Introduction
Lung cancer is the predominant reason of
cancer-associated mortality in developed and developing countries.
Lung adenocarcinoma is the most common histological type of lung
cancer, and accounts for ~50% of all lung cancers (1). Although the management and treatment of
surgery, radiotherapy and chemotherapy have improved, the
therapeutic efficacy is poor. One of the major reasons is the lack
of adequate treatment against lung adenocarcinoma invasion
(2,3).
Therefore, the development of novel adjuvant therapeutic strategies
specifically targeting the progression of invasion is of critical
importance for improving the prognosis of patients with lung
adenocarcinoma.
Invasion occurs through a complex pathophysiological
process involving multiple genetic alterations (4,5). Matrix
metalloproteinases (MMPs), particularly MMP-2 and MMP-9, play an
important role in the invasion process of numerous malignant tumors
by degrading the basement membrane and extracellular matrix (ECM)
(6–8).
Previous studies have suggested that MMP-2 and MMP-9 are associated
with invasion in lung cancer (4,5). In
addition, numerous studies have demonstrated that MMPs may be
regulated by tissue inhibitor of metalloproteinase (TIMP) (6,9).
Disturbing the balance of MMPs and TIMPs may affect the remodeling,
formation and degradation of matrix protein and induce invasion of
cancer cells.
The phosphoinositide 3-kinase (PI3K)/AKT signaling
pathway performs a key role in the control of cell differentiation
and proliferation. Previously, activation of the PI3K/AKT signaling
pathway was suggested to be associated with the invasion of
numerous tumor types, including prostate, ovarian, colon and breast
cancers (10–13). In lung cancer, the PI3K/AKT signaling
pathway is also considered as a crucial activator of intracellular
signaling cascades in invasion progression and is useful as a
therapeutic target for anticancer drug development.
Allicin, the main active principle associated with
Allium sativum chemistry, possesses therapeutic potential,
with antioxidant, anti-inflammatory and antitumor activities.
Previous studies demonstrated that allicin may induce tumor cell
apoptosis, inhibit the tumor cell cycle and regulate angiogenesis
(14–17). Numerous mechanisms are involved in the
biological activities of allicin, including unfolded protein
response, p53-mediated autophagy and p38 mitogen-activated protein
kinase/caspase-3 signaling (14–18).
However, to the best of our knowledge, the effects and mechanisms
of allicin on lung adenocarcinoma remain undefined. In addition,
the role of allicin in inhibiting invasion has not been reported.
In the present study, it was revealed that allicin may suppress
migration and invasion of lung adenocarcinoma cells by altering
TIMP/MMP balance via reduction of the activity of the PI3K/AKT
signaling pathway in vitro.
Materials and methods
Reagents
Lipofectamine® 2000 reagents and fetal
bovine serum (FBS) were purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Allicin and specific activator
of PI3K (insulin-like growth factor-1; IGF-1) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Specific
inhibitor of PI3K (LY294002) was purchased from Beyotime Institute
of Biotechnology (Shanghai, China). MTT was purchased from Beyotime
Institute of Biotechnology. Anti-phospho-AKTSer473
(anti-p-AKTS473) and anti-AKT were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-MMP-2,
anti-MMP-9, anti-TIMP-1, anti-TIMP-2 and anti-β-actin were
purchased from Santa Cruz Biotechnology, Inc. Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit (cat no. ZDR-5307;
dilution, 1:2,000) and anti-mouse immunoglobulin (cat no. ZDR-5307;
dilution, 1:2,000) were obtained from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China).
Cell lines and cell culture
The two lung adenocarcinoma cell lines A549 and
H1299 were purchased from the Cell Bank of Type Culture Collection
of Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Dulbecco's modified Eagle's medium with 10% FBS, 100
U/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Cell viability assay
The effects of allicin on cell viability were
determined by an MTT assay. MTT was purchased from Beyotime
Institute of Biotechnology. Cells (104 cells per well)
were seeded onto 96-well plates, incubated overnight at 37°C and
then incubated in various concentrations of allicin (0, 1.0, 2.5,
5.0, 7.5, 10.0, 15.0 and 20.0 µM) for 24 h. The medium was then
removed and the MTT solution (0.5 mg/ml) was added to the cell
culture. Following incubation for 4 h at 37°C, the reaction was
stopped by adding dimethyl sulfoxide (0.5 mg/ml). At the end,
absorbance was measured spectrophotometrically at 570 nm (Bio-Tek
ELX800UV; Omega Bio-Tek Inc., Norcross, GA, USA).
Cell adhesion assay
The 96-well plates were prepared for coating with 5
mg/ml fibronectin (Sigma-Aldrich; EMD Millipore, Billerica, MA,
USA) and blocking with 1% bovine serum albumin (BSA) for 4 h. A549
and H1299 cells were incubated for 48 h with various concentrations
of allicin (0, 5.0, 7.5 and 10.0 µM) at 37°C. Cells (20,000
cells/well) were then allowed to attach to fibronectin coated
plates for 1 h at 37°C. The unattached cells were washed away with
PBS. Attached cells were quantified by MTT assay.
Transwell migration and invasion
assays
Cell invasion experiments were assayed using 6.5-mm
Transwell chambers (8-µm pore size; Corning-Costar Inc., Corning,
NY, USA). The filters were precoated with 1–2 mg/ml Matrigel
(reconstituted basement membrane; BD Biosciences, Franklin Lakes,
CA, USA). Cells were pretreated with 0, 5.0, 7.5 and 10.0 µM
allicin or IGF-1 (50 ng/ml) or LY294002 (25 µM). Surviving cells in
100 µl of serum-free medium were seeded in the upper chamber.
Medium supplemented with 10% FBS was added to the lower chamber as
the chemoattractant. Following 24 h of incubation at 37°C, the
cells on the upper side were wiped with a cotton bud. The cells
that had migrated into the lower compartment were fixed with
methanol, stained with hematoxylin and eosin (Beyotime Institute of
Biotechnology) and counted in 5 random fields of the insert under a
light microscope (magnifcation, ×200). A migration assay was
performed as described for the invasion assay, but with a shorter
incubation period (12 h) and no Matrigel coating.
Western blot analysis
The A549 and H1299 cells were extracted with
radioimmunoprecipitation assay buffer [1 mg/ml phenylmethylsulfonyl
fluoride, 1 Mm aprotinin, 1 mg/ml leupeptin, 1 mM EDTA, 150 mM
NaCl, 0.25% Na-deoxycholate, 1 mg/ml pepstatin and 50 mM Tris-HCl
(pH 7.4)] following treatment with various concentrations (0, 5.0,
7.5 and 10.0 µM) of allicin, IGF-1 or LY294002. Total proteins were
quantified using the bicinchoninic acid method. Equal amounts of
protein were separated on SDS-PAGE gels and electrophoretically
transferred to polyvinylidene fluoride membranes. Subsequent to
blocking in 5% BSA for 1 h at room temperature, membranes were
incubated overnight at 4°C with antibodies against MMP-2 (dilution,
1:500; cat no. sc-13594), MMP-9 (dilution, 1:500; cat no.
sc-12759), TIMP-1 (dilution, 1:500; cat no. sc-6832), TIMP-2
(dilution, 1:500; cat no. sc-365671), p-AKTS473
(dilution, 1:1,000; cat no. sc-33437), AKT (dilution, 1:1,000; cat
no. sc-24500) or β-actin (dilution, 1:1,000; cat no. sc-10731). The
membranes were then incubated with the appropriate HRP-conjugated
goat anti-rabbit (cat no. ZDR-5307; dilution, 1:2,000) and
anti-mouse immunoglobulin (cat no. ZDR-5307; dilution, 1:2,000) for
1 h at room temperature. The bands were visualized by enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). The
densitometry analysis was performed using Quality One analysis
software (version 6.0; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 and H1299 cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
subsequent to treatment with various concentrations of allicin (0,
5.0, 7.5 and 10.0 µM). cDNA was synthesized and RT-qPCR was
performed in accordance with previously described protocols
(19). The primers for human MMP-2
(forward, 5′-GGTTGTCTGAAGTCACTGCACAGT-3′ and reverse,
5′-CTCGGTAGGGACATGCTAAGTAGAG-3′), MMP-9 (forward,
5′-GCTGGGCTTAGATCATTCCTCA-3′ and reverse,
5′-CTGGCGACGCAAAAGAAGA-3′), TIMP-1 (forward,
5′-GAGAACCCACCATGGCCC-3′ and reverse,
5′-TATCAGCCACAGCAACAACAGG-3′), TIMP-2 (forward,
5′-CCACCCAGAAGAAGAGCCTG-3′ and reverse, 5′-CAGCGCGTGATCTTGCAC-3′)
and GAPDH (forward, 5′-CCTCCCGCTTCGCTCTCT-3′ and reverse,
5′-CTGGCGACGCAAAAGAAGA-3′) were used for RT-qPCR. The average
expression level of genes was normalized to the reference gene
GAPDH. Data analysis was performed using the 2-ΔΔCq
method (19).
Statistical analysis
Each experiment was repeated at least three times.
Data are presented as the mean ± standard deviation. SPSS version
16.0 statistical software (SPSS, Inc., Chicago, IL, USA) and
Student's t-test were used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Allicin inhibits proliferation of lung
adenocarcinoma cells
To determine the antitumor effect of allicin against
lung adenocarcinoma cells, the ability of allicin to inhibit
proliferation in lung adenocarcinoma A549 and H1299 cells was first
examined by the cell viability assay. As shown in Fig. 1, cell proliferation was significantly
reduced in A549 and H1299 cells following treatment with 15.0 and
20.0 µM allicin (P<0.000). However, no significant reduction in
proliferation was observed when lung adenocarcinoma cells were
treated with allicin at concentrations below 15.0 µM. Therefore, a
concentration range of allicin <15.0 µM was selected for all
subsequent experiments in order to exclude the effect of
cellular cytotoxicity on invasion.
Allicin inhibits adhesion, migration
and invasion of lung adenocarcinoma cells
Cell migration and invasion are critical events in
the development of lung adenocarcinoma. The present study first
examined the cell adhesion ability following incubation of A549 and
H1299 cells with allicin. It was observed that allicin treatment
decreased tumor cell adhesion to fibronectin in a
concentration-dependent manner (Fig. 2A
and B). The effect of allicin on migration and invasion was
then examined in A549 and H1299 cells that were exposed to various
concentrations of allicin for 12 h (cell migration) and 24 h (cell
invasion). The number of migratory or invasive lung adenocarcinoma
cells decreased in a dose-dependent manner (Fig. 2C and D). The aforementioned data
demonstrated that allicin inhibited adhesion, migration and
invasion of lung adenocarcinoma cells.
Allicin alters TIMP/MMP balance in
lung adenocarcinoma cells
It has been demonstrated that MMPs, particularly
MMP-2 and MMP-9, are involved in the invasion of lung cancer.
Therefore, the present study then investigated whether allicin
regulates the expression of MMP-2 and MMP-9. Allicin was revealed
to dose-dependently inhibit MMP-2 and MMP-9 mRNA and protein levels
in H1299 cells (Fig. 3). Previous
studies indicated that the expression of MMPs was regulated by
their endogenous tissue inhibitors (TIMPs) (7–9). Thus, the
expression levels of TIMP-1 and TIMP-2 in H1299 cells were examined
by RT-qPCR and western blot analysis following treatment with 0,
5.0, 7.5 and 10.0 µM of allicin for 48 h. It was identified that
allicin upregulated the RNA and protein levels of TIMP-1 and TIMP-2
in H1299 cells in a concentration-dependent manner (Fig. 3). These results indicated that allicin
regulates TIMP/MMP balance and stimulates H1299 cell invasion.
 | Figure 3.Allicin alters TIMP/MMP balance in
H1299 cells. H1299 cells were treated with 0, 5.0, 7.5 and 10.0 µM
allicin for 48 h and the mRNA levels of (A) MMP-2 and MMP-9, and
(B) TIMP-1 and TIMP-2 were assayed by reverse
transcription-quantitative polymerase chain reaction and normalized
to GAPDH. H1299 cells were incubated with various concentrations of
allicin (0, 5.0, 7.5 and 10.0 µM) for 48 h. The protein levels of
(C) MMP-2, MMP-9, (D) TIMP-1 and TIMP-2 in cell lysates were
analyzed by western blot analysis and band intensity was quantified
by densitometry and normalized to β-actin for (E) MMP-2 and MMP-9
and (F) TIMP-1 and TIMP-2. Values are presented as the mean ±
standard deviation of three independent experiments. *P<0.05,
compared with control (0 µM). MMP, matrix metalloproteinase; TIMP,
tissue inhibitor of metalloproteinase. |
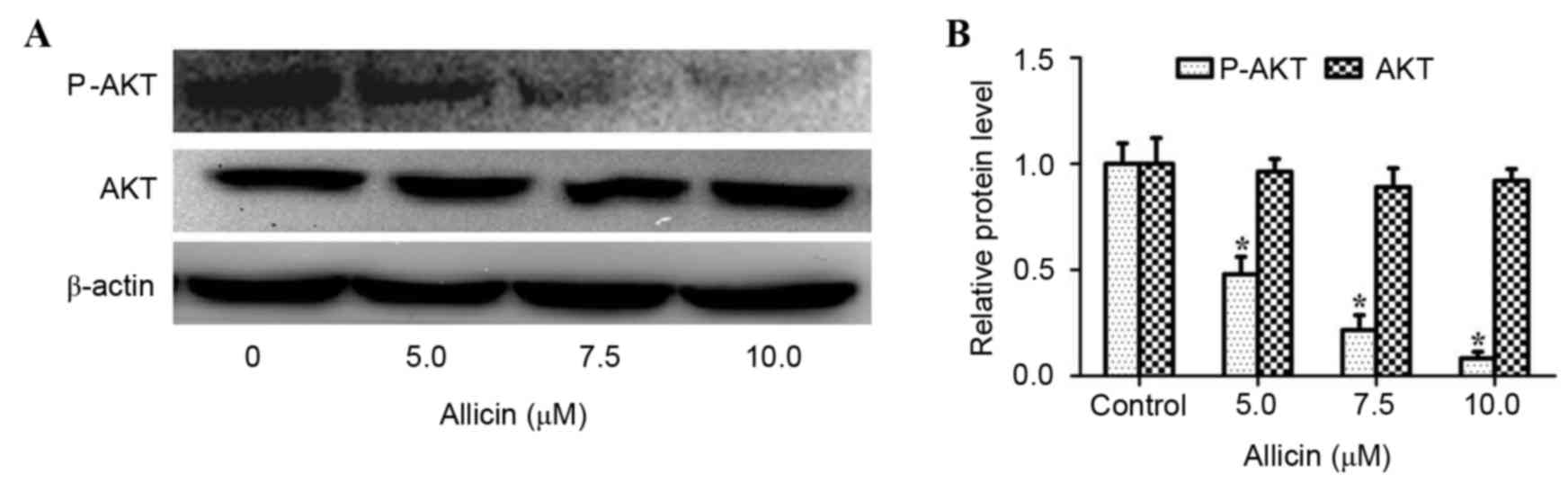
The PI3K/AKT signaling pathway is
associated with the anti-invasive mechanism of allicin
Studies have shown that activation of PI3K/AKT
signaling plays a vital role in the invasion process of lung cancer
(12,20). Thus, the effect of allicin on the
PI3K/AKT signaling pathway in H1299 cells was investigated. As
shown in Fig. 4, allicin
significantly suppressed the phosphorylation of AKT in a
concentration-dependent manner (P<0.05). However, the total
protein expression of AKT was not altered by allicin. To confirm
whether the inhibitory effect of allicin on cell invasion and
TIMP/MMP balance was associated with inhibition of the PI3K/AKT
signaling pathway, H1299 cells were pretreated with or without PI3K
inhibitor (LY294002, 25 µM) for 1 h, and then exposed to allicin (0
or 7.5 µM) for 48 h. It was identified that treatment with LY294002
and allicin significantly inhibited cell invasion (P<0.015;
Fig. 5A), decreased MMP-2
(P<0.002) and MMP-9 (P<0.000) protein expression and
increased TIMP-1 (P<0.000) and TIMP-2 (P<0.000) protein
expression (Fig. 5B and C) compared
with the allicin treated group. Furthermore, H1299 cells were
pretreated with PI3K activator (IGF-1; 0 or 50 ng/ml) for 1 h and
then exposed to various concentrations of allicin (0 or 7.5 µM) for
48 h. It was revealed that the effects of allicin on cell invasion
and protein expression of MMP-2 (P<0.001), MMP-9 (P<0.001),
TIMP-1 (P<0.000) and TIMP-2 (P<0.000) were significantly
reversed by IGF-1 (Fig. 5D-F). These
results revealed that allicin inhibited the invasion of lung
adenocarcinoma cells by altering TIMP/MMP balance via regulation of
PI3K/AKT signaling.
 | Figure 5.Involvement of the PI3K/AKT pathway in
allicin-induced TIMP/MMP imbalance and cell invasion. (A) Cells
were pretreated with LY294002 (25 µM) for 1 h and then incubated in
the presence or absence of allicin (7.5 µM) for 48 h. Cellular
invasiveness was measured using the Transwell invasion assay. The
invasion rate was expressed as a percentage of control. (B) The
protein levels of MMP-2, MMP-9, TIMP-1 and TIMP-2 were analyzed in
treated H1299 cells by western blotting. (C) Values are expressed
as the mean ± SD of three independent experiments. *P<0.05,
compared with the allicin treated group. (D) Cells were pretreated
with IGF-1 (50 ng/ml) for 1 h and then incubated in the presence or
absence of allicin (7.5 µM) for 48 h. Cellular invasiveness was
measured using the Transwell invasion assay. The invasion rate was
expressed as a percentage of the control. (E) The protein levels of
MMP-2, MMP-9, TIMP-1 and TIMP-2 were analyzed in treated H1299
cells by western blot analysis. (F) Band intensity was quantified
by densitometry and normalized to β-actin. Values are expressed as
the mean ± SD of three independent experiments. *P<0.05 compared
with the allicin treated group. MMP, matrix metalloproteinase;
TIMP, tissue inhibitor of metalloproteinase; IGF-1, insulin-like
growth factor-1; SD, standard deviation; PI3K, phosphoinositide
3-kinase. |
Discussion
Abnormal invasion of cancer cells is considered to
be the crucial biological feature of cancer. The presence of
invasion is the major reason of recurrence and mortality in
patients with lung cancer (1,20). In the present study, allicin was found
to inhibit lung adenocarcinoma cell adhesion, migration and
invasion. It also provided evidence that the mechanism underlying
the aforementioned effects was associated with altering TIMP/MMP
balance, which was regulated by the PI3K/AKT signaling pathway. The
present study shed light on the investigation of allicin in lung
adenocarcinoma invasion.
Increasing studies have demonstrated that allicin
exhibits a cytotoxic effect in several human cancer cells,
including glioma U87, liver cancer G2 and gastric cancer MGC803
cells (15–17). These high specificities make allicin a
promising anticancer agent for lung adenocarcinoma. In the present
study, it was identified that treatment with allicin was able to
suppress cell viability, indicating that allicin also possesses
cytotoxicity against lung adenocarcinoma. To the best of our
knowledge, there are only a small number of studies on the
association between allicin and the invasion of cancer cells. Thus,
the role of allicin in the invasion of lung adenocarcinoma cells
was also analyzed. It was revealed that allicin is associated with
decreased adhesion, migration and invasion of lung adenocarcinoma
cells. This indicated that allicin may suppress invasiveness in
lung adenocarcinoma.
MMPs, particularly MMP-2 and MMP-9, control
cell-cell and cell-matrix interactions. Normally, TIMPs
specifically combine with MMPs and keep their activity in a dynamic
balance. However, once this balance is broken, invasion and
metastasis of cancer cells is induced (6–9). The
imbalance between TIMPs and MMPs has been recognized as the main
mechanism for promoting the invasive processes of lung cancer. Hu
et al reported that hypoxia may affect the invasiveness of
lung cancer cells by regulating MMP-9 and TIMP-2 expression
(21). Ylisirnio et al
demonstrated that serum MMP-2, MMP-9, TIMP-1 and TIMP-2 were
associated with the clinical outcome of patients with lung cancer
and may serve as prognostic markers (22). To explore the possible mechanism of
allicin in the inhibition of lung adenocarcinoma invasion, the
effects of allicin on the expression level of MMP-2, MMP-9, TIMP-1
and TIMP-2 were investigated. It was revealed that allicin
dose-dependently downregulated mRNA and protein levels of MMP-2 and
MMP-9 and then enhanced mRNA and protein levels of TIMP-1 and
TIMP-2 in a dose-dependent manner. These data indicated that
allicin may cause inhibition of lung adenocarcinoma invasion by
inducing an imbalance of expression between MMPs (MMP-2 and MMP-9)
and TIMPs (TIMP-1 and TIMP-2).
Previous studies established that the PI3K/AKT
pathway is activated in numerous tumors, including breast cancers,
pituitary adenoma and prostate cancer (10,13). An
increasing number of studies have shown that the PI3K/AKT signaling
pathway may modulate the expression of MMPs, as well as TIMPs, to
promote the degradation of ECM proteins, and this mechanism was
essential for invasion of human tumors, including lung cancer
(13,23). To the best of our knowledge, allicin
has been confirmed to inhibit the PI3K/AKT signaling pathway in the
HepG2 cell line; however, it remains unknown whether such an effect
also exists in lung adenocarcinoma (17). Therefore, the effect of allicin on the
PI3K/AKT signaling pathway was investigated in H1299 cells. The
results demonstrated that allicin may decrease the phosphorylation
of AKT in H1299 cells, whereas no significant changes were observed
in the total protein expression of AKT. In addition, allicin
combined with LY294002 (an inhibitor of PI3K) significantly reduced
H1299 cell invasion (P<0.05) and was accompanied by upregulation
of TIMP-1 and TIMP-2 and downregulation of MMP-2 and MMP-9.
Whereas, in H1299 cells, the PI3K/AKT signaling activator
(IGF-1) reversed the effect produced by allicin on invasion, as
well as the protein expression of MMP-2, MMP-9, TIMP-1 and TIMP-2.
These findings indicated that the regulation of cell invasion and
TIMP-1, TIMP-2, MMP-2 and MMP-9 expression by allicin occurred via
the suppression of the PI3K/AKT signaling pathway.
In summary, to the best of our knowledge, the
present data demonstrated for the first time that allicin inhibits
the invasion of lung adenocarcinoma cells by altering TIMP/MMP
balance, via reducing the activity of the PI3K/AKT signaling
pathway. Allicin may be recognized as an anti-invasive agent for
lung adenocarcinoma treatment.
References
|
1
|
Zhang XD, Li W, Zhang N, Hou YL, Niu ZQ,
Zhong YJ, Zhang YP and Yang SY: Identification of adipophilin as a
potential diagnostic tumor marker for lung adenocarcinoma. Int J
Clin Exp Med. 7:1190–1196. 2014.PubMed/NCBI
|
|
2
|
Pannu BS and Iyer VN: Lung adenocarcinoma
presenting with isolated ‘chronic cough’ of 3 years duration-a
cautionary tale. Respir Med Case Rep. 16:157–159. 2015.PubMed/NCBI
|
|
3
|
Sasada S, Miyata Y, Mimae T, Tsutani Y,
Mimura T and Okada M: Application of PET/CT to adjuvant
chemotherapy for early lung adenocarcinoma. J Cardiothorac Surg. 10
Suppl 1:A1982015. View Article : Google Scholar
|
|
4
|
Wang L, Zhan W, Xie S, Hu J, Shi Q, Zhou
X, Wu Y, Wang S, Fei Z and Yu R: Over-expression of Rap2a inhibits
glioma migration and invasion by down-regulating p-AKT. Cell Biol
Int. 38:326–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang LC and Hueng DY: CD97 and glioma
invasion. J Neurosurg. 120:579–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dung TD, Feng CC, Kuo WW, Pai P, Chung LC,
Chang SH, Hsu HH, Tsai FJ, Lin YM and Huang CY: Suppression of
plasminogen activators and the MMP-2/−9 pathway by a Zanthoxylum
avicennae extract to inhibit the HA22T human hepatocellular
carcinoma cell migration and invasion effects in vitro and in vivo
via phosphatase 2A activation. Biosci Biotechnol Biochem.
77:1814–1821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutschalk CM, Yanamandra AK, Linde N,
Meides A, Depner S and Mueller MM: GM-CSF enhances tumor invasion
by elevated MMP-2, −9, and −26 expression. Cancer Med. 2:117–129.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Li Y, Qian ZJ, Lee SH, Li YX and
Kim SK: Dieckol from Ecklonia cava Regulates Invasion of Human
Fibrosarcoma Cells and Modulates MMP-2 and MMP-9 Expression via
NF-κB Pathway. Evid Based Complement Alternat Med. 2011:1404622011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krengel S, Alexander M, Brinckmann J and
Tronnier M: MMP-2, TIMP-2 and MT1-MMP are differentially expressed
in lesional skin of melanocytic nevi and their expression is
modulated by UVB-light. J Cutan Pathol. 29:390–396. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YR, Park J, Yu HN, Kim JS, Youn HJ and
Jung SH: Up-regulation of PI3K/Akt signaling by 17beta-estradiol
through activation of estrogen receptor-alpha, but not estrogen
receptor-beta, and stimulates cell growth in breast cancer cells.
Biochem Biophys Res Commun. 336:1221–1226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thamilselvan V, Craig DH and Basson MD:
FAK association with multiple signal proteins mediates
pressure-induced colon cancer cell adhesion via a Src-dependent
PI3K/Akt pathway. FASEB J. 21:1730–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng Q, Xia C, Fang J, Rojanasakul Y and
Jiang BH: Role of PI3K and AKT specific isoforms in ovarian cancer
cell migration, invasion and proliferation through the p70S6K1
pathway. Cell Signal. 18:2262–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni J, Cozzi P, Hao J, Beretov J, Chang L,
Duan W, Shigdar S, Delprado W, Graham P, Bucci J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo R, Fang D, Hang H and Tang Z: Recent
progress of allicin on cell growth inhibition and Apoptosis in
gastric cancer cells. Anticancer Agents Med Chem. 16:802–809. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhu Y, Duan W, Feng C and He X:
Allicin induces apoptosis of the MGC-803 human gastric carcinoma
cell line through the p38 mitogen-activated protein
kinase/caspase-3 signaling pathway. Mol Med Rep. 11:2755–2760.
2015.PubMed/NCBI
|
|
16
|
Chu YL, Ho CT, Chung JG, Rajasekaran R and
Sheen LY: Allicin induces p53-mediated autophagy in Hep G2 human
liver cancer cells. J Agric Food Chem. 60:8363–8371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha JH, Choi YJ, Cha SH, Choi CH and Cho
WH: Allicin inhibits cell growth and induces apoptosis in U87MG
human glioblastoma cells through an ERK-dependent pathway. Oncol
Rep. 28:41–48. 2012.PubMed/NCBI
|
|
18
|
Bat-Chen W, Golan T, Peri I, Ludmer Z and
Schwartz B: Allicin purified from fresh garlic cloves induces
apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 62:947–957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou QX, Jiang XM, Wang ZD, Li CL and Cui
YF: Enhanced expression of suppresser of cytokine signaling 3
inhibits the IL-6-induced epithelial-to-mesenchymal transition and
cholangiocarcinoma cell metastasis. Med Oncol. 32:1052015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Yu S, Li H, Liu C, Li J, Lin W,
Gao A, Wang L, Gao W and Sun Y: ILT4 drives B7-H3 expression via
PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates
with poor prognosis in non-small cell lung cancer. FEBS Lett.
589:2248–2256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Z, Huang J, Li Q, Yang J, Zhong L and
Zeng Q: Effect of hypoxia on infiltration and migration of lung
cancer cells and expression of MMP-2 and TIMP-2. Zhongguo Fei Ai Za
Zhi. 8:270–273. 2005.(In Chinese). PubMed/NCBI
|
|
22
|
Ylisirnio S, Höyhtyä M and
Turpeenniemi-Hujanen T: Serum matrix metalloproteinases −2, −9 and
tissue inhibitors of metalloproteinases −1, −2 in lung
cancer-TIMP-1 as a prognostic marker. Anticancer Res. 20:1311–1316.
2000.PubMed/NCBI
|
|
23
|
Chen YY, Liu FC, Chou PY, Chien YC, Chang
WS, Huang GJ, Wu CH and Sheu MJ: Ethanol extracts of fruiting
bodies of Antrodia cinnamomea suppress CL1-5 human lung
adenocarcinoma cells migration by inhibiting matrix
metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling
pathways. Evid Based Complement Alternat Med.
2012:3784152012.PubMed/NCBI
|