Introduction
In China, the morbidity and mortality of colorectal
carcinoma (CRC), are next only to lung and liver cancer, and
increasing each year especially in younger population (1). The pathogenesis of CRC is related to
bacterial and viral infection, immunological disorders, hereditary
mechanisms, living habits, diet, hormonal abnormality, and weight
(2). The genesis and development of
CRC is a multi-genes evolution process, which is caused by vascular
endothelial growth factor (VEGF), epidermal growth factor receptor
(EGFR), tumor suppressor gene, proto-oncogene, abnormality of
signal transduction and other molecular events. Treatment targeting
molecular level is most promising (3). Bevacizumab is the first humanized
monoclonal antibody used for inhibiting blood vessel growth. With
endogenous VEGF competing for binding with VEGF receptor, it can
limit tumor growth effectively and reduce chemotherapy resistance
(4). Currently, it is effective as
second-line or first line chemotherapy regimens in treating
progressive CRC (5,6). Endoscopic resection of early CRC and
precancerous lesions has high success rate of resection (7), but there is no unified understanding on
whether it is with chemotherapy combined or not. Clinical data from
5-year follow-up suggest that lymph node metastasis rate is 20–40%
(8) and early combined chemotherapy
can improve survival outcomes (9).
Based on this, we analyzed the application value of bevacizumab in
the early stage and chemotherapy regimen containing 5-fluorouracil
regularly in treating early CRC after laparoscopic surgery.
Materials and methods
General material
Ninety-two patients diagnosed with early CRC being
admitted to Hongqi Hospital from January 2013 to January 2016 were
included in the study. They were treated with endoscopic mucosal
resection for the first time and diagnosis was confirmed
pathologically. Patients with colorectal metastases, primary tumors
in other parts, serious primary diseases, such as organ
dysfunctions of heart, liver, lung, kidney and brain, incomplete
clinical data, and other reasons were excluded. Our investigation
obtained approval of the Ethics Committee of Hongqi Hospital and
written informed consent of patients or their family was obtained.
Patients were randomly divided into the control group and the
observation with 46 cases each. In the control group, there were 25
males and 21 females with an average age of 52.6±10.3 years. There
were 28 cases of TNM stage I and 18 cases of stage II; there were
30 cases of adenocarcinoma, 14 cases of squamous cell carcinoma and
2 cases of others. Out of the total cases in control group 4 were
of poor differentiation, 18 of moderate differentiation and 24 of
high differentiation. The location was, 19 cases of colon and 27
cases of rectum, and the largest diameter of tumors was 0.5–2.6 cm,
and the average was 1.7±0.5 cm; the average number of tumors is
1.2±0.3. In the observation group, there were 23 males and 23
females with an average age of 53.6±12.4 years. There were 26 cases
of TNM stage I and 20 cases of stage II; 31 cases were of
adenocarcinoma, 12 cases of squamous cell carcinoma and 3 cases of
others. There were 5 cases of poor differentiation, 19 cases of
moderate differentiation and 22 cases of high differentiation.
Based on the location, there were 18 cases of colon and 28 cases of
rectum, and the largest diameter of tumors was 0.6–3.0 cm, and the
average was 1.9±0.7 cm; the average number of tumors is 1.3±0.4.
Baseline data of these two groups was comparable.
Research methods
The same surgical and nursing team treated both
groups according to standard medical procedure. Endoscopic
resection was completed in these two groups successfully, and
surgical margin was pathologically confirmed as negative during the
surgery. After the surgery, convention chemotherapy regimen was
adopted in the control group as: 90 mg/m2 oxaliplatin in
intravenous guttae (ivgtt) for 4 h, 300 mg/m2 calcium
folinate in ivgtt for 2 h, 400 mg/m2 5-FU as i.v. and
2,400 mg/m2 micro-pump in ivgtt for 46 h; one course was
for two weeks and at least three courses were needed; 5 mg/kg
bevacizumab was administered in the observation group (bevacizumab,
Avastin®, 100 mg/4 ml; Yangze Pharma, Taizhou, China);
i.v. for the first time for no less than 1.5 h, and i.v. for the
second time to be finished within 1 h, it could be finished within
0.5 h if patients showed good tolerance. Bevacizumab was stopped
after i.v. once a day for two weeks, and patients with adverse
effects of nausea and vomiting were treated by rehydration and
parenteral nutrition support.
Observation index and evaluation
criteria
In the two groups, follow-up period was 5–40 months,
and the median time was 30 months. Treatment effects and the
incidence of adverse drug reactions were compared. The therapeutic
effective evaluation was divided into complete remission (CR),
partial remission (PR), stable disease (SD), progressive disease
(PD) according to the Criteria for the Evaluation of Therapeutic
Effect of Solid Carcinoma (10).
Objective response rate (ORR) is calculated as ORR = (CR+PR)/total
cases × 100%, disease control rate (DCR) as DCR = (CR+PR+SD)/total
cases × 100%. Disease-free survival (DFS), the recurrence rates and
the survival rates were compared. VEGF expression of serum before
and after treatment were compared by ELISA method. ELISA kits were
from Sigma-Aldrich (St. Louis, MO, USA). Manual steps were followed
strictly. Positive expressions of VEGF in the samples during the
surgery were compared by immunohistochemical staining method, and
brown-yellow suggested positivity. On each section, different 9
fields of view were chosen randomly at high-power (x400), and 100
cells were counted in each field. Semi-quantitative scoring method
was adopted. According to the ratio of positive cells in every
section: 0, the positive cells <5%; 1, 5–25%; 2, 26–50%; 3,
51–75%; and 4, more than 75% positivity. The cell staining: When
cells were colorless, score 0; when light yellow, score 1; brown
yellow, score 2; and brown, score 3. Total scores were added by
these two scores; ≤3 suggested that it was negative; 4–5 that it
was positive; and ≥6 strongly positive.
Statistical analysis
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) software was
used for statistical analysis and measurement data were expressed
by the mean ± standard deviation. Comparisons among groups were
analyzed by independent samples t-test; comparisons within groups
underwent paring t-test; countable data are expressed by the rate
and analyzed by χ2 test; ordinal data underwent rank-sum
test; survival periods were analyzed by Kaplan-Meier method and
log-rank χ2 test; p<0.05 indicated that the
difference was statistically significant.
Results
Comparisons of treatment effects
ORR and DCR of the observation group were
significantly higher than those of the control group, and the
differences had statistical significance (p<0.05) (Table I).
 | Table I.Comparisons of treatment effects
[cases, n (%)]. |
Table I.
Comparisons of treatment effects
[cases, n (%)].
| Groups | Cases | CR | PR | SD | PD | ORR | DCR |
|---|
| Control | 46 | 25 (54.3) | 7 (15.2) | 4 (8.7) | 10 (21.7) | 32 (69.6) | 36 (78.3) |
| Observation | 46 | 32 (69.6) | 8 (17.4) | 3 (6.5) | 3 (6.5) | 40 (87.0) | 43 (93.5) |
| χ2 |
|
|
|
|
| 4.089 | 4.389 |
| P-value |
|
|
|
|
| 0.043 | 0.036 |
Comparisons of adverse reaction
rates
Adverse reaction rate of the observation group was
lower than that of the control group, and the difference had
statistical significance (p<0.05) (Table II).
 | Table II.Comparisons of adverse reaction rates
[cases, n (%)]. |
Table II.
Comparisons of adverse reaction rates
[cases, n (%)].
| Groups | Cases | Liver and kidney
damage | Severe diarrhea and
vomiting | Bone marrow
transplant | Infection | Electrolyte
disturbance | Overall
incidence |
|---|
| Control | 46 | 3 | 3 | 2 | 2 | 3 | 13 (28.3) |
| Observation | 46 | 1 | 1 | 1 | 1 | 1 | 5 (10.9) |
| χ2 |
|
|
|
|
|
| 4.420 |
| P-value |
|
|
|
|
|
| 0.036 |
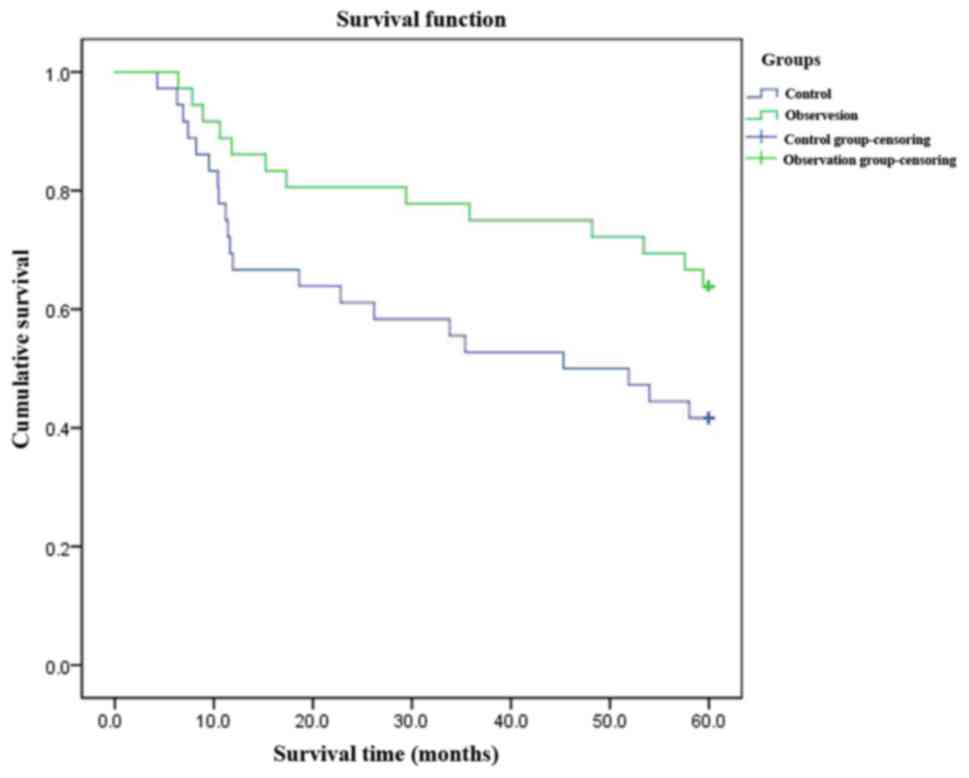
Comparisons of DFS, recurrence rates
and survival rates
In the observation group, DFS was prolonged (38.6
vs. 30.5 months, log-rank χ2=8.234, p=0.002), the
recurrence rate reduced [13.0% (6/46) vs. 30.4% (14/46),
χ2=4.089, p=0.043], and the survival rate improved
[91.3% (42/46) vs. 76.1% (35/46), χ2=3.903, p=0.048].
The differences were statistically significant (p<0.05)
(Fig. 1).
Comparisons of VEGF expression in
serum and positive expressions in tissue VEGF
VEGF expressions was lower in both groups but the
VEGF expression in observation group was significantly lower than
that in the control group. The difference was statistically
significant (p<0.05). There was no statistical difference in
comparisons of positive expressions in tissue VEGF (p>0.05)
(Table III).
 | Table III.Comparisons of VEGF expressions in
serum and positive expressions in tissue VEGF. |
Table III.
Comparisons of VEGF expressions in
serum and positive expressions in tissue VEGF.
| Groups | Cases | VEGF before the
operation (pg/ml) | Follow-up VEGF | Stain negative in
tissue VEGF | Positive | Strong positive |
|---|
| Control | 46 | 422.3±32.2 | 67.4±19.8 | 6 (13.0) | 24 (52.2) | 16 (34.8) |
| Observation | 46 | 418.2±31.3 | 241.8±26.7 | 8 (17.4) | 18 (39.1) | 20 (43.5) |
| t-test |
| 0.125 | 86.235 | 1.587 | 1.587 | 1.587 |
| P-value |
| 0.869 | <0.001 | 0.452 | 0.452 | 0.452 |
Discussion
VEGF (vascular permeability factor), is a highly
specific tissue factor and the dimer is composed of two different
subunits. It increases angiogenesis by promoting endothelial cell
proliferation and provides nutrition for tumor cells. Moreover, it
can inhibit antigen-presenting function of dendritic cells and
reduce immunogenic functions of T-cells and B-cells, which leads to
immune escape of colorectal neoplasms and incomplete cell clearance
of residual tumor cells which are important factors causing
recurrence (11). Sustainable
expression in CRC can be taken as an effective target for CRC
treatment. VEGFR, a protease-activated receptor, can maintain the
growth and proliferation of cells. Its excessive activation will
lead to rapid proliferation and metastasis of cancer cells and
inhibit apoptosis of cancer cells (12). VEGF and its receptor, VEGFR,
participates in genesis and development of colorectal neoplasms and
plays an important role in angiogenesis. Its high expression can
stimulate angiogenesis, which is the basis of recurrence and
metastasis of colorectal neoplasms (13). Detecting its expression can be an
important marker of recurrence and prognosis of CRC (14).
This study concluded that VEGF expressions of
follow-up serum in the two groups were lower, and VEGF expression
of the control group was significantly lower than that of the
observation group, and the difference was statistically
significant. The difference of comparison of positive degrees in
tissue VEGF had no statistical significance, and the positive rate
was 82.6% (38/46) vs. 87.0% (40/46). In the observation group, VEGF
expressions of follow-up serum decreased obviously, and it was
closely related to treatment effects and survival outcomes. ORR and
DCR of the observation group were significantly higher than those
of the control group; the incidence of adverse reaction was lower;
DFS was prolonged (38.6 vs. 30.5 months); the recurrence was
reduced (13.0 vs. 30.4%); the survival rate improved (91.3 vs.
76.1%); the differences had statistical significance. These results
suggested the safety and effectiveness of bevacizumab in
chemotherapy after early CRC operation.
The mechanisms of bevacizumab targeting therapy
include: i) Interference with tumor vessels (15): it breaks binding and activation
directly, regulates or inhibits vasculature of tumors and prevents
proliferation of tumor vessels. ii) Inhibit tumor differentiation
(11): Tumor differentiation factors
can operate in the micro-environment of vessel endothelium in the
human body and produce angiogenesis effects; bevacizumab can
inhibit secretion of tumor differentiation factors effectively and
control the formation of new blood vessels from the source, such as
cellular hypoxia to cause apoptosis, prevents normalization process
of pseudo-vessels. iii) Antitumor effects (16): Bevacizumab reduces tumor interstitial
pressure, decrease tumor vascular bed, change permeability, reduce
seepage and make chemotherapeutic drugs released into the tumor
cells more effective. iv) Inhibit the growth of cancer stem cells
(CSC) (17): It can inhibit the
microenvironment of CSC for growth, inhibit the activity of ABC
transporter and block signaling transduction.
As suggested above, after treating early CRC with
bevacizumab targeting therapy, VEGF expressions is reduced;
treatment effects improve; adverse drug reaction is reduced;
survival period is prolonged; the recurrence is lower; the survival
rate improves. Therefore, it has good application values. However,
because the sample size was small and the follow-up time was short,
further observation and verification is needed.
References
|
1
|
Yung KW, Yung TT, Chung CY, Tong GT, Liu
Y, Henderson J, Welbeck D and Oseni S: Principles of cancer
staging. Asian Pac J Surg Oncol. 1:1–16. 2015.
|
|
2
|
Zheng YF, Tan LK, Tan BH, Sterling H and
Kane R: Principles of surgical oncology. Asian Pac J Surg Oncol.
1:17–26. 2015.
|
|
3
|
Nasir A, Reising LO, Nedderman DM, Fulford
AD, Uhlik MT, Benjamin LE, Schade AE and Holzer TR: Heterogeneity
of vascular endothelial growth factor receptors 1, 2, 3 in primary
human colorectal carcinoma. Anticancer Res. 36:2683–2696.
2016.PubMed/NCBI
|
|
4
|
Cabart M, Frénel JS, Campion L, Ramée JF,
Dupuis O, Senellart H, Hiret S, Douillard JY and Bennouna J:
Bevacizumab efficacy is influenced by primary tumor resection in
first-line treatment of metastatic colorectal cancer in a
retrospective multicenter study. Clin Colorectal Cancer. 7:15–16.
2016.
|
|
5
|
Nagasaka T, Mishima H, Sawaki A, Shimokawa
M, Inukai M, Shinozaki K, Tanioka H, Nasu J, Nishina T, Hazama S,
et al: Protocol of a randomised phase III clinical trial of
sequential capecitabine or 5-fluorouracil plus bevacizumab
(Cape/5-FU-Bmab) to capecitabine or 5-fluorouracil plus oxaliplatin
plus bevacizumab (CapeOX/mFOLFOX6-Bmab) versus combination
CapeOX/mFOLFOX6-Bmab in advanced colorectal cancer: the C-cubed
(C3) study. BMJ Open. 6:e0114542016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ocvirk J, Moltara ME, Mesti T, Boc M,
Rebersek M, Volk N, Benedik J and Hlebanja Z: Bevacizumab plus
chemotherapy in elderly patients with previously untreated
metastatic colorectal cancer: single center experience. Radiol
Oncol. 50:226–231. 2016.PubMed/NCBI
|
|
7
|
Silva GL, de Moura EG, Bernardo WM, de
Castro V Leite, Morais C, Baba ER and Safatle-Ribeiro AV:
Endoscopic versus surgical resection for early colorectal cancer-a
systematic review and meta-analysis. J Gastrointest Oncol.
7:326–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Probst A and Messmann H: Diagnosis and
endoscopic treatment of early colorectal cancer. MMW Fortschr Med.
158:47–49. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonetti L Reggiani, Di Gregorio C, de
Gaetani C, Pezzi A, Barresi G, Barresi V, Roncucci L and de Leon M
Ponz: Lymph node micrometastasis and survival of patients with
stage I (Dukes' A) colorectal carcinoma. Scand J Gastroenterol.
46:881–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verdaguer H, Tabernero J and Macarulla T:
Ramucirumab in metastatic colorectal cancer: Evidence to date and
place in therapy. Ther Adv Med Oncol. 8:230–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gasser M and Waaga-Gasser AM: Therapeutic
antibodies in cancer therapy. Adv Exp Med Biol. 917:95–120. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suenaga M, Mashima T, Kawata N, Wakatsuki
T, Horiike Y, Matsusaka S, Dan S, Shinozaki E, Seimiya H, Mizunuma
N, et al: Serum VEGF-A and CCL5 levels as candidate biomarkers for
efficacy and toxicity of regorafenib in patients with metastatic
colorectal cancer. Oncotarget. 7:34811–34823. 2016.PubMed/NCBI
|
|
14
|
Zong S, Li H, Shi Q, Liu S, Li W and Hou
F: Prognostic significance of VEGF-C immunohistochemical expression
in colorectal cancer: a meta-analysis. Clin Chim Acta. 458:106–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yue GG, Kwok HF, Lee JK, Jiang L, Wong EC,
Gao S, Wong HL, Li L, Chan KM, Leung PC, et al: Combined therapy
using bevacizumab and turmeric ethanolic extract (with absorbable
curcumin) exhibited beneficial efficacy in colon cancer mice.
Pharmacol Res. 111:43–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michl M, Stintzing S, von Weikersthal L
Fischer, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE,
Heintges T, Lerchenmueller C, Kahl C, et al: FIRE-3 Study Group:
CEA response is associated with tumor response and survival in
patients with KRAS exon 2 wild-type and extended RAS wild-type
metastatic colorectal cancer receiving first-line FOLFIRI plus
cetuximab or bevacizumab (FIRE-3 trial). Ann Oncol. 27:1565–1572.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshida M, Takagane A, Miyake Y, Shimada
K, Nagata N, Sato A, Ogata Y, Fukunaga M, Otsuka K, Takahashi T, et
al: A phase II study of third-line combination chemotherapy with
bevacizumab plus S-1 for metastatic colorectal cancer with mutated
KRAS (SAVIOR Study). Oncology. 91:24–30. 2016. View Article : Google Scholar : PubMed/NCBI
|