Introduction
Ovarian cancer is the leading cause of mortality in
women with gynecological cancer (1).
Advanced stage cancer, which is associated with increased morbidity
and mortality, is diagnosed in >70% of patients with ovarian
cancer (2). In spite of initial
responsiveness to conventional chemotherapy with platinum- and
taxane-based drugs, the majority of patients develop chemoresistant
tumors and succumb to the disease (2). Therefore, treatment failure is often
attributed to primary or acquired resistance to chemotherapeutic
agents, representing a considerable problem in the management of
the majority of patients with cancer (2).
In a previous study, the present authors
characterized nucleus accumbens-1 (NAC1) as a candidate protein
involved in chemoresistance in ovarian cancer (3). NAC1 is a nuclear protein belonging to
the bric-a-brac-tramtrack-broad complex/pox virus and zinc finger
domain family (4). In ovarian
carcinomas, NAC1 expression is markedly increased in recurrent
tumors following chemotherapeutic intervention compared with that
in primary tumors prior to treatment (3,4).
Upregulation of NAC1 contributes to tumor cell growth, survival,
migration, invasion and resistance to chemotherapeutic drugs
(3–6).
Previously, the present authors demonstrated that NAC1 protein
negatively regulates the expression of growth arrest and DNA
damage-inducible 45γ (GADD45G) (4)
and GADD45G-interacting protein 1 (GADD45GIP1) (6). GADD45GIP1 has been demonstrated to
interact with all isoforms of GADD45; this interaction enhances the
functions of the GADD45 complex (7).
NAC1 contributes to tumor growth and survival by inhibiting
GADD45GIP1 expression (6).
Furthermore, NAC1 has been demonstrated to contribute to
chemoresistance to paclitaxel and carboplatin in ovarian cancer
through the inactivation of the GADD45 pathway (4). However, the specific functions of NAC1
in tumor development, recurrence and chemoresistance remain
unclear.
NAC1 has been identified as a negative regulator of
cellular senescence (8). Furthermore,
suppression of senescence by NAC1 serves an important role in
promoting tumorigenesis and improved treatment outcomes (8). Cellular senescence is defined as the
irreversible growth arrest of cells in the G1-phase of
the cell cycle, and is frequently characterized by flattened and
enlarged cell morphology, increased cytoplasmic granularity, and
elevated activity of senescence-associated β-galactosidase (SAβgal)
(8–10). Senescence may occur following a number
of cell divisions or be induced by various stimuli, including DNA
damage, oncogene activation, telomere shortening, and treatment
with DNA-damaging drugs or irradiation (10,11).
Furthermore, senescent cells require decreased doses of
chemotherapeutic drugs to induce cell death compared with those
required to drive non-senescent cells into apoptosis. This may
substantially improve anticancer strategies and reduce the side
effects of many treatment procedures (10–12).
Therefore, therapy-induced senescence may influence the outcome of
treatments, whereas evasion of senescence may induce tumorigenesis,
cancer recurrence and treatment failure.
In the present study, the role of NAC1 in ovarian
cancer was investigated by examining the association between NAC1
and GADD45GIP1 protein expression in tissue samples from patients
with ovarian carcinoma, and evaluating the prognostic significance
of NAC1/GADD45GIP1 expression. The underlying molecular mechanisms
of NAC1-mediated senescence through GADD45GIP1 in ovarian cancer
cells were also investigated.
Materials and methods
Tissue samples
A total of 49 paraffin-embedded tumor tissues from
female patients with advanced (stages III or IV) ovarian cancer
were obtained from the Department of Obstetrics and Gynecology at
Shimane University Hospital (Izumo, Japan), all of whom underwent
surgery at Shimane University Hosipital between January 1998 and
December 2008. The 49 patients with ovarian cancer were aged from
46 to 76 years (median, 61 years). All tissue specimens were
collected after obtaining written consent from patients with the
approval of the Facility Ethical Committee (Shimane University
Hospital; approval no. 2004–0381). Diagnosis was based on the
conventional morphological examination of sections stained with
hematoxylin and eosin (H&E), and tumors were classified
according to the World Health Organization classification (13). All patients were primarily treated
with cytoreductive surgery, and adjuvant platinum and taxane
chemotherapy (5 mg/ml × min carboplatin with 175 mg/m2
paclitaxel or 70 mg/m2 docetaxel). All patients received
between 6 and 12 courses of this regimen. The acquisition of tumor
tissues was approved by the Shimane University Institutional Review
Board (Izumo, Japan). The paraffin tissue blocks were organized
into tissue microarrays, each made by removing cores (3 mm in
diameter) of tumor tissues from the block. Selection of the area to
core was made by a gynecological oncologist and pathology
technician, and was based on a review of the H&E slides.
Immunohistochemistry
Briefly, tissue sections were dewaxed in xylene for
10 min at 20°C, rehydrated in graded ethanol, washed in
phosphate-buffered solution (pH 7.25) for 5 min and quenched in
peroxidase-blocking reagent for 5 min at 20°C to remove endogenous
peroxidase activity. Following antigen retrieval in sodium citrate
buffer (pH 7.0), slides were incubated overnight at 4°C with mouse
monoclonal anti-NAC1 antibody (cat. no. NB110-77345; Novus
Biologicals, LLC, Littleton, CO, USA) and mouse monoclonal
anti-GADD45GIP1 antibodies (cat. no. LS-C120010; LifeSpan
BioSciences, Inc., Seattle, WA, USA) at a dilution of 1:100
followed by detection using the peroxidase method with the
EnVision+ System (Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) according to the manufacturer's protocol.
Immunohistochemical signal intensity was scored by two
investigators using a four-tier system: 0, undetectable; 1+, weakly
positive; 2+, moderately positive; and 3+, intensely positive
(2). Scores of 0 and 1+ indicated
negative results, whereas scores of 2+ and 3+ were regarded as
positive results.
Cell lines and cell culture
The SKOV3 (serous carcinoma) and TOV-21G (clear cell
carcinoma) human ovarian carcinoma cell lines were obtained from
the American Type Culture Collection (Manassas, VA, USA). All cell
lines were maintained in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin.
All cells were seeded into Cellstars® tissue culture
plates (Greiner Bio-One GmbH, Frickenhausen, Germany) in a
humidified incubator containing 5% CO2 at 37°C.
Transfection with NAC1 small
interfering RNA (siRNA)
Two siRNAs targeting NAC1 were designed with the
following sense sequences: 5′-UGAUGUACACGUUGGUGCCUGUCACCA-3′ and
5′-GAGGAAGAACUCGGUGCCCUUCUCCAU-3′. Control siRNA (luciferase siRNA)
was purchased from Invitogen (cat. no. 12935-146; Thermo Fisher
Scientific, Inc.). A total of 5,000 SKOV3 cells/well were seeded in
96-well plates and transfected with siRNAs using
Oligofectamine™ (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
Retroviral transfection and generation
of NAC1
The TOV-21G cells were used for transfection when
the confluence reached 70%. The NAC1 retroviral vector (pWZL-Hygro
retroviral vector) was donated by Dr Ie-Ming Shih (Johns Hopkins
Medical Institutions, Baltimore, MD, USA). Packaging cells (Phoenix
cells; Invitrogen; Thermo Fisher Scientific, Inc.) were transiently
transfected with the NAC1 construct or empty vector using
Lipofectamine™ (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The following day,
the supernatant was harvested and passed through a 0.45 µm syringe
filter. The filtered viral supernatant was resuspended in 4 µg/ml
polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and added
to the TOV-21G ovarian cancer cell cultures. Cells were incubated
for 24 h following infection at 37°C and subsequently harvested
using Trypsin-EDTA for use in assays.
SAβgal assay
A total of 5,000 TOV-21G and SKOV3 cells were seeded
into 96-well plates. Following appropriate exposure to 1, 2 or 5 µM
cisplatin, cells at 37°C for 24 h. Cells were stained for
β-galactosidase activity as previously described by Dimri et
al (9). Cells were washed twice
with PBS, fixed with 2% formaldehyde and 0.2% glutaraldehyde at
room temperature for 1 h, washed three times with PBS, and
incubated at 37°C overnight in X-gal staining solution using a
Senescent β-Galactosidase Staining kit (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's protocol. Blue-stained senescent
cells were counted using a light microscope. Cell morphology was
analyzed using a light microscope.
Western blot analysis
Cell lysates were prepared from siRNA-transfected
SKOV3 cells and NAC1-transfected TOV-21G cells following exposure
to 1, 2 or 5 µM cisplatin at 37°C for 24 h. Equal amounts of total
protein (20 µg/well) from each lysate were separated on 10%
Tris-glycine-SDS-polyacrylamide gels (Novex; Thermo Fisher
Scientific, Inc.) using SDS/PAGE and transferred using
electroblotting onto Immobilon-P polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
probed overnight at 4°C with anti-NAC1 antibodies (cat. no.
NB110-77345; 1:1,000 dilution; Novus Biologicals, Littleton, CO,
USA) and anti-GADD45GIP1 antibodies (cat. no. LS-C120010; 1:500
dilution; LifeSpan Biosciences, Inc.) followed by incubation with
horseradish peroxidase-conjugated anti-mouse immunoglobulin (cat.
no. 715-035-1500; 1:10,000 dilution; Jackson ImmunoReasearch
Laboratories, Inc., West Grove, PA, USA) at room temperature for 1
h. The same membranes were probed with anti-GAPDH antibodies
overnight at 4°C (cat no. 5174; 1:10,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA) as a loading control. Western
blots were developed by chemiluminescence, according to the
manufacturer's protocol (cat. no. 62242; Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
Statistical analyses were conducted using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). Results are
presented as the mean ± standard deviation from triplicate
determinations. Progression-free and overall survival rates were
calculated between the date of diagnosis and the date of first
relapse or last follow-up. Survival data were plotted as
Kaplan-Meier estimator curves, and the statistical significance was
determined using the log-rank test. Data were censored when
patients were lost to follow-up. The Student's t test was used to
analyze the significance of differences. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of NAC1 and GADD45GIP1
protein is inversely associated in ovarian carcinoma
Among 49 ovarian carcinomas, increased NAC1
expression (immunohistochemical intensity of 2+ or 3+) was observed
in 12 cases (24%), and increased GADD45GIP1 expression was
identified in 13 cases (26%). NAC1 immunoreactivity was determined
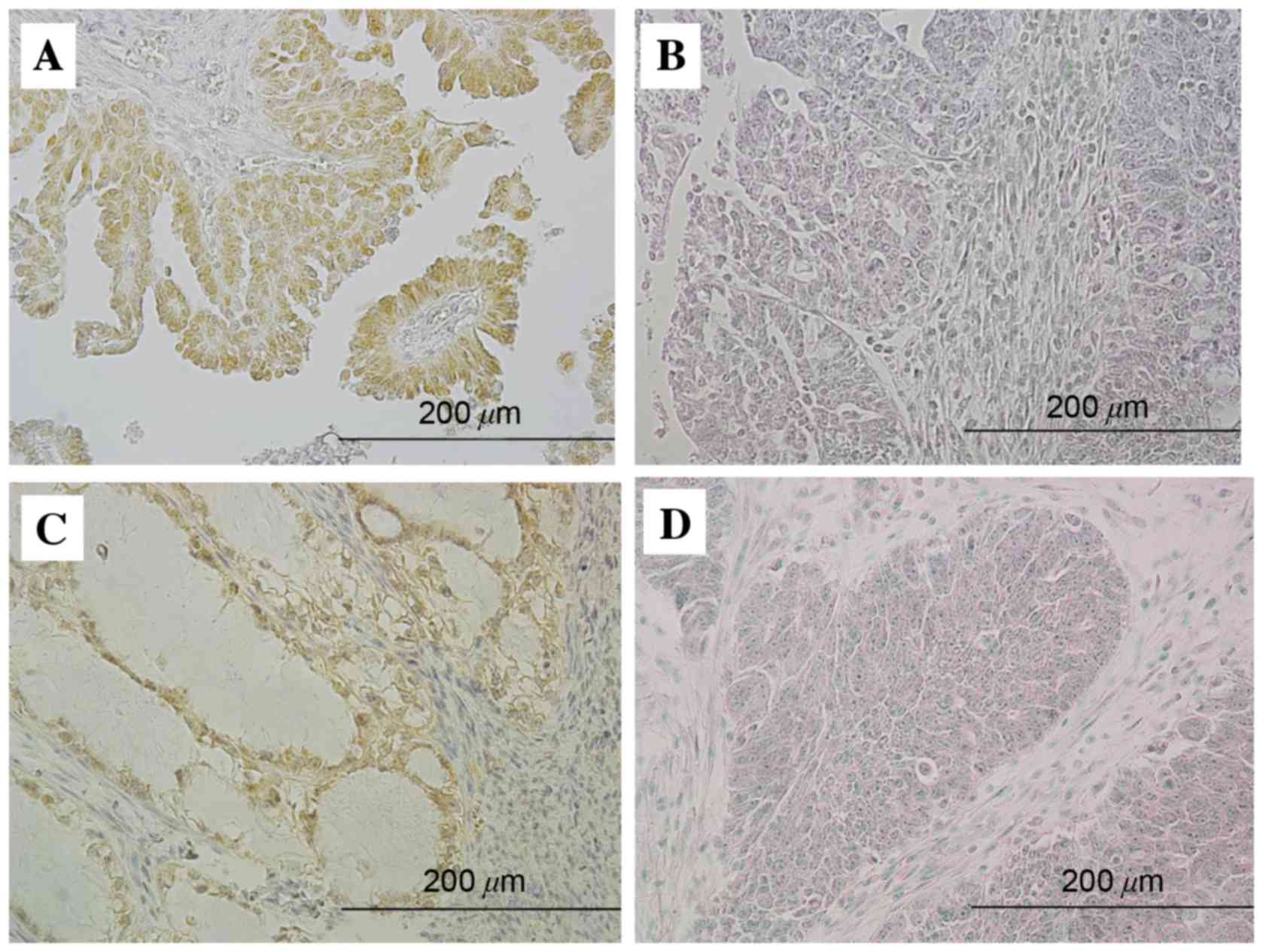
in tumor cell nuclei (Fig. 1A and B).
GADD45GIP1 immunoreactivity was detected in the cytoplasm (Fig. 1C and D). All 12 cases with increased
NAC1 expression exhibited decreased GADD45GIP1 expression (Table I), whereas the remaining 37 cases with
decreased NAC1 expression exhibited decreased (24 cases) or
increased (13 cases) GADD45GIP1 expression. Increased NAC1
expression was significantly inversely associated with decreased
GADD45GIP1 expression in ovarian carcinomas (P<0.05).
 | Table I.Association between NAC1 and
GADD45GIP1 expression. |
Table I.
Association between NAC1 and
GADD45GIP1 expression.
|
| NAC1 expression |
|---|
|
|---|
| GADD45GIP1
expression | Increased | Decreased | P-value |
|---|
| Increased | 0 | 13 | P<0.05 |
| Decreased | 12 | 24 |
|
Increased NAC1 and decreased
GADD45GIP1 protein expression are associated with decreased
overall/progression-free survival
Of the 49 ovarian carcinoma samples examined in the
present study, 45 were used for clinicopathological and prognostic
analysis. Using immunohistochemical analysis, NAC1 and GADD45GIP1
were identified to be markedly negatively associated with each
other. When patients with ovarian carcinomas treated with
platinum-based chemotherapy were classified using a two-tier system
based on expression level (decreased or increased), patients with
increased NAC1 expression or decreased GADD45GIP1 expression
exhibited significantly decreased progression-free survival
compared with patients exhibiting decreased NAC1 expression or
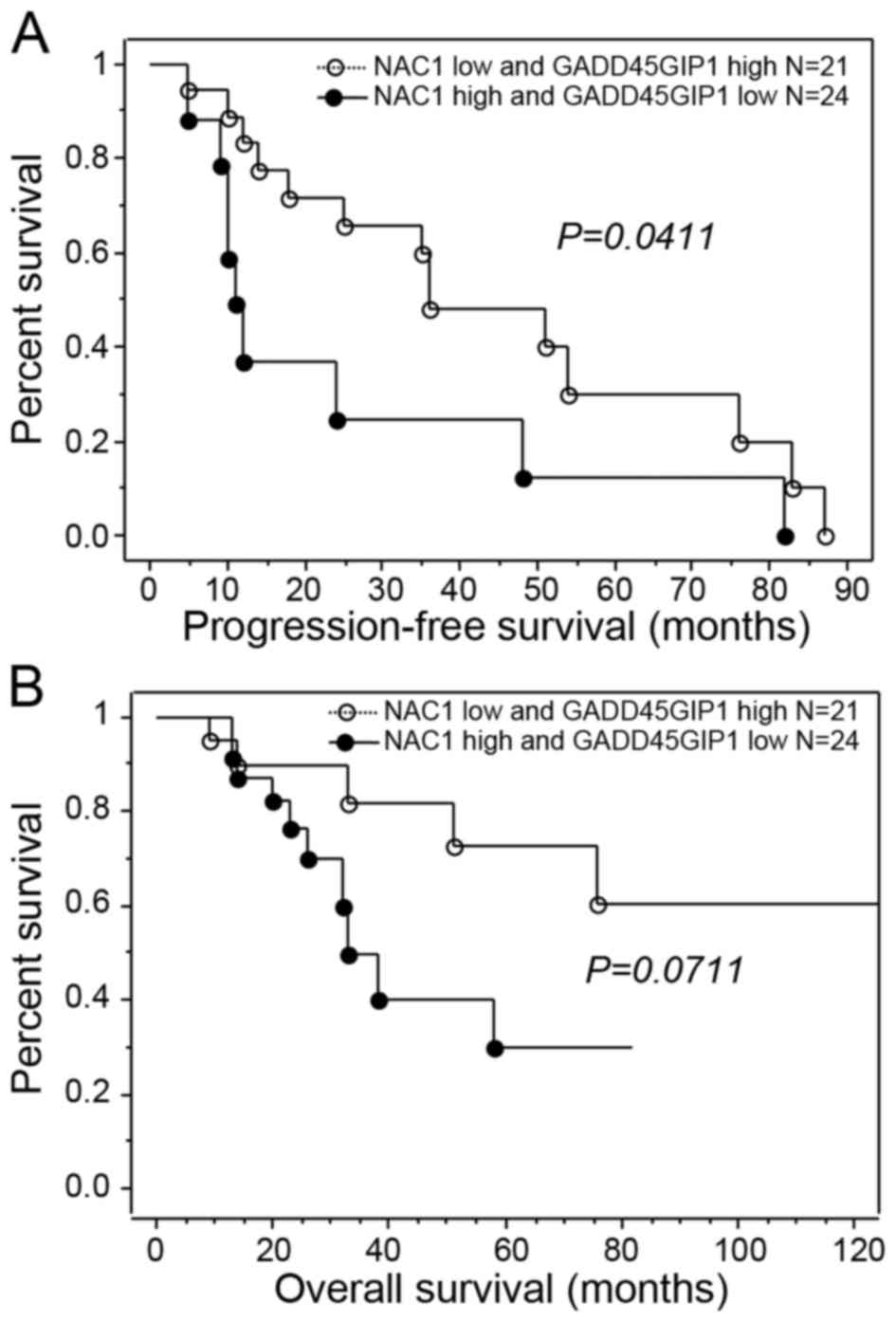
increased GADD45GIP1 expression (P=0.0411, log-rank test; Fig. 2A). The presence of increased NAC1
expression or decreased GADD45GIP1 expression tended to be
associated with decreased overall survival compared with decreased
NAC1 expression or increased GADD45GIP1 expression; however, this
was not identified to be significant (P=0.0711; Table II; Fig.
2B). When the data were stratified using multivariate analysis,
either increased NAC1 expression or decreased GADD45GIP1 expression
and residual tumor (≥1 cm) remained a significant predictor for
decreased progression-free survival (P=0.0405 and 0.0457,
respectively; Table III).
 | Table II.Univariate analysis of overall
prognostic factors in patients with ovarian cancer. |
Table II.
Univariate analysis of overall
prognostic factors in patients with ovarian cancer.
|
|
| Univariate
analysis |
|---|
|
|---|
| Factor | n | Hazard ratio | 95% CI | P-value |
|---|
| FIGO stage |
| 2.8 |
0.4–21.7 |
0.3154 |
| I/II | 10 |
|
|
|
|
III/IV | 35 |
|
|
|
| Histology |
| 1 | 0.3–3.6 |
0.9903 |
|
Serous | 33 |
|
|
|
|
Others | 12 |
|
|
|
| Age, years |
| 0.9 | 0.3–3.0 |
0.9169 |
|
<60 | 12 |
|
|
|
| ≥60 | 33 |
|
|
|
| Residual tumor size,
cm |
| 3.9 |
0.9–17.7 | 0.08 |
|
<1 | 9 |
|
|
|
| ≥1 | 36 |
|
|
|
| NAC1/GADD45GIP1
status |
| 2.8 | 0.9–8.4 |
0.0711 |
| Increased
NAC1 or decreased GADD45GIP1 | 24 |
|
|
|
|
Others | 21 |
|
|
|
 | Table III.Univariate and multivariate analysis
of progression-free prognostic factors in patients with ovarian
cancer. |
Table III.
Univariate and multivariate analysis
of progression-free prognostic factors in patients with ovarian
cancer.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Factor | n | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| FIGO stage |
| 1 | 0.3–2.9 | 0.9389 | NA | NA | NA |
|
III | 10 |
|
|
|
|
|
|
| IV | 35 |
|
|
|
|
|
|
| Histology |
| 1.6 | 0.6–3.8 | 0.3185 | NA | NA | NA |
|
Serous | 33 |
|
|
|
|
|
|
|
Others | 12 |
|
|
|
|
|
|
| Age, years |
| 0.8 | 0.3–1.8 | 0.5532 | NA | NA | NA |
|
<60 | 12 |
|
|
|
|
|
|
|
≥60 | 33 |
|
|
|
|
|
|
| Residual tumor
size, cm |
| 2.8 | 1.0–7.9 | 0.0457 | 2.9 | 1.0–8.6 | 0.0514 |
|
<1 | 9 |
|
|
|
|
|
|
| ≥1 | 36 |
|
|
|
|
|
|
| NAC1/GADD45GIP1
status |
| 2.5 | 1.0–5.9 | 0.0411 | 2.5 | 1.0–6.2 | 0.0405 |
|
Increased NAC1 or decreased
GADD45GIP1 | 24 |
|
|
|
|
|
|
|
Others | 21 |
|
|
|
|
|
|
NAC1 suppresses therapy-induced
cellular senescence in ovarian cancer cells
A previous study demonstrated that NAC1 negatively
regulates senescence in tumor cells (8). In the present study, this phenomenon was
investigated further following treatment of ovarian cancer cells
with cisplatin, the standard chemotherapeutic drug used for ovarian
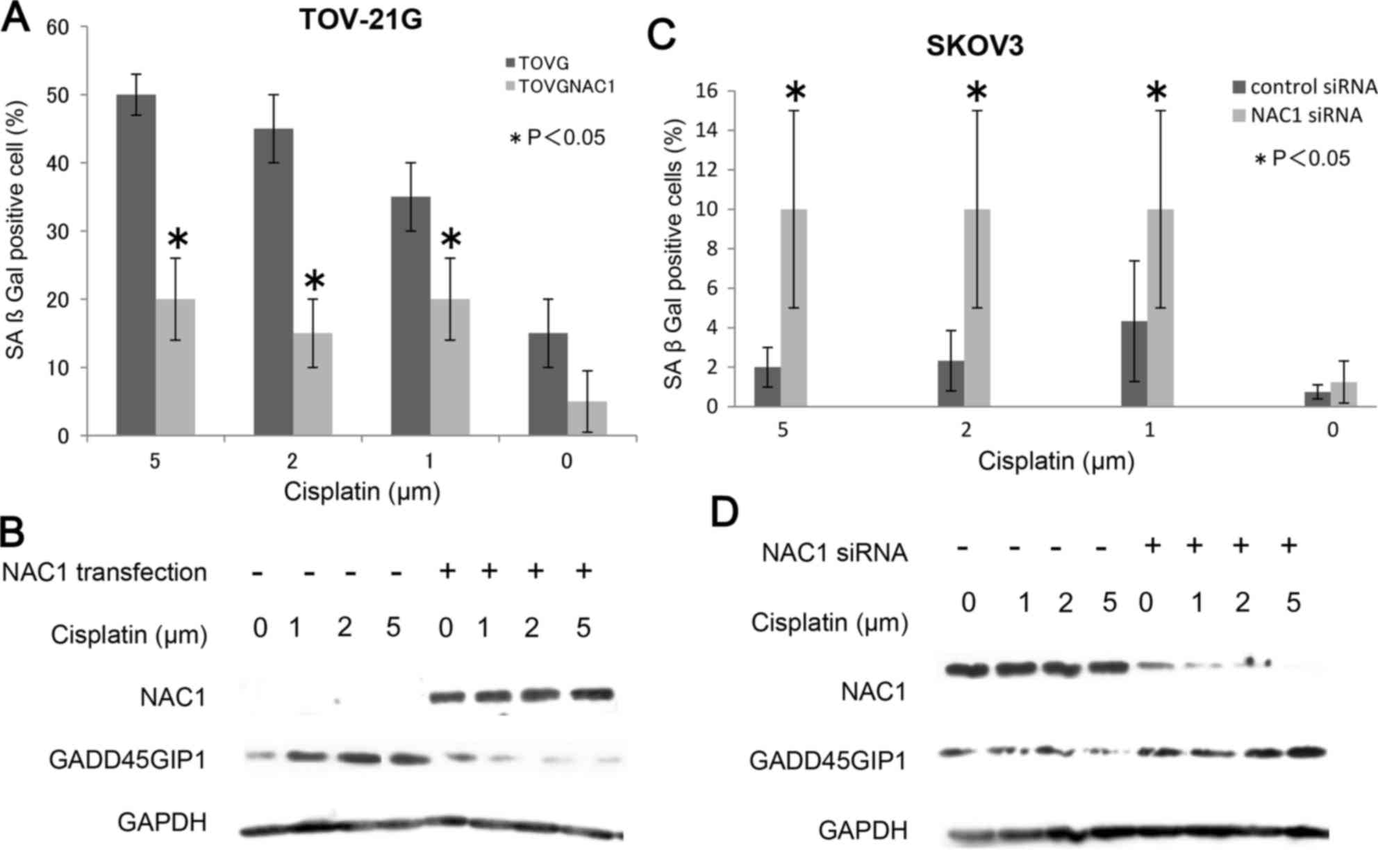
cancer treatment. Various sublethal doses of cisplatin (1, 2 and 5
µM) were used to induce senescence. Sublethal doses of cisplatin
significantly increased the number of blue-stained senescent cells
(P<0.05; Fig. 3A and B), and the
number of cells with flattened and enlarged morphology compared
with that in untreated cells (data not shown).
As presented in Fig.
3A, NAC1 transfection of cisplatin-treated TOV-21G cells caused
a significant decrease in SAβgal staining compared with that in
untransfected cisplatin-treated cells. To confirm this result, the
SAβgal assay was repeated using NAC1 siRNA transfection in SKOV3
cells, which have higher endogenous NAC1 expression. As presented
in Fig. 3B, NAC1 knockdown in
cisplatin-treated SKOV3 cells caused a significant increase in the
number of Saβgal-positive cells compared with that in control
siRNA-transfected SKOV3 cells (P<0.05). These results further
confirmed that NAC1 inactivates stress-induced cellular senescence
following therapeutic intervention.
NAC1 prevents therapy-induced
senescence possibly through inactivation of GADD45GIP1
The gene coding for GADD45GIP1 is a downstream
target regulated by NAC1 (6), and
acts as a negative regulatory factor for cell cycle progression and
cell growth (7). Therefore, it was
hypothesized that GADD45GIP1 contributes to the NAC1-mediated
suppression of cellular senescence. It was identified that
NAC1-transfected TOV-21G cells exhibited decreased GADD45GIP1
protein expression, whereas NAC1-deficient TOV-21G cells exhibited
increased GADD45GIP1 protein expression following treatment with
the same sublethal doses of cisplatin that were used in the SAβgal
assay (Fig. 3C). Similarly, knockdown
of NAC1 in SKOV3 cells followed by treatment with cisplatin
enhanced GADD45GIP1 expression compared with that in
NAC1-overexpressing SKOV3 cells (Fig.
3D).
Discussion
The majority of patients with ovarian cancer are
initially responsive to carboplatin-paclitaxel combination
chemotherapy; however, the majority of patients eventually develop
recurrent chemoresistant tumors, contributing to increased
mortality rates in patients with ovarian cancer (2). In a previous study, the authors
demonstrated that NAC1 upregulation in ovarian serous carcinomas
was significantly associated with early tumor recurrence following
cytoreduction therapy and carboplatin-paclitaxel combined
chemotherapy (3).
In the present study, a previously unrecognized role
for NAC1 in regulating cellular senescence was identified, which
was demonstrated to result in cisplatin resistance. Furthermore, a
potential underlying molecular mechanism was investigated for the
contribution of NAC1 upregulation, as observed in ovarian cancer,
to early recurrence in patients following chemotherapy, as
previously reported (2,14). Treatment with the chemotherapeutic
drug cisplatin was demonstrated to activate cellular senescence in
ovarian cancer cells, and inactivation and gene silencing of NAC1
inhibited the activation of cellular senescence by cisplatin. It
was further demonstrated that regulation of cellular senescence by
NAC1 was mediated through its effects on GADD45GIP1. Previously,
the present authors demonstrated that NAC1 negatively regulates the
expression of GADD45GIP1 (6) and
GADD45G (4). Additionally, Zhang
et al (15) recently
demonstrated that GADD45G promotes cellular senescence in
hepatocellular carcinoma (HCC) cells and markedly suppresses tumor
growth in vivo. It was demonstrated that GADD45 G induces
HCC cell senescence independently of the functional presence of
p16, p53 and retinoblastoma protein and that downregulation of
Janus kinase (Jak)/signal transducer and activator of transcription
3 (Stat3) is the key event for GADD45G-induced cell senescence and
tumor suppression. GADD45GIP1 has been demonstrated to directly
bind to all GADD45 isoforms, particularly GADD45G, and the
interaction between GADD45GIP1 and GADD45 members enhances GADD45
function in a cell culture system (7). Therefore, NAC1 may affect cisplatin
resistance, resulting in cellular senescence through the
suppression of GADD45GIP1 expression; this would inhibit Gadd45G
activity, thereby preventing activation of cellular senescence
through the Jak and Stat3 signaling pathways. Further studies are
required to clarify the specific underlying molecular mechanisms
mediating this cellular senescence cascade between
GADD45GIP1/GADD45G, and the Jak or Stat3 signaling pathway.
Biomarkers that are able to predict clinical
prognosis, including treatment response and overall survival, have
substantial clinical impact on the management of patients with
ovarian cancer (16). To further
explore the clinical relevance of NAC1/GADD45GIP1 axis alterations
in ovarian carcinomas, the association between NAC1/GADD45GIP1
expression and progression-free/overall survival was examined in a
population of patients with ovarian carcinoma in the present study.
A marked association between poor prognosis and NAC1/GADD45GIP1
axis expression was identified in patients who received
platinum-based chemotherapy. The underlying molecular mechanism for
the association between NAC1/GADD45GIP1 axis expression and
decreased survival remains unclear; however, as the mortality of
patients with ovarian cancer is directly associated with recurrence
of the disease following chemotherapy, it is hypothesized that
expression of NAC1 and GADD45GIP1 may confer resistance to
platinum-based chemotherapy and/or enhance cell proliferation in
chemoresistant recurrent tumors, as demonstrated in the present
study and in previous studies (2,3,5).
The results of the present study suggested that
targeting NAC1 to restore the senescence response may be
investigated, as a novel strategy for the treatment of
platinum-resistant ovarian cancer. Furthermore, the NAC1-mediated
suppression of senescence may also influence other aspects of
cancer, including tumor dormancy, response to therapeutic
intervention and metastasis. Exploring the effects of NAC1-mediated
senescence on these features of ovarian cancer may provide insights
into the importance of NAC1 and senescence in the treatment and
management of platinum-resistant ovarian cancer. In addition, NAC1
has been identified to be associated with Nanog in a protein
complex that is necessary for maintaining the stemness of mouse
embryonic stem cells (17,18). Nanog is able to prevent terminal
differentiation of embryonic stem cells and sustain their
pluripotency through a protein network involving NAC1 (19). The interaction between NAC1 with Nanog
(19), as part of a multimember
family required for maintaining the stemness of mouse embryonic
stem cells, suggested that NAC1 serves a role in preventing or
determining the terminal differentiation of cells. Further research
is required to investigate whether this function of NAC1 in stem
cells is associated with its effects on cellular senescence.
In conclusion, the results of the present study
identify NAC1 as a negative regulator of cellular senescence and
demonstrate that NAC1-mediated prevention of senescence, which is
regulated through GADD45GIP1, serves an important role in promoting
cisplatin resistance. The identification of the NAC1/GADD45GIP1
axis as a regulator of senescence, and the elucidation of the
signaling pathways involved, may improve understanding of the
molecular and cellular functions of this nuclear factor in ovarian
cancer. Therefore, the NAC1/GADD45GIP1 axis may be a target in the
treatment of ovarian cancer, particularly in the context of
platinum resistance.
Glossary
Abbreviations
Abbreviations:
|
BTB
|
bric-a-brac-tramtrack-broad
complex
|
|
CDDP
|
cisplatin
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishibashi M, Nakayama K, Yeasmin S,
Katagiri A, Iida K, Nakayama N, Fukumoto M and Miyazaki K: A
BTB/POZ gene, NAC-1, a tumor recurrence-associated gene, as a
potential target for Taxol resistance in ovarian cancer. Clin
Cancer Res. 14:3149–3155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakayama K, Nakayama N, Davidson B, Sheu
JJ, Jinawath N, Santillan A, Salani R, Bristow RE, Morin PJ, Kurman
RJ, et al: A BTB/POZ protein, NAC-1, is related to tumor recurrence
and is essential for tumor growth and survival. Proc Natl Acad Sci
USA. 103:pp. 18739–18744. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jinawath N, Vasoontara C, Yap KL,
Thiaville MM, Nakayama K, Wang TL and Shih IM: NAC-1, a potential
stem cell pluripotency factor, contributes to paclitaxel resistance
in ovarian cancer through inactivating Gadd45 pathway. Oncogene.
28:1941–1948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama K, Rahman MT, Rahman M, Yeasmin
S, Ishikawa M, Katagiri A, Iida K, Nakayama N and Miyazaki K:
Biological role and prognostic significance of NAC1 in ovarian
cancer. Gynecol Oncol. 119:469–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama K, Nakayama N, Wang TL and Shih
IeM: NAC-1 controls cell growth and survival by repressing
transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer
Res. 67:8058–8064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung HK, Yi YW, Jung NC, Kim D, Suh JM,
Kim H, Park KC, Song JH, Kim DW, Hwang ES, et al: CR6-interacting
factor 1 interacts with Gadd45 family proteins and modulates the
cell cycle. J Biol Chem. 278:28079–28088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Cheng Y, Ren X, Hori T,
Huber-Keener KJ, Zhang L, Yap KL, Liu D, Shantz L, Qin ZH, et al:
Dysfunction of nucleus accumbens-1 activates cellular senescence
and inhibits tumor cell proliferation and oncogenesis. Cancer Res.
72:4262–4275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:pp.
9363–9367. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saretzki G: Cellular senescence in the
development and treatment of cancer. Curr Pharm Des. 16:79–100.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechel A, Satyanarayana A, Ju Z, Plentz
RR, Schaetzlein S, Rudolph C, Wilkens L, Wiemann SU, Saretzki G,
Malek NP, et al: The cellular level of telomere dysfunction
determines induction of senescence or apoptosis in vivo. EMBO Rep.
6:275–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Havelka AM, Berndtsson M, Olofsson MH,
Shoshan MC and Linder S: Mechanisms of action of DNA-damaging
anticancer drugs in treatment of carcinomas: Is acute apoptosis an
‘off-target’ effect? Mini Rev Med Chem. 7:1035–1039. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Odicino F, Pecorelli S, Zigliani L and
Creasman WT: History of the FIGO cancer staging system. Int J
Gynaecol Obstet. 101:205–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih IeM, Nakayama K, Wu G, Nakayama N,
Zhang J and Wang TL: Amplification of the ch19p13.2 NACC1 locus in
ovarian high-grade serous carcinoma. Mod Pathol. 24:638–645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Yang Z, Ma A, Qu Y, Xia S, Xu D,
Ge C, Qiu B, Xia Q, Li J and Liu Y: Growth arrest and DNA damage
45G down-regulation contributes to Janus kinase/signal transducer
and activator of transcription 3 activation and cellular senescence
evasion in hepatocellular carcinoma. Hepatology. 59:178–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scott M and Hall PA: Prognostic and
predictive factors. Methods Mol Med. 97:1–11. 2004.PubMed/NCBI
|
|
17
|
Kim J, Chu J, Shen X, Wang J and Orkin SH:
An extended transcriptional network for pluripotency of embryonic
stem cells. Cell. 132:1049–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Rao S, Chu J, Shen X, Levasseur
DN, Theunissen TW and Orkin SH: A protein interaction network for
pluripotency of embryonic stem cells. Nature. 444:364–368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Levasseur DN and Orkin SH:
Requirement of Nanog dimerization for stem cell self-renewal and
pluripotency. Proc Natl Acad Sci USA. 105:pp. 6326–6331. 2008;
View Article : Google Scholar : PubMed/NCBI
|