Introduction
Lung cancer was the leading cause of
cancer-associated mortality in Japan and worldwide in 2013
(1). Recently, oncogenic driver
mutations in non-small cell lung cancer (NSCLC) patients, including
gene alterations in epidermal growth factor receptor (EGFR) and
fusion of the anaplastic lymphoma kinase (ALK) gene, have been
identified (2–4). Several tyrosine kinase inhibitors (TKIs)
are currently approved or under clinical development for the
treatment of NSCLC, particularly lung adenocarcinoma (ADC)
(2–4).
However, although the recent beneficial use of molecular targeted
therapy for advanced ADC prolonged progression-free survival,
patient prognosis is still poor due to drug resistance (5–7).
Furthermore, the prognosis of NSCLC patients without specific
driver mutations is even more unfavorable (8). Therefore, the identification of
sensitive and specific biomarkers for prognosis and drug resistance
will be of great clinical benefit to ADC patients.
The ability of lung cancer to recur despite systemic
therapy is correlated with the presence of a small number of
residual cancer cells termed cancer stem cells (CSCs), which
consist of a population with the capacity for self-renewal and
differentiation, biological functions that are generally limited to
normal somatic stem cells (5). The
CSC theory is based on a myriad of experimental and clinical
observations suggesting that the malignant phenotype is sustained
by a subset of cells characterized by their capacity for
self-renewal, differentiation, and innate resistance to
chemotherapy and radiation (5). CSCs
may be responsible for resistance to anticancer agents and disease
recurrence following definitive therapy such as chemotherapy and
molecular-targeted therapy in solid tumors (5–7). Several
putative CSC-associated markers for NSCLC, including ATP binding
cassette subfamily B member 1 [ABCB1, also known as multidrug
resistance protein 1 (MDR1)], aldehyde dehydrogenase 1 family
member A1 (ALDH1A1) and cluster of differentiation (CD) 44, have
been identified (6). A previous study
demonstrated that CSCs were involved in the acquired resistance to
EGFR TKIs in mutant EGFR NSCLC (6).
Our group recently reported that overexpression of ABCB1 was
associated with CSCs as a mechanism of resistance to
mesenchymal-epithelial transition factor (MET) inhibition in NSCLC
cells (7). However, the potential
correlation between these CSC-associated marker proteins and
patient survival remains to be clarified.
In the present study, the prognostic significance of
the above three CSC-associated markers was evaluated in ADC
patients by immunohistochemical (IHC) analysis. It was noticed that
ABCB1 could be useful for the prognosis of stage I ADC patients.
Furthermore, ABCB1 overexpression was associated with recurrence,
and could be used as a post-operative recurrence prediction factor
in ADC patients harboring wild-type EGFR. The present findings may
be useful for the selection of stage I ADC patients who would
benefit from adjuvant chemotherapy, particularly those with
wild-type EGFR.
Materials and methods
Clinical samples
A total of 194 stage I–III NSCLC patients who
received surgical treatment from February 2001 to December 2009 at
Nippon Medical School Hospital (Tokyo, Japan) were enrolled in the
present study. In total, 128 specimens were collected from ADC
patients, while 66 were collected from lung squamous cell carcinoma
(SCC) patients. All tissues were freshly collected during surgery,
snap-frozen and stored at −80°C. Tumor-node-metastasis (TNM) stage
and grade were classified according to the World Health
Organization TNM staging system, 7th edition (9,10).
Information on patient survival and recurrence during 5 years of
follow-up was available for all the 194 cases. EGFR mutation status
was examined using the peptide nucleic acid-locked nucleic acid
polymerase chain reaction clamp method, which was conducted by LSI
Medience Corporation (Tokyo, Japan). IHC staining of the NSCLC
samples was carried out in accordance with the principles embodied
in the 2008 Declaration of Helsinki (11). All included patients provided written
informed consent for the use of their tissue specimens for medical
research. The study protocol was approved by an ethics committee
review board at Nippon Medical School Hospital.
IHC
IHC staining was performed on snap-frozen surgical
samples, which were fixed in 10% neutral-buffered formalin and
paraffin-embedded. Following deparaffinization, antigen retrieval
was carried out with 10 mmol/l citrate buffer (pH 6.0) (LSI
Medience Corporation) using an autoclave at 120°C for 15 min. Upon
blocking with swine serum albumin (Vector Laboratories, Inc.,
Burlingame, CA, USA) at room temperature for 20 min, the sections
were washed with PBS and incubated with mouse anti-human CD44
monoclonal antibody (cat. no. 156-3c11; dilution, 1:100; Cell
Signaling Technology, Inc., Danvers, MA, USA), mouse anti-human
MDR1 monoclonal antibody (cat. no. D-11; 1:100 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or rabbit anti-human ALDH1A1
antibody (cat. no. EP1933Y; 1:100 dilution; Abcam, Cambridge, UK)
at 4°C overnight. Upon washing with PBS, the sections were
incubated with biotinylated goat anti-mouse immunoglobulin (Ig) G
(cat. no. BA-9200; dilution, 1:200; Vector Laboratories, Inc.) or
biotinylated goat anti-rabbit IgG (cat. no. BA-1000; dilution,
1:200; Vector Laboratories, Inc.) for 30 min at room temperature.
Visualization was then conducted with the ABC Peroxidase Staining
kit (Funakoshi Co., Ltd, Tokyo, Japan). Negative controls were
prepared by omitting the primary antibody under the same
experimental conditions.
Evaluation of ABCB1, CD44 and ALDH1A1
protein expression
IHC scoring was performed using the Histoscore
(H-score) (12,13). CD44 expression level was scored on a
scale according to a previous study as follows: No expression, 0;
low expression, 1+; and high expression, 2+ and 3+ (14). ABCB1 and ALDH1A1 expression were
scored on a scale according to a previous study (15) as follows: A semi-quantitative H-score
for each tissue sample was calculated by multiplying the staining
intensity of tumor cells (0, no staining; 1, weak staining; 2,
moderate staining; and 3, strong staining) by a proportion score
based on the percentage of positive tumor cells (0, ≤10%; 1,
10–39%; 2, 40–69%; and 3, ≥70%). For ABCB1 expression, the score
was graded as follows: Low expression, H-score ≤3; and high
expression, H-score >3. ALDH1A1 expression was graded as
follows: Low expression, H-score ≤2; and high expression, H-score
>2. IHC status was determined independently by two investigators
(F.Z. and R.N.), who were blinded to the clinical data, and
consensus was reached for any discordant cases.
Statistical analyses
Correlations between protein expression and
patients' characteristics were assessed by χ2 tests.
Overall survival (OS) and disease-free survival (DFS) were
calculated from the date of surgery. Kaplan-Meier survival curves
were represented for OS and DFS, and the results were compared by
log-rank test. Univariate and multivariate analyses were performed
using the Cox regression model as previously described (16). For each analysis, P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were carried using SPSS version 21 (IBM SPSS,
Inc., Armonk, NY, USA).
Results
Expression and prognostic significance
of ABCB1, ALDH1A1 and CD44 in NSCLC
Samples from 194 NSCLC patients were available for
IHC analysis of ABCB1, ALDH1A1 and CD44. The expression levels of
ABCB1, ALDH1A1 and CD44 were high in 25 (13%), 61 (31%) and 117
(60%) of the 194 NSCLC specimens, respectively (Fig. 1). The correlations between ABCB1,
ALDH1A1 and CD44 protein expression and patients' characteristics
were evaluated. Significant positive correlations of CD44
expression with sex (P=0.02), tobacco smoking (P=0.02) and
histology (P<0.01) were observed (data not shown). No
significant correlations were noticed between ABCB1 or ALDH1 and
patients' characteristics. Next, the prognostic significance of the
expression of the aforementioned three CSC-associated proteins was
evaluated. Positive staining for ABCB1 demonstrated a trend toward
worse survival compared with negative staining in stage I–III and
stage I NSCLC. Negative staining for ALDH1 or CD44 exhibited a
trend toward worse survival compared with positive staining in
stage I–III and stage I NSCLC. However, these differences were not
statistically significant (data not shown).
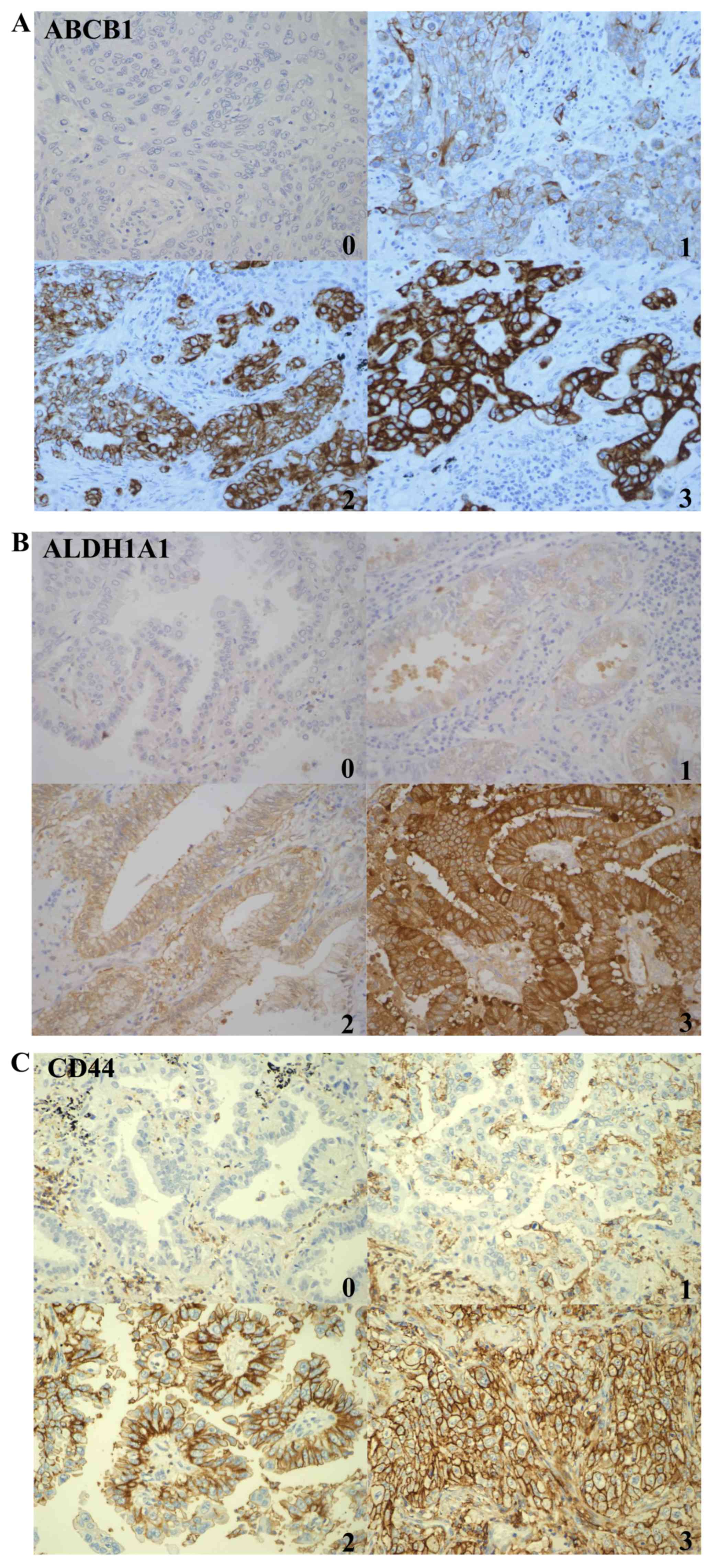 | Figure 1.IHC staining for ALDH1A1, CD44 and
ABCB1 from different patients. IHC staining for (A) ABCB1, (B)
ALDH1A1 and (C) CD44 protein expression in tumor cells. Scores 0,
1, 2 and 3 correspond to negative, weak, moderate and strong
staining, respectively (magnification, ×20). IHC,
immunohistochemical; ABCB1, ATP binding cassette subfamily B member
1; ALDH1A1, aldehyde dehydrogenase 1 family member A1; CD, cluster
of differentiation. |
Correlation between CSC-associated
marker expression and patient survival in ADC
The prognostic significance of CSC-associated marker
expression was next examined based on the histological types. The
194 NSCLC samples evaluated included 128 ADC and 66 SCC specimens.
The characteristics of the 128 ADC patients are shown in Table I. There were significant positive
correlations of ALDH1A1 expression with age (P=0.03) and disease
grade (P=0.01). CD44 expression was associated with T stage
(P=0.02) and pathological stage (P=0.02; Table I). Next, the prognostic significance
of the expression of the aforementioned three CSC-associated
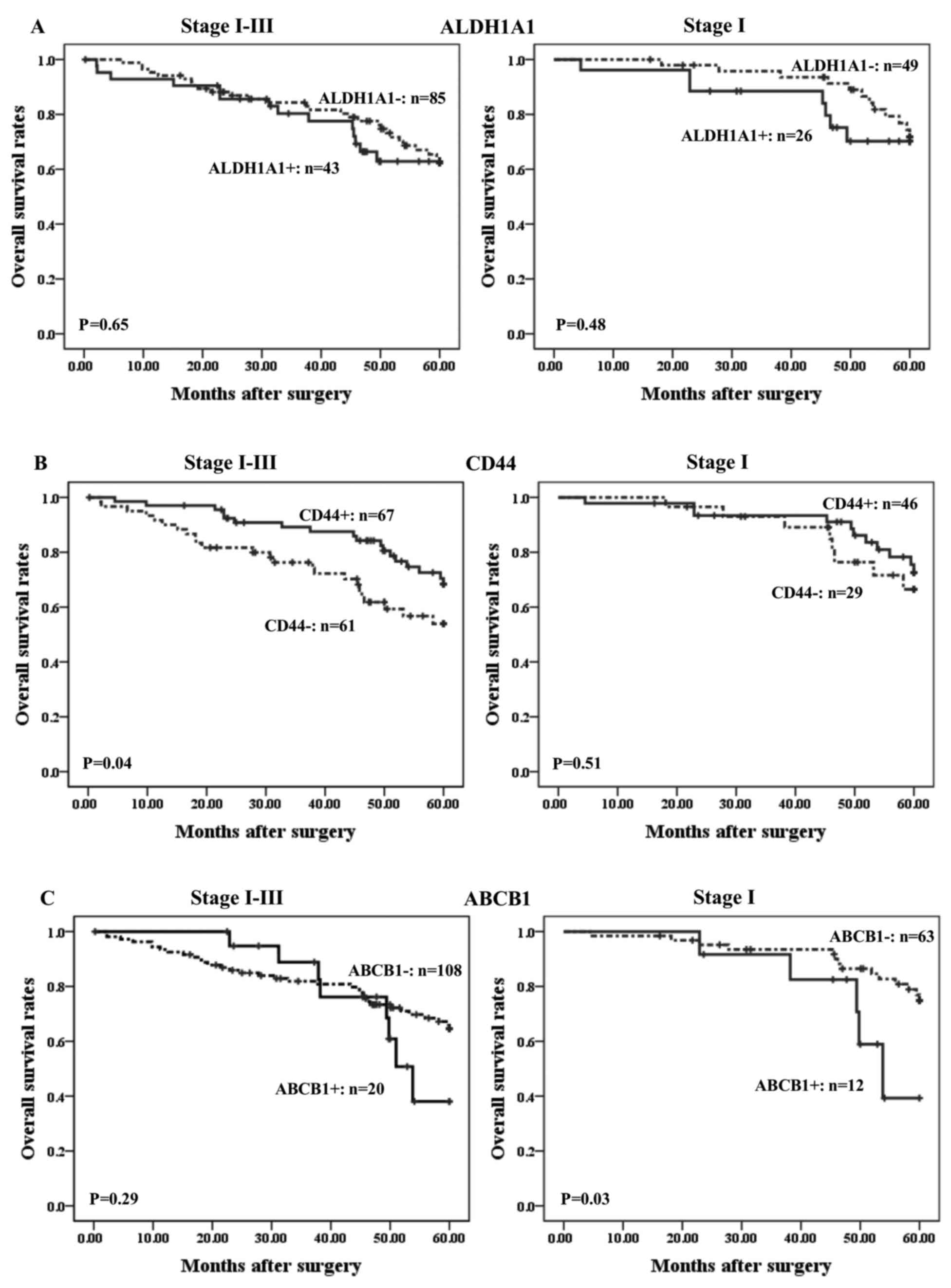
markers was evaluated in ADC patients (Fig. 2). ALDH1A1 expression did not show any
significant association with ADC patient survival (Fig. 2A). Stage I–III patients with a
CD44-positive status displayed significantly better survival than
those who were negative for CD44 expression (P=0.04; Fig. 2B). However, CD44 status did not show
any significant correlation with survival in stage I cases. By
contrast, positivity for ABCB1 expression in stage I ADC patients
was significantly associated with poorer survival than in
ABCB1-negative cases (P=0.03; Fig.
2C).
 | Table I.Associations between ABCB1, ALDH1A1
and CD44 expression levels and characteristics of patients with
lung adenocarcinoma. |
Table I.
Associations between ABCB1, ALDH1A1
and CD44 expression levels and characteristics of patients with
lung adenocarcinoma.
|
|
| ABCB1
expression |
| ALDH1A1
expression |
| CD44
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | Total no. (%) | Positive [no.
(%)] | Negative [no.
(%)] | P-value | Positive [no.
(%)] | Negative [no.
(%)] | P-value | Positive [no.
(%)] | Negative [no.
(%)] | P-value |
|---|
| No. of cases | 128 (100) | 20 (16) | 108 (84) |
| 43 (34) | 85 (66) |
| 67 (52) | 61 (48) |
|
| Age, years |
|
|
| 0.40 |
|
| 0.03 |
|
| 0.38 |
|
<65 | 47
(37) | 9
(19) | 38
(81) |
| 10 (21) | 37 (79) |
| 27 (57) | 20 (43) |
|
|
≥65 | 81
(63) | 11 (14) | 70
(86) |
| 33 (41) | 48 (59) |
| 40 (49) | 41 (51) |
|
| Sex |
|
|
| 0.22 |
|
| 0.09 |
|
| 0.16 |
|
Male | 67
(52) | 13 (19) | 54
(81) |
| 27 (40) | 40 (60) |
| 39 (58) | 28 (42) |
|
|
Female | 61
(48) | 7
(11) | 54
(89) |
| 16 (26) | 45 (74) |
| 28 (46) | 33 (54) |
|
| Tobacco
smoking |
|
|
| 0.84 |
|
| 0.97 |
|
| 0.08 |
|
Yes | 80
(63) | 13 (16) | 67
(84) |
| 27 (34) | 53 (66) |
| 47 (59) | 33 (41) |
|
| No | 47
(37) | 7
(15) | 40
(85) |
| 16 (34) | 31 (66) |
| 20 (43) | 27 (57) |
|
|
Unknown | 1
(1) | 0 (0) | 1
(1) |
| 0 (0) | 1 (1) |
| 0 (0) | 1 (1) |
|
| Grade |
|
|
| 0.18 |
|
| 0.01 |
|
| 0.13 |
| 1 | 42
(17) | 4
(10) | 38
(90) |
| 7
(17) | 35 (83) |
| 26 (62) | 16 (38) |
|
|
2+3 | 86
(83) | 16 (19) | 70
(81) |
| 36 (42) | 50 (58) |
| 41 (48) | 45 (52) |
|
| T stage |
|
|
| 0.36 |
|
| 0.56 |
|
| 0.02 |
| T1 | 40
(31) | 8
(20) | 32
(80) |
| 12 (30) | 28 (70) |
| 27 (68) | 13 (33) |
|
|
T2+T3 | 88
(69) | 12 (14) | 76
(86) |
| 31 (35) | 57 (65) |
| 40 (45) | 48 (55) |
|
| N stage |
|
|
| 0.51 |
|
| 0.81 |
|
| 0.36 |
| N0 | 91
(71) | 13 (14) | 78
(86) |
| 30 (33) | 61 (67) |
| 50 (55) | 41 (45) |
|
|
N1+N2 | 37
(29) | 7
(19) | 30
(81) |
| 13 (35) | 24 (65) |
| 17 (46) | 20 (54) |
|
| Pathological
stage |
|
|
| 0.89 |
|
| 0.76 |
|
| 0.02 |
| I | 75
(59) | 12 (16) | 63
(84) |
| 26 (35) | 49 (65) |
| 46 (61) | 29 (39) |
|
|
II+III | 53
(41) | 8
(15) | 45
(85) |
| 17 (32) | 36 (68) |
| 21 (40 | 32 (60) |
|
| EGFR mutation
status |
|
|
| 0.55 |
|
| 0.47 |
|
| 0.32 |
|
Positive | 28
(22) | 4
(14) | 24
(86) |
| 11 (39) | 17 (61) |
| 17 (61) | 11 (39) |
|
|
Negative | 100 (78) | 16 (16) | 84
(84) |
| 32 (32) | 68 (68) |
| 50 (50) | 50 (50) |
|
Prognostic significance of ABCB1
expression in stage I ADC
The present study further evaluated the prognostic
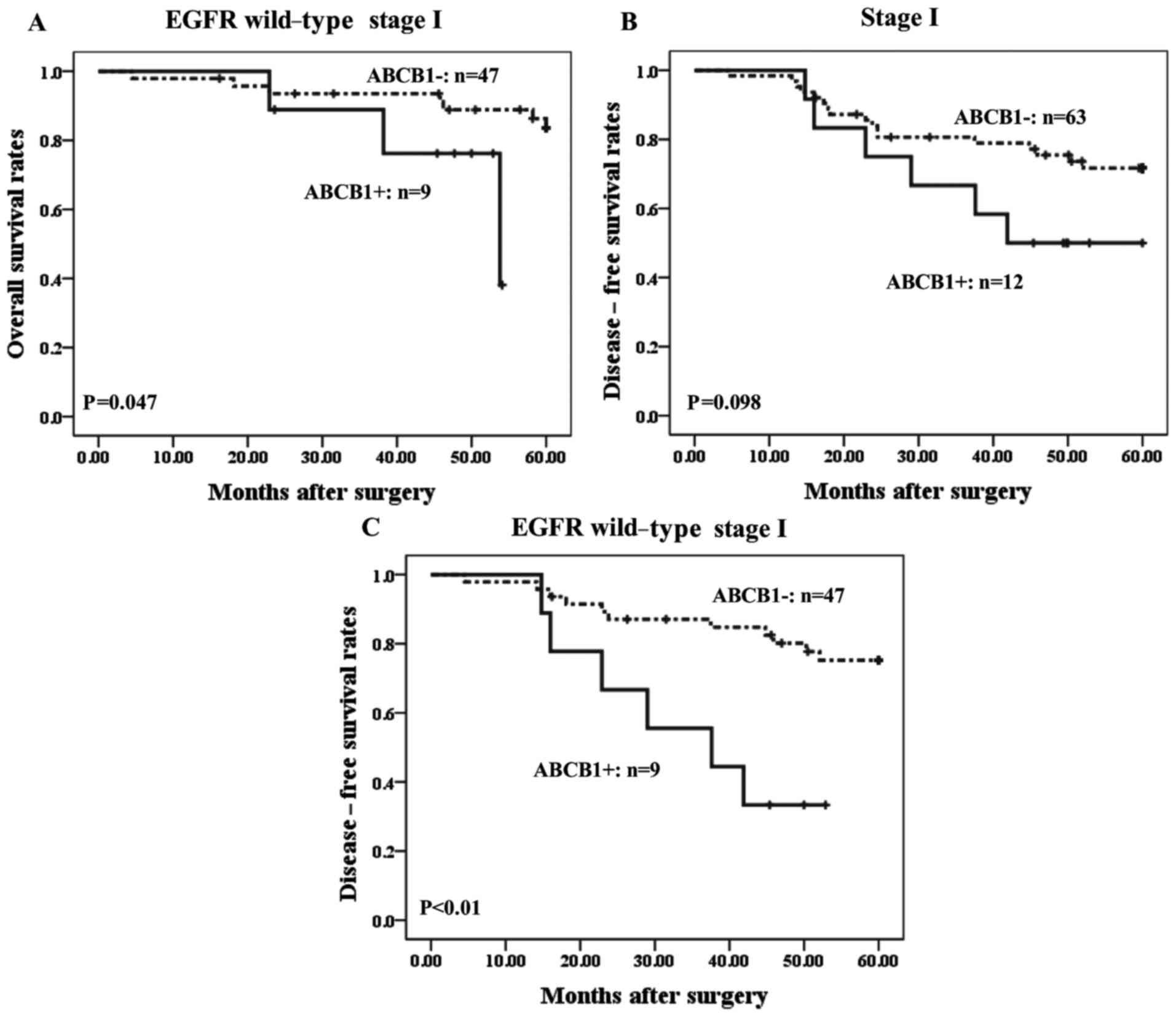
significance of ABCB1 expression in stage I ADC cases. Among 75 ADC
patients with stage I disease, 56 cases harbored wild-type EGFR.
Positivity for ABCB1 expression in stage I ADC patients with
wild-type EGFR was correlated with significantly poorer prognosis
than in ABCB1-negative cases (P<0.05) (Fig. 3A). By contrast, no significant
correlation between ABCB1 staining and patient prognosis was
observed in 19 stage I ADC patients with mutant EGFR (data not
shown). The present study also evaluated the correlation between
ABCB1 expression and DFS in ADC patients with stage I disease, and
it was observed that ABCB1-positive cases with stage I disease
exhibited a trend toward worse DFS compared with patients who are
ABCB1-negative, although the difference was not statistically
significant (P<0.10; Fig. 3B).
However, among stage I ADC patients with wild-type EGFR,
ABCB1-positive cases exhibited significantly worse DFS than
ABCB1-negative cases (P<0.01; Fig.
3C).
Univariate analysis and multivariate
Cox proportional hazards models for factors associated with
mortality and DFS in ADC patients with stage I disease
The present study further investigated whether the
prognostic ability of ABCB1 was affected by underlying clinical
variables by performing univariate and multivariate Cox
proportional hazards survival analyses in stage I cases (Table II). Univariate analysis revealed that
EGFR and ABCB1 statuses were significantly associated with
mortality [hazard ratio (HR), 2.98 and 3.11; P=0.02 and P=0.04,
respectively]. However, multivariate analysis revealed that EGFR
and ABCB1 statuses were not significantly associated with mortality
(HR, 2.64 and 2.53; P=0.08 and P=0.13, respectively; Table II). Univariate and multivariable
analyses were also performed in stage I ADC patients with wild-type
EGFR, but no significant associations were detected (Table II).
 | Table II.Univariate and multivariate Cox
proportional hazards models for factors associated with mortality
in stage I lung adenocarcinoma patients. |
Table II.
Univariate and multivariate Cox
proportional hazards models for factors associated with mortality
in stage I lung adenocarcinoma patients.
| A, Stage I
(n=75) |
|---|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | <65 vs. ≥65 | 0.93 | 0.37–2.30 | 0.87 | 0.96 | 0.33–2.79 | 0.94 |
| Sex | Female vs.
male | 0.95 | 0.39–2.34 | 0.91 | 1.94 | 0.60–6.22 | 0.27 |
| Tobacco
smoking | No vs. yes | 0.57 | 0.23–1.42 | 0.23 | 0.49 | 0.15–1.56 | 0.23 |
| Grade | G1 vs. G2+G3 | 1.59 | 0.57–4.43 | 0.37 | 1.41 | 0.41–4.83 | 0.59 |
| T stage | T1 vs. T2-T4 | 1.29 | 0.51–3.28 | 0.59 | 1.44 | 0.46–4.53 | 0.53 |
| UFT | No vs. yes | 1.19 | 0.46–3.08 | 0.72 | 1.00 | 0.36–2.81 | 1.00 |
| EGFR
expression | EGFR−
vs. EGFR+ | 2.98 | 1.21–7.36 | 0.02 | 2.64 | 0.88–7.92 | 0.08 |
| ABCB1 status | ABCB1−
vs. ABCB1+ | 3.11 | 1.08–8.97 | 0.04 | 2.53 | 0.76–8.47 | 0.13 |
|
| B, EGFR wild-type
(n=56) |
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
|
Characteristics | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age, years | <65 vs. ≥65 | 5.50 | 0.69–43.14 | 0.11 | 4.73 | 0.55–40.71 | 0.16 |
| Sex | Female vs.
male | 1.27 | 0.33–4.91 | 0.73 | 1.91 | 0.36–10.16 | 0.45 |
| Tobacco
smoking | No vs. yes | 1.56 | 0.33–7.33 | 0.58 | 1.30 | 0.18–9.29 | 0.80 |
| Grade | G1 vs. G2+G3 | 1.27 | 0.33–4.92 | 0.73 | 0.54 | 0.12–2.47 | 0.42 |
| T stage | T1 vs. T2-T4 | 5.86 | 0.74–46.42 | 0.09 | 6.82 | 0.67–69.08 | 0.10 |
| UFT | No vs. yes | 0.57 | 0.15–2.20 | 0.42 | 0.26 | 0.06–1.09 | 0.07 |
| ABCB1 status | ABCB1−
vs. ABCB1+ | 3.92 | 0.92–16.78 | 0.07 | 3.58 | 0.70–18.41 | 0.13 |
Furthermore, univariate and multivariate Cox
proportional hazards models for factors associated with DFS in
stage I ADC patients were also performed. No significant
associations between DFS and clinical variables were observed in
stage I cases by multivariate analysis (Table III). In stage I patients with
wild-type EGFR, age and ABCB1 status were significantly correlated
with DFS by univariate analysis (HR, 4.71 and 4.29; P=0.04 and
P=0.01, respectively; Table III).
Multivariate Cox proportional hazards model analysis demonstrated
that only ABCB1 status was an independent prognostic indicator of
DFS in stage I cases with wild-type EGFR (HR, 3.49; P<0.05;
Table III).
 | Table III.Univariate and multivariate Cox
proportional hazards models for factors associated with
disease-free survival in stage I lung adenocarcinoma patients. |
Table III.
Univariate and multivariate Cox
proportional hazards models for factors associated with
disease-free survival in stage I lung adenocarcinoma patients.
| A, Stage I
(n=75) |
|---|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | <65 vs. ≥65 | 1.87 | 0.74–4.74 | 0.19 | 1.94 | 0.70–5.38 | 0.20 |
| Sex | Female vs.
male | 1.97 | 0.81–4.80 | 0.13 | 2.47 | 0.78–7.88 | 0.13 |
| Tobacco
smoking | No vs. yes | 1.62 | 0.60–4.38 | 0.34 | 1.49 | 0.44–5.06 | 0.52 |
| Grade | G1 vs. G2+G3 | 1.36 | 0.54–3.47 | 0.51 | 0.75 | 0.27–2.07 | 0.57 |
| T stage | T1 vs. T2-T4 | 2.70 | 1.00–7.28 | 0.05 | 2.08 | 0.62–7.01 | 0.24 |
| UFT | No vs. yes | 2.54 | 1.06–6.08 | 0.04 | 2.22 | 0.85–5.77 | 0.10 |
| EGFR
expression | EGFR−
vs. EGFR+ | 1.17 | 0.46–2.96 | 0.75 | 2.03 | 0.63–6.46 | 0.23 |
| ABCB1 status | ABCB1−
vs. ABCB1+ | 2.16 | 0.85–5.53 | 0.11 | 1.47 | 0.51–4.25 | 0.47 |
|
| B, EGFR wild-type
(n=56) |
|
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
|
Characteristics | Comparison | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age, years | <65 vs. ≥65 | 4.71 | 1.07–20.64 | 0.04 | 4.31 | 0.96–19.40 | 0.06 |
| Sex | Female vs.
male | 2.66 | 0.76–9.27 | 0.12 | 1.91 | 0.44–8.31 | 0.39 |
| Tobacco
smoking | No vs. yes | 3.30 | 0.75–14.45 | 0.11 | 2.30 | 0.39–13.61 | 0.36 |
| Grade | G1 vs. G2+G3 | 1.23 | 0.43–3.52 | 0.69 | 0.51 | 0.15–1.77 | 0.29 |
| T stage | T1 vs. T2-T4 | 3.08 | 0.88–10.73 | 0.08 | 2.36 | 0.49–11.35 | 0.29 |
| UFT | No vs. yes | 1.58 | 0.61–4.10 | 0.35 | 0.84 | 0.29–2.47 | 0.75 |
| ABCB1 status | ABCB1−
vs. ABCB1+ | 4.29 | 1.54–11.92 | 0.01 | 3.49 | 1.03–11.89 | 0.05 |
Expression and clinical significance
of ABCB1, ALDH1A1 and CD44 in lung SCC
The present study also evaluated the prognostic
significance of ABCB1 in lung SCC. The characteristics of 66 SCC
patients were evaluated. There were significant positive
correlations of ABCB1 expression with N stage and p-stage in lung
SCC samples (P=0.04 and P=0.02, respectively; data not shown).
ABCB1-positive cases with stage I–III disease exhibited a trend
toward worse survival compared with patients who are
ABCB1-negative; however, the difference was not statistically
significant (data not shown). The prognostic significance of ABCB1
in stage I SCC could not be evaluated, since none of these cases
exhibited positivity for ABCB1. Thus, these data indicated that
ABCB1 expression was associated with metastatic ability in lung SCC
patients.
Discussion
The present study examined by IHC the prognostic
significance of the protein expression of the CSC-associated
markers ABCB1, ALDH1A1 and CD44 in NSCLC patients. It was
demonstrated that ABCB1 protein expression could be used as a
prognostic marker in stage I ADC patients. In particular, ABCB1
expression was associated with recurrence in stage I ADC patients
with wild-type EGFR. In SCC patients, ABCB1 expression was
associated with lymph node metastasis (data not shown).
ABCB1 encodes the transport protein P-glycoprotein
and is localized in the plasma membrane to protect sensitive
tissues from potentially toxic xenobiotics (17,18).
However, ABCB1 is considered a ‘double-edged sword’, since the
ABCB1 protein can also remove therapeutic agents out of cancer
cells and diminish the efficacy of anticancer drugs (19–21). Thus,
ABCB1 can affect the pharmacokinetics of administered drugs and the
efficacy of chemotherapy agents for cancer. In fact, previous
studies reported that increased ABCB1 expression conferred
resistance to chemotherapeutic agents in several cancers (21,22). ABCB1
is closely associated with CSC-like properties and is considered
one of the CSC-associated markers (6). Cancer cells with CSC-like properties,
which are characterized by their capacity for pluripotency and
self-renewal, have been attracting interest as a source of cancer
cells (23). The therapeutic
significance of CSC-like properties has been reported in NSCLC
patients (6,7,24,25); however, the prognostic significance of
CSC-associated markers remains to be clarified. Embryonic signaling
pathways, including Hedgehog, Notch, WNT and B lymphoma MLV
insertion region 1, are associated with the renewal of normal stem
cells and the maintenance of tissue homeostasis (26). CSCs exhibit similar properties to
those of normal stem cells, suggesting that these signaling
pathways are important in maintaining CSCs in a variety of cancers,
including lung (5,27). Previous studies reported that ABCB1
expression was affected by the above signaling pathways (28–30).
However, the association between ABCB1 and these signaling pathways
should be further confirmed in NSCLC patients.
The 5-year survival rate of stage I NSCLC patients
who undergo complete resection is ~70% (31). However, 20–30% of early stage NSCLC
patients undergo a relapse even upon complete surgical resection of
their tumor (32,33). Platinum-based adjuvant chemotherapy
for NSCLC patients with stage IB-IIIA disease has been investigated
in several clinical trials (34–37). In
Japan, NSCLC patients with stage IB lung ADC can benefit from
uracil-tegafur (UFT) treatment following complete resection of the
tumor (38). However, only 5–15% of
NSCLC patients could benefit from UFT or platinum-based adjuvant
chemotherapy (38,39). Therefore, the identification of
prognostic biomarkers to select NSCLC patients with poor prognosis
and to develop tailored treatment strategies may have a clinical
benefit. A previous study indicated that evaluation of excision
repair cross-complementation group 1 (ERCC1) and ribonucleotide
reductase 1 proteins has prognostic value in NSCLC patients with
stage I disease (40). Although
several studies have evaluated the expression levels of ERCC1 by
IHC, no consensus has been reached due to the difficulty in
detecting the unique functional ERCC1 isoform (41). Actin 4 has been identified as a
potential prognostic biomarker in stage I lung adenocarcinoma
(42). The present study revealed
that ABCB1 could be used as a prognostic marker in lung ADC
patients with stage I disease, and is associated with recurrence in
stage I ADC patients with wild-type EGFR. EGFR mutations or ALK
rearrangement contributes to the therapeutic effects of certain
cancer treatments, resulting in significant improvement of patient
prognosis (2–4). By contrast, the therapeutic strategy for
NSCLC patients without specific driver mutations is still
undeveloped. ABCB1 status may be useful for selecting ADC patients
with poor prognosis and identifying ADC patients harboring
wild-type EGFR who may benefit from post-surgical adjuvant
therapy.
In conclusion, the present study demonstrated that
ABCB1 protein expression may have prognostic value in stage I ADC
patients. In particular, overexpression of ABCB1 was observed to be
associated with recurrence and was recognized as a post-operative
recurrence prediction factor in ADC patients with wild-type EGFR.
The present findings may be useful for the selection of stage I
NSCLC patients who would benefit from adjuvant chemotherapy.
Acknowledgements
The authors gratefully acknowledge Ms. K. Matsuda
and Ms. C. Soeno of Nippon Medical School (Tokyo, Japan) for their
technical assistance. The present study was supported in part by a
grant-in-aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (grant no. 24591179 awarded to Dr
M. Seike and grant no. 25461172 awarded to Dr A. Gemma), and the
Clinical Rebiopsy Bank Project for Comprehensive Cancer Therapy
Development in Nippon Medical School (grant no. S1311022). The
authors utilized the present study as a doctor of philosophy (PhD)
dissertation.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alamgeer M, Peacock CD, Matsui W, Ganju V
and Watkins DN: Cancer stem cells in lung cancer: Evidence and
controversies. Respirology. 18:757–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shien K, Toyooka S, Yamamoto H, Soh J,
Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, et al:
Acquired resistance to EGFR inhibitors is associated with a
manifestation of stem cell-like properties in cancer cells. Cancer
Res. 73:3051–3061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugano T, Seike M, Noro R, Soeno C, Chiba
M, Zou F, Nakamichi S, Nishijima N, Matsumoto M, Miyanaga A, et al:
Inhibition of ABCB1 overcomes cancer stem cell-like properties and
acquired resistance to MET inhibition in non-small lung cancer. Mol
Cancer Ther. 14:2433–2440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalikaki A, Koutsopoulos A, Hatzidaki D,
Trypaki M, Kontopodis E, Stathopoulos E, Mavroudis D, Georgoulias V
and Voutsina A: Clinical outcome of patients with non-small cell
lung cancer receiving front-line chemotherapy according to EGFR and
K-RAS mutation status. Lung Cancer. 69:110–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee, : Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCarty KS, Miller LS, Cox EB, Konrath J
and McCarty KS Sr: Estrogen receptor analyses. Correlation of
biochemical and immunohistochemical methods using monoclonal
antireceptor antibodies. Arch Pathol Lab Med. 109:716–721.
1985.PubMed/NCBI
|
|
13
|
Noro R, Seike M, Zou F, Soeno C, Matsuda
K, Sugano T, Nishijima N, Matsumoto M, Kitamura K, Kosaihira S, et
al: MET FISH-positive status predicts short progression-free
survival and overall survival after gefitinib treatment in lung
adenocarcinoma with EGFR mutation. BMC Cancer. 15:312015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu WY, Hunag YY, Liu XG, He JY, Chen DD,
Zeng F, Zhou JH and Zhang YK: Prognostic evaluation of CapG,
gelsolin, P-gp, GSTP1, and Topo-II proteins in non-small cell lung
cancer. Anat Rec (Hoboken). 295:208–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seike M, Yanaihara N, Bowman ED, Zanetti
KA, Budhu A, Kumamoto K, Mechanic LE, Matsumoto S, Yokota J,
Shibata T, et al: Use of a cytokine gene expression signature in
lung adenocarcinoma and the surrounding tissue as a prognostic
classifier. J Natl Cancer Inst. 99:1257–1269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moitra K, Lou H and Dean M: Multidrug
efflux pumps and cancer stem cells: Insights into multidrug
resistance and therapeutic development. Clin Pharmacol Ther.
89:491–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Neill AJ, Prencipe M, Dowling C, Fan Y,
Mulrane L, Gallagher WM, O'Connor D, O'Connor R, Devery A, Corcoran
C, et al: Characterisation and manipulation of docetaxel resistant
prostate cancer cell lines. Mol Cancer. 10:1262011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Liu C, Nadiminty N, Lou W, Tummala
R, Evans CP and Gao AC: Inhibition of ABCB1 expression overcomes
acquired docetaxel resistance in prostate cancer. Mol Cancer Ther.
12:1829–1836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szilvassy SJ, Humphries RK, Lansdorp PM,
Eaves AC and Eaves CJ: Quantitative assay for totipotent
reconstituting hematopoietic stem cells by a competitive
repopulation strategy. Proc Natl Acad Sci USA. 87:pp. 8736–8740.
1990; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di A, Conticello C, Ruco L, Peschle C and De Maria R:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA and Katz RL: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karamboulas C and Ailles L: Developmental
signaling pathways in cancer stem cells of solid tumors. Biochim
Biophys Acta. 1830:2481–2495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takebe N and Ivy SP: Controversies in
cancer stem cells: Targeting embryonic signaling pathways. Clin
Cancer Res. 16:3106–3112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou H, Ma H, Wei W, Ji D, Song X, Sun J,
Zhang J and Jia L: B4GALT family mediates the multidrug resistance
of human leukemia cells by regulating the hedgehog pathway and the
expression of p-glycoprotein and multidrug resistance-associated
protein 1. Cell Death Dis. 4:e6542013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang CC, Yan Z, Zong Q, Fang DD, Painter
C, Zhang Q, Chen E, Lira ME, John-Baptiste A and Christensen JG:
Synergistic effect of the γ-secretase inhibitor PF-03084014 and
docetaxel in breast cancer models. Stem Cells Transl Med.
2:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen DY, Zhang W, Zeng X and Liu CQ:
Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein
and reverses multi-drug resistance of cholangiocarcinoma. Cancer
Sci. 104:1303–1308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese Lung Cancer Registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lou F, Huang J, Sima CS, Dycoco J, Rusch V
and Bach PB: Patterns of recurrence and second primary lung cancer
in early-stage lung cancer survivors followed with routine computed
tomography surveillance. J Thorac Cardiovasc Surg. 145:75–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu JF, Feng XY, Zhang XW, Wen YS, Lin P,
Rong TH, Cai L and Zhang LJ: Time-varying pattern of postoperative
recurrence risk of early-stage (T1a-T2bN0M0) non-small cell lung
cancer (NSCLC): Results of a single-center study of 994 Chinese
patients. PLoS One. 9:e1066682014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somerfield MR, Brouwers MC, Darling G, Ellis
PM, et al: Cancer Care Ontario and American Society of Clinical
Oncology adjuvant chemotherapy and adjuvant radiation therapy for
stages I–IIIA resectable non small-cell lung cancer guideline. J
Clin Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Douillard J-Y, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer [Adjuvant Navelbine
International Trialist Association (ANITA)]: A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Winton T, Livingston R, Johnson D, Rigas
J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E,
et al: Vinorelbine plus cisplatin vs. observation in resected
non-small-cell lung cancer. N Engl J Med. 352:2589–2597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kato H, Ichinose Y, Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, et al:
A randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zheng Z, Chen T, Li X, Haura E, Sharma A
and Bepler G: DNA synthesis and repair genes RRM1 and ERCC1 in lung
cancer. N Engl J Med. 356:800–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Friboulet L, Olaussen KA, Pignon JP,
Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour
L, Pirker R, et al: ERCC1 isoform expression and DNA repair in
non-small-cell lung cancer. N Engl J Med. 368:1101–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Noro R, Honda K, Tsuta K, Ishii G,
Maeshima AM, Miura N, Furuta K, Shibata T, Tsuda H, Ochiai A, et
al: Distinct outcome of stage I lung adenocarcinoma with ACTN4 cell
motility gene amplification. Ann Oncol. 24:2594–2600. 2013.
View Article : Google Scholar : PubMed/NCBI
|