patients exhibited low liver
function impairment 3 months following the start of the KORTUC II
radiosensitization treatment, this impairment was also observed
prior to treatment in 3/4 of these individuals. Therefore, only 1
patient, patient 9, who exhibited persistent impairment of liver
function as determined by blood examination following treatment
with KORTUC II, was considered to be a possible candidate for an
adverse treatment event. However, no clinical conditions requiring
medical treatment were observed as a result of the KORTUC II
treatment for any patient. There was no evident mutual association
between KORTUC II treatment and the impairment of liver function,
and the patient was evaluated as experiencing grade I complications
(CTCAE criteria version 4.0). The details of possible adverse
events following KORTUC II treatment are presented in Tables II, III and IV.
 | Table II.Details of KORTUC II treatment and
adverse events (1). |
Table II.
Details of KORTUC II treatment and
adverse events (1).
|
|
| Injection of KORTUC
II |
|
|
| Abnormalities of
chest or abdomen |
|---|
|
|
|
|
|
|
|
|
|---|
| Pt. | Age, years | Number of
doses | Dose/time, ml | Total dose, ml | Pain during the
injection | Pain on the next
day of injection | Change of blood
pressure | Pre | Under | Post≤3M | Post>3M |
|---|
| 1 | 54 | 5 | 3 | 15 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 2 | 67 | 5 | 3 | 15 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 3 | 46 | 5 | 3 | 15 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 4 | 70 | 5 | 3 | 15 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 5 | 48 | 5 | 2 | 10 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 6 | 40 | 5 | 3 | 15 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 7 | 62 | 5 | 3 | 15 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 8 | 63 | 5 | 3 | 15 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 9 | 76 | 5 | 3 | 15 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 10 | 43 | 5 | 1.5 | 7.5 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 11 | 50 | 5 | 1.5 | 7.5 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 12 | 60 | 6 | 3 | 18 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 13 | 61 | 6 | 3 | 18 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 14 | 63 | 4 | 3 | 12 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
| 15 | 73 | 5 | 3 | 15 | Low | (−) | (−) | (−) | (−) | (−) | (−) |
 | Table III.Details of KORTUC II treatment and
adverse events (2). |
Table III.
Details of KORTUC II treatment and
adverse events (2).
|
|
| Impairment of
kidney function | Impairment of liver
function |
|
|---|
|
|
|
|
|
|
|---|
| Pt. | Age, years | Pre | Under | Post≤3M | Post> 3M | Pre | Under | Post≤ 3M | Post> 3M | Max value of
impairment of liver function |
|---|
| 1 | 54 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 2 | 67 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 3 | 46 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 4 | 70 | (−) | (−) | (−) | (−) | (+) | (−) | (+) | (+) | LDH max, 322; CK
max, 449; ALP, 435 |
| 5 | 48 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 6 | 40 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 7 | 62 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 8 | 63 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 9 | 76 | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (+) | CHE max,562 |
| 10 | 43 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 11 | 50 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 12 | 60 | (−) | (−) | (−) | (−) | (+) | (−) | (−) | (+) | AST max, 40; ALT
max, 67 |
| 13 | 61 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 14 | 63 | (−) | (−) | (−) | (−) | (+) | (+) | (+) | (+) | LDH max, 253; ALP
max, 529 |
 | Table IV.Details of cases of impaired liver
function. |
Table IV.
Details of cases of impaired liver
function.
| Pt. | Pre | Under | Post≤3M | Post>3M |
|---|
| 4 | CK max, 449 | (−) | ALP max, 346 | ALP max, 435 |
|
| LDH max, 297 |
| LDH max, 297 | LDH max, 322 |
| 9 | (−) | (−) | CHE max, 520 | CHE max, 562 |
| 12 | ALT max, 67 | (−) | (−) | ALT max, 45 |
|
| AST max, 40 |
|
| AST max, 36 |
| 14 | ALP max, 367 | ALP max, 373 | ALP max, 346 | ALP max, 529 |
|
|
|
|
| LDH max, 253 |
All lesions exhibited a complete response; the
response rate was 100% and no local recurrence was experienced
during the follow-up period. The overall survival rate was 100% at
five years. All patients with estrogen receptor positive tumors
(identified in the patient specimens obtained by pre-treatment
biopsy) received hormone therapy for 5 years following KORTUC II
treatment to prevent recurrence, according to the updated St. Galle
consensus. The treatment outcomes were satisfactory and the adverse
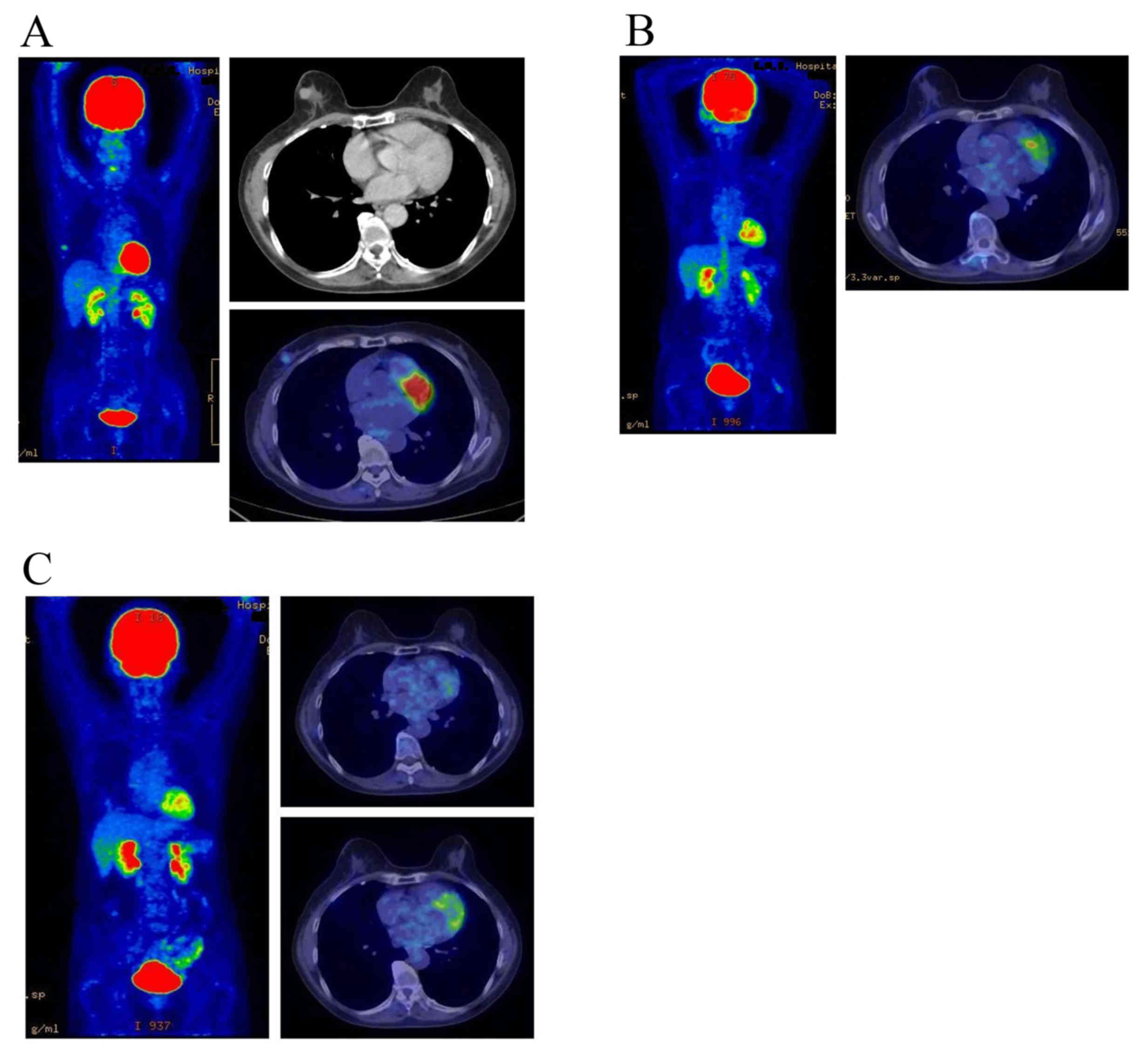
events were within the acceptable range. Images of representative
PET-CT examinations are presented in Figs. 2 and 3.
The mean follow-up period of the patients at the end of March 2015
was 53 months. No patients succumbed to the disease or exhibited
disease recurrence over the course of this study.
Discussion
At present, the five-year survival rate for stage I
breast cancer is >85% worldwide (28,29). With
the use of multidisciplinary therapy, consisting of limited
surgery, hormonal therapy and radiotherapy, the majority of
patients with stage I breast cancer can be cured (14). In addition, with the use of
radiotherapy following breast-conserving surgery, the rates of
local recurrence and mortality have been markedly reduced (28,29).
Treatment using the non-surgical method KORTUC II exhibited a
response rate of 100% and an overall 5-year survival rate of 100%
in the present study. At present, the treatment outcomes of KORTUC
II are satisfactory, and adverse events, as assessed with visual
inspection methods, are within an acceptable range.
The majority of cancer types contain numerous
hypoxic cells and/or large quantities of antioxidative enzymes,
leading to radiotherapy resistance (16–26).
KORTUC II exerts a marked therapeutic effect on these types of
cancer. The results of previous studies using KORTUC II to treat
supraclavicular lymph node metastasis, recurrent breast cancer and
stage IV primary breast cancer were considered to be equal to or
improved, compared with the therapeutic results of the
commonly-used multidisciplinary therapy administered to patients
with advanced conditions originating from primary breast cancer
(2–6).
Although the sample size was small in the present
study, the positive therapeutic effects of KORTUC II were also
reported in previous studies (16–26).
Therefore, KORTUC II may be considered as a novel effective
therapeutic approach for the treatment of certain types of cancer.
KORTUC II consists of sodium hyaluronate and hydrogen peroxide;
therefore, this treatment is inexpensive and widely available. In
addition, KORTUC II produces positive therapeutic effects and its
mechanism of action is simple (Fig.
1). Therefore, the novel radiosensitizer KORTUC II may be used
for the treatment of various types of cancer globally. However,
prospective randomized clinical trials are required to establish
the therapeutic efficacy of KORTUC II.
Acknowledgements
The authors of the present study would like to thank
Forte Science Communications (Tokyo, Japan) for their editorial
assistance. The present study was partially supported by a
Grant-in-Aid for Scientific Research from the Japanese Ministry of
Education, Culture, Sports, Science and Technology (grant no.
25461916).
Glossary
Abbreviation
Abbreviations:
|
KORTUC
|
Kochi oxydol-radiation therapy for
unresectable carcinomas
|
References
|
1
|
Harris JR: Fifty years of progress in
radiation therapy for breast cancer. Am Soc Clin Oncol Educ Book.
21–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danforth DN Jr, Zujewski J, O'Shaughnessy
J, Riseberg D, Steinberg SM, McAtee N, Noone M, Chow C, Chaudhry U,
Lippman M, et al: Selection of local therapy after neoadjuvant
chemotherapy in patients with Stage IIIA, B breast cancer. Ann Surg
Oncol. 5:150–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu XC, Zhang J, Xu BH, Cai L, Ragaz J,
Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, et al: Cisplatin plus
gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomized, open-label, multicenter, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karasawa K, Saito M, Hirowatari H, Izawa
H, Furuya T, Ozawa S, Ito K, Suzuki T and Mitsuhashi N: The role of
chemoradiotherapy in patients with unresectable T4 breast tumors.
Breast Cancer. 20:254–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin Y, Zhang P, Xu BH, Zhang BL, Li Q,
Yuan P, Cai RG, Wang JY, Wang X and Xu XZ: Unfavorable pathological
complete response rate of neoadjuvant chemotherapy epirubicin plus
taxanes for locally advanced triple-negative breast cancer. J
Huazhong Univ Sci Technolog Med Sci. 33:262–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaughnessy JN, Meena RA, Dunlap NE, Jain
D, Riley EC, Quillo AR and Dragun AE: Efficacy of concurrent
chemoradiotherapy for patients with locally recurrent or advanced
inoperable breast cancer. Clin Breast Cancer. 15:135–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bollet MA, Sigal-Zafrani B, Gambotti L,
Extra JM, Meunier M, Nos C, Dendale R, Campana F, Kirova YM, Diéras
V, et al: Pathological response to preoperative concurrent
chemo-radiotherapy for breast cancer: Results of a phase II study.
Eur J Cancer. 42:2286–2295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matuschek C, Bölke E, Roth SL, Orth K,
Lang I, Bojar H, Janni JW, Audretsch W, Nestle-Kraemling C,
Lammering G, et al: Long-term outcome after neoadjuvant
radiochemotherapy in locally advanced noninflammatory breast cancer
and predictive factors for a pathologic complete remission: Results
of a multivariate analysis. Strahlenther Onkol. 188:777–781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niibe Y, Kuranami M, Matsunaga K, Takaya
M, Kakita S, Hara T, Sekiguchi K, Watanabe M and Hayakawa K: Value
of high-dose radiation therapy for isolated osseous metastasis in
breast cancer in terms of oligo-recurrence. Anticancer Res.
28:3929–3932. 2008.PubMed/NCBI
|
|
10
|
Niibe Y and Hayakawa K: Oligometastases
and oligo-recurrence: The new era of cancer therapy. Jpn J Clin
Oncol. 40:107–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milano MT, Zhang H, Metcalfe SK, Muhs AG
and Okunieff P: Oligometastatic breast cancer treated with
curative-intent stereotactic body radiation therapy. Breast Cancer
Res Treat. 115:601–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Genet D, Lejeune C, Bonnier P, Aubard Y,
Venat-Bouvet L, Adjadj DJ, Martin J, Labourey JL, Benyoub A,
Clavère P, et al: Concomitant intensive chemoradiotherapy induction
in non-metastatic inflammatory breast cancer: Long-term follow-up.
Br J Cancer. 97:883–887. 2007.PubMed/NCBI
|
|
13
|
Mukai H, Watanabe T, Mitsumori M, Tsuda H,
Nakamura S, Masuda N, Yamamoto N, Shibata T, Sato A, Iwata H and
Aogi K: Final results of a safety and efficacy trial of
preoperative sequential chemoradiation therapy for the nonsurgical
treatment of early breast cancer: Japan Clinical Oncology Group
Study JCOG0306. Oncology. 85:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmon R, Garbey M, Moore LW and Bass BL:
Interrogating a multifactorial model of breast conserving therapy
with clinical data. PLoS One. 10:e01250062015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Massa M, Meszaros P, Baldelli I, Bisso N
and Franchelli S: Aesthetic evaluation in oncoplastic and
conservative breast surgery: A comparative analysis. Plast Reconstr
Surg Glob Open. 3:e3392015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTUC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI
|
|
17
|
Tsuzuki A, Ogawa Y, Kubota K, Tokuhiro S,
Akima R, Yaogawa S, Itoh K, Yamada Y, Sasaki T, Onogawa M, et al:
Evaluation of changes in tumor shadows and microcalcifications on
mammography following KORTUC II, a new radiosensitization treatment
without any surgical procedure for elderly patients with stage I
and II breast cancer. Cancers (Basel). 3:3496–3505. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aoyama N, Ogawa Y, Kubota K, Ohgi K,
Kataoka Y, Miyatake K, Tadokoro M, Yamanishi T, Ohnishi T, Hamada
N, et al: Therapeutic response to a new enzyme-targeting
radiosensitization treatment (KORTUC-SC) for patients with
chemotherapy-resistant supraclavicular lymph node metastasis. J
Cancer Res Ther. 1:215–219. 2013. View Article : Google Scholar
|
|
19
|
Aoyama N, Ogawa Y, Yasuoka M, Takahashi M,
Iwasa H, Miyatake K, Yamanishi T, Hamada N, Tamura T, Nishioka A
and Yamagami T: Therapeutic response to a novel enzyme-targeting
radiosensitization treatment (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas) in patients with recurrent breast cancer.
Oncol Lett. 12:29–34. 2016.PubMed/NCBI
|
|
20
|
Aoyama N, Ogawa Y, Yasuoka M, Iwasa H,
Miyatake K, Yoshimatsu R, Yamanishi T, Hamada N, Tamura T,
Kobayashi K, et al: Therapeutic response to a novel
enzyme-targeting radiosensitization treatment (KORTUC II) for
residual lesions in patients with stage IV primary breast cancer,
following induction chemotherapy with epirubicin and
cyclophosphamide or taxane. Oncol Lett. 13:69–76. 2017.PubMed/NCBI
|
|
21
|
Ogawa Y, Kubota K, Ue H, Nishioka A,
Kariya S, Yokota N, Sasaki T, Suzuki K, Nakatani K, Yamanishi T, et
al: Development and clinical application of a new radiosensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection-a new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas, Type II). Strahlenther Onkol. 183:100–101.
2007.
|
|
22
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radiosensitization treatment (KORTUC I) using hydrogen
peroxide solution-soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
23
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme-targeting radiosensitization treatment, kochi
oxydol-radiation therapy for unresectable carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyatake K, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical care for locally advanced
breast cancer: Radiologically assessed therapeutic outcome of a
new-enzyme-targeting radiosensitization treatment, Kochi
oxydol-radiation therapy for unresectable carcinomas, type II
(KORTUC II) with systemic chemotherapy. Oncol Rep. 24:1161–1168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a novel
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer-radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010.PubMed/NCBI
|
|
26
|
Hitomi J, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical therapy and radiologic
assessment of stage I breast cancer treatment with novel
enzyme-targeting radiosensitization: Kochi Oxydol-radiation therapy
for unresectable carcinomas, type II (KORTUC II). Exp Ther Med.
1:769–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gnant M, Thomssen C and Harbeck N: St.
Gallen/Vienna 2015: A Brief Summary of the Consensus Discussion.
Breast Care (Basel). 10:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
EORTC Breast Cancer Cooperative Group;
EORTC Radiotherapy Group; Bijker N, Meijnen P, Peterse JL, Bogaerts
J, van Hoorebeeck I, Julien JP, Gennaro M, Rouanet P, et al:
Breast-conserving treatment with or without radiotherapy in ductal
carcinoma-in-situ: Ten-year results of European Organisation for
research and treatment of cancer randomized phase III trial 10853-a
study by the EORTC breast cancer cooperative group and EORTC
radiotherapy group. J Clin Oncol. 24:3381–3387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|