Introduction
The National Comprehensive Cancer Network guidelines
for the management of colorectal cancer recommend the initial
treatment of metastatic colorectal cancer (mCRC) with oxaliplatin,
fluorouracil and leucovorin (FOLFOX) or irinotecan (CPT-11),
fluorouracil and leucovorin (FOLFIRI) in combination with
bevacizumab (1). This is followed by
second-line treatment with the regimen not selected for initial
treatment in the event of disease progression (1).
Alternatives to FOLFOX and FOLFIRI include the
combination of oral fluorouracil and a venous drip infusion of
oxaliplatin (administered as XELOX, the combination of capecitabine
and oxaliplatin) or CPT-11 (administered as XELIRI, the combination
of capecitabine and irinotecan), which are widely used for patients
with mCRC, as first-line therapy (2–4). XELOX and
XELIRI regimens in combination with bevacizumab typically have the
benefit of not needing a central venous access device (CVAD) or a
home infusion pump, and require less frequent outpatient visits. In
a first-line setting, the XELIRI-bevacizumab regimen has been
demonstrated to exhibit efficacy equivalent to FOLFIRI or XELOX in
combination with bevacizumab, according to the ACCORD 13 study
(3) and AIO trial 0604 (4). However, the efficacy of
XELIRI-bevacizumab for second-line therapy remains largely
unknown.
In a Phase III trial (ML18147), the tri-weekly
XELIRI-bevacizumab regimen, as used in AIO trial 0604, was selected
for 12% of patients, while a CPT-11-based regimen was selected for
~35% of patients as second-line therapy (5). This indicates that XELIRI-bevacizumab is
recognized as a viable treatment option alongside
FOLFOX-bevacizumab and FOLFIRI-bevacizumab for European patients.
In Japan, a Phase I/II study (the BIX study) was conducted to
evaluate the benefits of the dosage regimen used in the AIO trial
0604 in patients with mCRC who had previously been treated with
oxaliplatin and bevacizumab. The BIX study showed that
XELIRI-bevacizumab was safe and effective in Japanese patients
(6).
Sequential administration of XELOX and XELIRI in
combination with bevacizumab in a first- to second-line setting
would allow patients to be managed more easily in an outpatient
unit. However, only a small number of studies have considered the
benefits of this strategy (7), and
concerns regarding cumulative adverse events as a consequence of
continuous use of capecitabine remain (8).
In the present study, the efficacy and feasibility
of sequential administration of XELOX and XELIRI (X–X) in
combination with bevacizumab in a first- to second-line setting was
evaluted, and compared with the FOLFOX-FOLFIRI (F-F) regimen.
Materials and methods
Patients and treatments
Bevacizumab and capecitabine were purchased from
Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan); folinic acid from
Pfizer Japan, Inc. (Tokyo, Japan); fluorouracil (5-FU) from Kyowa
Hakko Kirin Co., Ltd. (Tokyo, Japan) and oxaliplatin and irinotecan
(CPT-11) from Yakult Honsha Co., Ltd. (Tokyo, Japan).
A total of 81 consecutive patients with mCRC, all of
Asian descent, were recruited to the study from at the Saitama
Medical Center, Jichi Medical University (Saitama, Japan) between
January 2006 and December 2012. The patients were treated with the
F-F regimen, with or without bevacizumab (n=40) or the X–X regimen
with or without bevacizumab (n=41) as first- to second-line
chemotherapy.
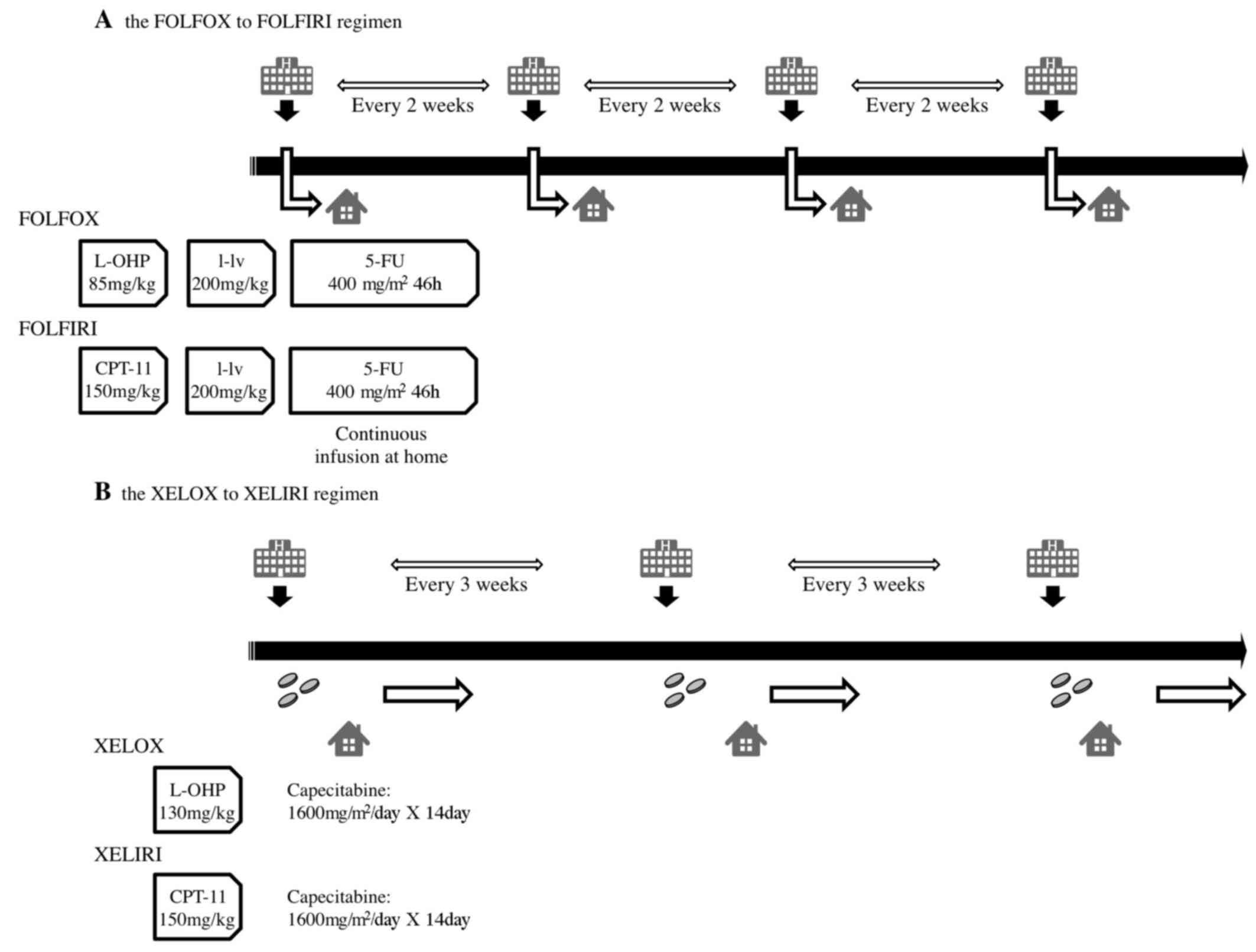
Individuals described as receiving the FOLFOX
regimen received either a FOLFOX4 or mFOLFOX6 regimen. The FOLFOX4
regimen consisted of folinic acid (200 mg/m2),
fluorouracil (5-FU; 400 mg/m2) followed by 22 h of
continuous infusion with 5-FU 600 mg/m2 for 2
consecutive days every 2 weeks, with a 2-h infusion of oxaliplatin
(85 mg/m2) on day 1. The mFOLFOX6 regimen consisted of
folinic acid (200 mg/m2), fluorouracil (5-FU; 400
mg/m2) and oxaliplatin (85 mg/m2) on day 1,
followed by 46 h of continuous infusion with 5-FU 2,400
mg/m2 on days 1 and 2, with or without bevacizumab (5
mg/kg) on day 1 and every 14th day. The FOLFIRI regimen included
folinic acid (200 mg/m2), 5-FU (400 mg/m2)
and CPT-11 (150 mg/m2) on day 1, followed by 46 h of
continuous infusion with 5-FU (2,400 mg/m2) on days 1
and 2, with or without bevacizumab depending on the patient (5
mg/kg) on day 1 and q14d.
As a first-line chemotherapy, the XELOX regimen
consisted of capecitabine (1,600 mg/m2 for patients aged
≥65 years or 2,000 mg/m2 for patients aged <65 years)
on days 1–15 and oxaliplatin (130 mg/m2) on day 1, with
or without bevacizumab (7.5 mg/kg) depending on the patient, on day
1 and q21d.
As a second-line chemotherapy, the XELIRI regimen
consisted of CPT-11 (200 mg/m2) on day 1 in combination
with oral capecitabine (1,600 mg/m2 for patients aged
≥65 years or 2,000 mg/m2 for patients aged <65 years)
on days 1–15, with or without bevacizumab depending on the patient
(7.5 mg/kg) on day 1 and q21d. The gradual dose reduction from
first-line chemotherapy was also applied to second-line
chemotherapy. Schemas of the treatment schedule for the F-F and X–X
regimens are included in Fig. 1.
Efficacy and safety assessment
Progression free survival (PFS) rates, overall
survival (OS) rates and adverse events were assessed and compared
between the F-F and X–X regimens. In addition, the time to failure
of strategy (TFS), which was the period from the start of
first-line chemotherapy to the end of second-line chemotherapy, was
assessed. Lesions were evaluated every 3–4 cycles of chemotherapy
by a computed tomography scan. Tumor response and progression were
assessed as complete response (CR), partial response (PR), stable
disease (SD) or progressive disease (PD) according to the Response
Evaluation Criteria for Solid Tumors (9). Adverse events were graded as 0–4
according to the Common Terminology Criteria for Adverse Events,
version 3.0 (10). Treatment was
continued till disease progression, unacceptable toxicity (grade
3/4), deterioration of Eastern Cooperative Oncology Group
performance status (11) to >2, or
withdrawal of patient consent. The present study was approved by
the Research Ethics Committee of Jichi Medical University. Written
informed consent was obtained from all patients prior to
chemotherapy, according to institutional guidelines.
Statistical analysis
A χ2 test was used to assess the
association between two categorical variables. Continuous
comparisons between two groups were performed using a Student's
t-test for normally distributed data, and the non-parametric
Mann-Whitney U test for data that were not normally distributed.
P<0.05 was considered to indicate a statistically significant
difference. Values are shown as the mean ± standard deviation. PFS,
OS and TFS data are plotted as Kaplan-Meier curves, and the
differences among groups were compared with the log-rank test. STAT
View Version 5.0.1 (SAS Institute Inc., NC, USA) was used to
perform all analyses.
Results
Patient characteristics
Patient characteristics are summarized in Table I. In total, 40 patients received the
F-F regimen and 41 patients underwent the X–X regimen. In the F-F
group, 10 patients (25%) were treated with bevacizumab during first
or second line chemotherapy, whereas 20 patients (49%) received
bevacizumab in the X–X group. There was a significant difference in
the likelihood of bevacizumab administration between the two groups
(P=0.03); however, there were no significant differences in median
age, sex, primary tumor site, or the likelihood of resection of the
primary tumor (P=0.48, 0.58, 0.20 and 0.25, respectively). There
was a significant difference in the metastatic site between the two
groups (P=0.02). The median follow-up was 16.1 months (range,
5.1–39.0) in the F-F regimen and 19.9 months (range, 4.0–46.6) in
the X–X regimen.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | F-F (n=40) | X-X (n=41) | P-value |
|---|
| Age, years | 66 | 66 | 0.48a |
| [median (range)] | (40–86) | (37–85) |
|
| Gender, n |
|
| 0.58b |
| Male | 27 | 30 |
|
|
Female | 13 | 11 |
|
| Primary tumor site,
n |
|
| 0.20b |
|
Colon | 28 | 23 |
|
|
Rectum | 12 | 18 |
|
| Primary lesion,
n |
|
| 0.71b |
|
Resected | 38 | 36 |
|
|
Remained | 2 | 5 |
|
| Bevacizumab, n |
|
| 0.03b |
|
Included | 10 | 20 |
|
| not
included | 30 | 21 |
|
| Metastatic site,
n |
|
| 0.02b |
|
Liver | 19c | 19 |
|
| Lung | 4 | 6 |
|
| Lymph
nodes | 4 | 5 |
|
|
Peritoneum | 11 | 2 |
|
|
Other | 2 | 9 |
|
Relative dose intensity
During first-line treatment, the mean relative dose
intensities were 92.3% for 5-FU and 92.2% for oxaliplatin in the
F-F group, and 90.1% for capecitabine and 94.2% for oxaliplatin in
the X–X group. There were no significant differences in relative
dose intensity between groups (Table
II).
 | Table II.Relative dose intensity. |
Table II.
Relative dose intensity.
|
| F-F, % (n=40) | X-X, % (n=41) |
|---|
|
|
|---|
| FOLFOX | FOLFIRI | XELOX | XELIRI |
|---|
|
|
|
|
|---|
| Treatment | 5-FU | L-OHP | 5-FU | CPT-11 | Cap | L-OHP | Cap | CPT-11 |
|---|
| Relative dose
intensity | 92.3 | 92.2 | 93.8 | 90.2 | 90.1 | 94.2 | 85.3 | 86.4 |
| Full dose | 65.8 | 65.8 | 70.0 | 57.4 | 73.9 | 74.3 | 43.0 | 58.4 |
During second-line treatment, the average relative
dose intensities were 93.8% for 5-FU and 90.2% for CPT-11 in the
F-F group, and 85.3% for capecitabine and 86.4% for CTP-11 in the
X–X group. There were no significant differences in relative dose
intensity between groups. The rate of the full dose of capecitabine
in the XELIRI regimen being administered was 43.0%, which is
relatively low; this was due to a reduction of the capecitabine
dose in order to decrease the incidence of adverse events for some
patients during first-line treatment being carried over to
second-line treatment, resulting in a number of patients then
receiving 80% or 65% of 1,600 mg/m2 of capecitabine as
the initial dose in second-line treatment.
Efficacy
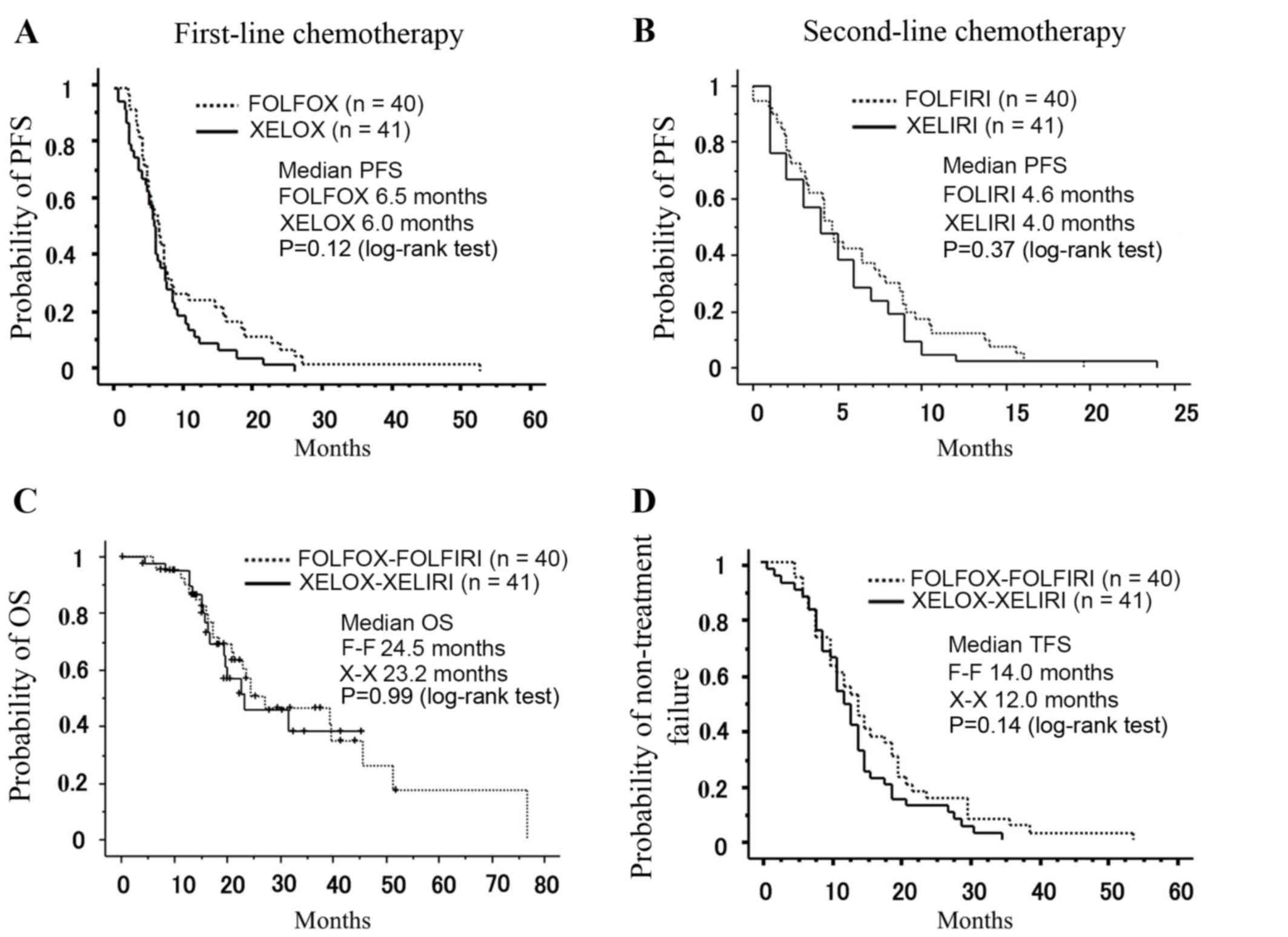
During first-line treatment, DCR was 85.0% for the
FOLFOX regimen and 58.5% for the XELOX regimen. The median PFS was
6.5 months for the FOLFOX regimen and 6.0 months for the XELOX
regimen (P=0.12, Fig. 2A). During
second-line treatment, DCR was 50.0% for the FOLFIRI regimen and
41.4% for the XELIRI regimen. The median PFS was 4.6 months for the
FOLFIRI regimen and 4.0 months for the XELIRI regimen (P=0.37,
Fig. 2B), which was not significantly
different. The median OS was 24.5 months in the F-F regimen and
23.2 months in the X–X regimen (P=0.99, Fig. 2C), which was not significantly
different. The median TFS was 14.0 months in the F-F regimen and
12.0 months in the X–X regimen (P=0.14, Fig. 2D). The relative dose intensity was low
in the XELIRI regimen, but this did not appear to affect median
PFS, OS and TFS. To eliminate the potential bias introduced by the
number of patients receiving bevacizumab in each group when
comparing the X–X and F-F regimens, subgroup analysis was performed
using patients who did not receive bevacizumab. There was no
difference in the median OS rate between these subgroups (P=0.27;
data not shown).
Feasibility
Regarding the most frequently reported grade 3–4
adverse events, the incidence of severe (grade ≥3) neutropenia was
47.5% in the F-F group, and 24.4% in the X–X group (P=0.030,
Table III). Febrile neutropenia was
detected in 1 case in the F-F group and 3 cases in the X–X group.
The incidence of severe (grade ≥3) hypertension was 0% in the F-F
group, and 14.6% in the X–X group (P=0.012, Table III). All hypertensive patients were
successfully treated with a single antihypertensive drug. No
treatment-associated mortality was reported.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| F-F (n=40) | X-X (n=41) |
P-valuea |
|---|
|
|
|
|---|
| Symptom | All, % | ≥G3, % | All, % | ≥G3, % | (≥G3) |
|---|
| Leucopenia | 85.0 | 27.5 | 78.0 | 19.5 | 0.40 |
| Neutropenia | 77.5 | 47.5 | 65.9 | 24.4 | 0.03 |
| Anemia | 67.5 | 7.50 | 65.9 | 7.30 | 0.98 |
|
Thrombocytopenia | 47.5 | 2.50 | 51.2 | 2.40 | 0.99 |
| Liver
dysfunction | 92.5 | 7.50 | 85.4 | 2.40 | 0.29 |
| Renal
dysfunction | 27.5 | 2.50 | 17.1 | 2.40 | 0.99 |
| Diarrhea | 52.5 | 5.00 | 58.5 | 2.40 | 0.54 |
| Constipation | 50.0 | 0 | 39.0 | 2.40 | 0.32 |
| Nausea | 72.5 | 2.50 | 63.4 | 0 | 0.31 |
| Vomiting | 32.5 | 2.50 | 39.0 | 2.40 | 0.99 |
| Fatigue | 52.5 | 2.50 | 61.0 | 4.90 | 0.57 |
| Stomatitis | 60.0 | 2.50 | 61.0 | 2.40 | 0.99 |
| Neuropathy | 87.5 | 2.50 | 92.7 | 4.90 | 0.58 |
| HFS | 30.0 | 0 | 53.7 | 0 | 1.00 |
| Hypertension | 2.50 | 0 | 34.1 | 14.60 | 0.01 |
Subsequent therapy
There was no significant difference in subsequent
therapies between groups (Table
IV).
 | Table IV.Subsequent therapies. |
Table IV.
Subsequent therapies.
| Therapy method | F-F, n (n=40) | X-X, n (n=41) |
|---|
| Anti-EGFR
antibody | 14 | 12 |
| (C-mab+CPT-11/C-mab
only/P-mab) | (6/2/6) | (1/0/12) |
| Other
therapiesa | 2 | 3 |
| Best supportive
care | 23 | 26 |
| Unknown | 1 | 0 |
Discussion
The findings of the present study have demonstrated
that sequential administration of X–X is as effective and feasible
as F-F, with the additional advantage of reducing the frequency of
infusion visits, and eliminating the need for a CVAD or home
infusion pump.
Regarding treatment efficacy, there was no
significant difference between the XELOX and FOLFOX regimens in
first-line therapy (6.5 vs. 6.0 months, P=0.12), which is
consistent with previous reports (12) or the XELIRI and FOLFIRI in second line
therapy, (4.6 vs. 4.0 months, P=0.37). In previous clinical trials,
FOLFIRI2 (continuous infusion of 5-FU, followed by CPT-11) had a
median PFS of 4.1 months (13), and
FOLFIRI3 (continuous infusion of 5-FU with CPT-11) had a median PFS
of 4.1 months (14), which is in
accordance with the present study. It was previously reported in a
non-randomized study that sequential administration of X–X (without
bevacizumab) resulted in a median PFS of 7.0 and 2.0 months in
first- and second-line chemotherapy, respectively (9). The time to second progression (described
as TFS in the present study) was 10 months, compared with 12 months
in the present study.
In the present study, a significant difference in
the number of patients receiving bevacizumab between the F-F and
X–X groups was identified, with 10 and 20 patients receiving
bevacizumab in the F-F and X–X groups, respectively. However, as
identified in the subgroup analysis, there was no difference in
median OS rate between patients who did and did not receive
bevacizumab, which suggests that X–X is as effective as the F-F
regimen without considering bevacizumab.
According to a systematic review (Phase II)
bevacizumab in combination with FOLFIRI prolongs the median PFS to
8.3 months compared with FOLFIRI alone, in the second-line setting
(15). XELIRI in combination with
bevacizumab was associated with a median PFS of 8.3 months in the
second-line setting in the BIX study (6), which is considerably longer than in the
present study, where the median PFS was 4.6 months (where 49% of
patients were treated with bevacizumab). However, we previously
reported a median PFS of 7.2 months when all patients were treated
with XELIRI with bevacizumab in the second-line setting (16).
The BIX study (6)
showed that the most common Grade 3–4 adverse events were nausea
(5.9%), diarrhea (5.9%), fatigue (2.9%) and neutropenia (8.8%). The
efficacy analysis revealed an overall response rate of 17.6% and a
PFS time of 8.3 months (6),
consistent with the data of the present study. Due to the dose
modification of capecitabine during first-line treatment, no
cumulative adverse events were observed as a consequence of the
continued use of capecitabine in the present study. Severe
hypertension (≥grade 3) was seen in 14.6% of patients in the X–X
group; however, all of the hypertensive patients were successfully
treated with a single antihypertensive drug. These findings suggest
that X–X plus bevacizumab is a safe and effective treatment for
patients with mCRC of Asian descent.
Unlike FOLFOX or FOLFIRI, the X–X regimen does not
require a central venous access device (CVAD), which itself may
cause complications. Indeed, CVAD-associated complications are
possible including pain, hematoma and hemorrhage, site infection
and catheter thrombosis, although no such complications occurred in
the present study. Another possible complication associated with
CVAD is pump malfunction, which may cause accelerated drug delivery
during contentious infusion of 5-FU and increase the risk of grade
3/4 neutropenia (17).
The tri-weekly X–X regimen is more convenient in
terms of administration and increases the time for work and other
activities compared with FOLFOX-6 (18). Sequential use of oral
fluoropyrimidines may be more convenient for patients with mCRC. In
addition, it is reported that XELOX significantly decreased the
direct treatment costs of patients with mCRC compared with the cost
of FOLFOX-6 (19). XELOX or XELIRI
can be introduced in an outpatient setting as the treatment can be
administered in a comparatively short time, limiting the use of
hospital resources as patients would not need to be admitted for
chemotherapy.
In conclusion, the present results should be
interpreted within the context of the study limitations; the study
was retrospective and only considered members from specific
treatment groups. Further studies, including multinational
randomized phase III studies, are required to draw definitive
conclusions. However, based on the results of the present study,
the administration of X–X has been demonstrated to be as effective
and feasible as F-F, with the advantage of reducing the frequency
of infusion visits with no need for a CVAD or home infusion
pump.
Acknowledgements
This work was supported in part by a grant-in-aid of
the post graduate students from Jichi Medical University, a
grant-in-aid from the Ministry of Education, Culture, Sports,
Science and Technology (grant no. JP16K10514), and the JKA
Foundation through its promotion funds from Keirin Racing (grant
no. 27-1-068).
References
|
1
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ducreux M, Adenis A, Pignon JP, François
E, Chauffert B, Ichanté JL, Boucher E, Ychou M, Pierga JY,
Montoto-Grillot C and Conroy T: Efficacy and safety of
bevacizumab-based combination regimens in patients with previously
untreated metastatic colorectal cancer: Final results from a
randomised phase II study of bevacizumab plus 5-fluorouracil,
leucovorin plus irinotecan versus bevacizumab plus capecitabine
plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer.
49:1236–1245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmiegel W, Reinacher-Schick A, Arnold D,
Kubicka S, Freier W, Dietrich G, Geißler M, Hegewisch-Becker S,
Tannapfel A, Pohl M, et al: Capecitabine/irinotecan or
capecitabine/oxaliplatin in combination with bevacizumab is
effective and safe as first-line therapy for metastatic colorectal
cancer: A randomized phase II study of the AIO colorectal study
group. Ann Oncol. 24:1580–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamamoto Y, Yamaguchi T, Nishina T,
Yamazaki K, Ura T, Nakajima T, Goto A, Shimada K, Nakayama N,
Sakamoto J, et al: A phase I/II study of XELIRI plus bevacizumab as
second-line chemotherapy for Japanese patients with metastatic
colorectal cancer (BIX Study). Oncologist. 19:1131–1132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakar B, Gumus M, Basaran M, Argon A,
Ustuner Z, Ustaoglu MA, Saglam S, Guney N, Tenekeci AN and Aykan
NF: XELOX followed by XELIRI or the reverse sequence in advanced
colorectal cancer. Oncology. 73:298–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lassere Y and Hoff P: Management of
hand-foot syndrome in patients treated with capecitabine (Xeloda).
Eur J Oncol Nurs. 8 Suppl 1:S31–S40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japanese translation of common terminology
criteria for adverse events (CTCAE) and instructions and
guidelines. Int J Clin Oncol. 9(Suppl 3): S1–S82. 2004.(In
Japanese).
|
|
11
|
Balducci L and Beghe C: The application of
the principles of geriatrics to the management of the older person
with cancer. Crit Rev Oncol Hematol. 35:147–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mabro M, Louvet C, André T, Carola E,
Gilles-Amar V, Artru P, Krulik M and de Gramont A: GERCOR:
Bimonthly leucovorin, infusion 5-fluorouracil, hydroxyurea and
irinotecan (FOLFIRI-2) for pretreated metastatic colorectal cancer.
Am J Clin Oncol. 26:254–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mabro M, Artru P, André T, Flesch M,
Maindrault-Goebel F, Landi B, Lledo G, Plantade A, Louvet C and de
Gramont A: A phase II study of FOLFIRI-3 (double infusion of
irinotecan combined with LV5FU) after FOLFOX in advanced colorectal
cancer patients. Br J Cancer. 94:1287–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beretta GD, Petrelli F, Stinco S, Cabiddu
M, Ghilardi M, Squadroni M, Borgonovo K and Barni S: FOLFIRI +
bevacizumab as second-line therapy for metastatic colorectal cancer
pretreated with oxaliplatin: A pooled analysis of published trials.
Med Oncol. 30:4862013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki K, Takaharu K, Muto Y, Ichida K,
Fukui T, Takayama Y, Tsujinaka S, Sasaki J, Horie H, Kawamura YJ,
et al: XELIRI regimen plus continuous treatment with bevacizumab is
well-tolerated and effective in metastatic colorectal cancer
patients in a second-line setting involving the sequential
administration of XELOX and XELIRI. Mol Clin Oncol. 2:827–832.
2014.PubMed/NCBI
|
|
17
|
Chu E, Haller D, Cartwright T, Twelves C,
Cassidy J, Sun W, Saif MW, McKenna E7, Lee S and Schmoll HJ:
Epidemiology and natural history of central venous access device
use and infusion pump function in the NO16966 trial. Br J Cancer.
110:1438–1445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conroy T, Hebbar M, Bennouna J, Ducreux M,
Ychou M, Llédo G, Adenis A, Faroux R, Rebischung C, Kockler L and
Douillard JY: Quality-of-life findings from a randomised phase-III
study of XELOX vs FOLFOX-6 in metastatic colorectal cancer. Br J
Cancer. 102:59–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perrocheau G, Bennouna J, Ducreux M,
Hebbar M, Ychou M, Lledo G, Conroy T, Dominguez S, Faroux R,
Florentin V and Douillard JY: Cost-minimisation analysis in
first-line treatment of metastatic colorectal cancer in France:
XELOX versus FOLFOX-6. Oncology. 79:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|