Introduction
Venous thromboembolism (VTE) includes pulmonary
thromboembolism (PE) and deep venous thrombosis. VTE that can be
diagnosed in clinics is known as dominant VTE. The clinical
spectrum of VTE is relatively wide, since VTE can occur in
different organs and tissues. However, dormant VTE, which is hard
to diagnose in clinics, is often revealed upon autopsy. PE has
become a global medical care problem due to its high morbidity,
misdiagnosis and mortality rates (1,2). VTE can
be divided into two categories: Genetic VTE and acquired VTE.
According to the results of epidemiological investigations, the
incidence of genetic VTE is relatively low, with the majority of
the VTEs being acquired VTEs (3). The
two types can be termed as symptomatic VTE when it is hard to
distinguish between them (3).
The American College of Chest Physicians published 9
editions of guidelines for VTE diagnosis, treatment and prevention
(4) between 1995 and 2012. Proposed
risk factors include advanced age, infection, malignancy,
autoimmune disease, surgery, trauma, pregnancy, long-trip syndrome
and family history.
Malignancy is one of the risk factors of VTE. The
prevalence of VTE in patients with malignancy is 4–7 times higher
than that of patients without malignancy (5,6). In
addition, the survival rate in patients with malignancies and
thrombi is 2–3 times lower than that of cancer patients without any
thrombus (7). Furthermore, 10–25% of
patients with idiopathic VTE as the first symptom are subsequently
diagnosed with cancer within 2 years, with the majority of the
diagnoses formed within 6 months, and the incidence of thrombosis
within 6 months of diagnosis being 4 times higher than that of
other periods (8). VTE is not only a
common complication of malignant tumors, but also the second
leading cause of mortality in cancer patients (9).
Certain issues with regard to VTE require
addressing, such as why patients with malignant tumors are often
also affected by VTE, why idiopathic VTE is always an early symptom
of occult cancer and the association between them.
Wang et al (10) reported that VTE is an inevitable
product in the proliferation phase of cancer cells, and acts as a
physical barrier by preventing cancer cells from hemorrhagic
metastasis. Therefore, there is necessarily a connection between
malignant tumors and the occurrence of VTE. To highlight the
association between malignant tumors and VTE, a novel mechanism of
VTE must be discussed.
Pathology of acute venous thrombosis
It has previously been reported (11) that the acute venous thrombus taken
from a pulmonary artery with a catheter presents as a red thrombus
to the naked eye, which is characterized as easily degraded. The
red thrombus is comprised of red blood cells, platelets, white
blood cells and plasma proteins, as shown by microscopy (Fig. 1).
Major proteins and core proteins of an acute
venous thrombus
Plasma proteins are important components that
combine with blood cells to form the material basis of thrombi. In
a previous study, mass spectrographic analyses demonstrated that a
large majority of the proteins identified were fibrinogen; the
remaining proteins included fibrin and cytoskeletal proteins
(12). Thrombi containing fibrinogen
degrade easily due to reversible combinations between fibrinogen
and their ligand proteins, which theoretically explains why an
acute venous thrombus is easy to autolyze, why delayed thrombolysis
is effective and why it is easy to lyse the thrombus through
interventional fragmentation (12).
An acute venous thrombus, also known as a red
thrombus, is composed of red blood cells, platelets, white blood
cells and fibrinogen. The manner in which fibrinogen binds to blood
cells during the formation of a venous thrombus is representative
of the mechanism of acute venous thrombosis. The use of tandem mass
spectrometry and the bioinformatic analysis of emboli in patients
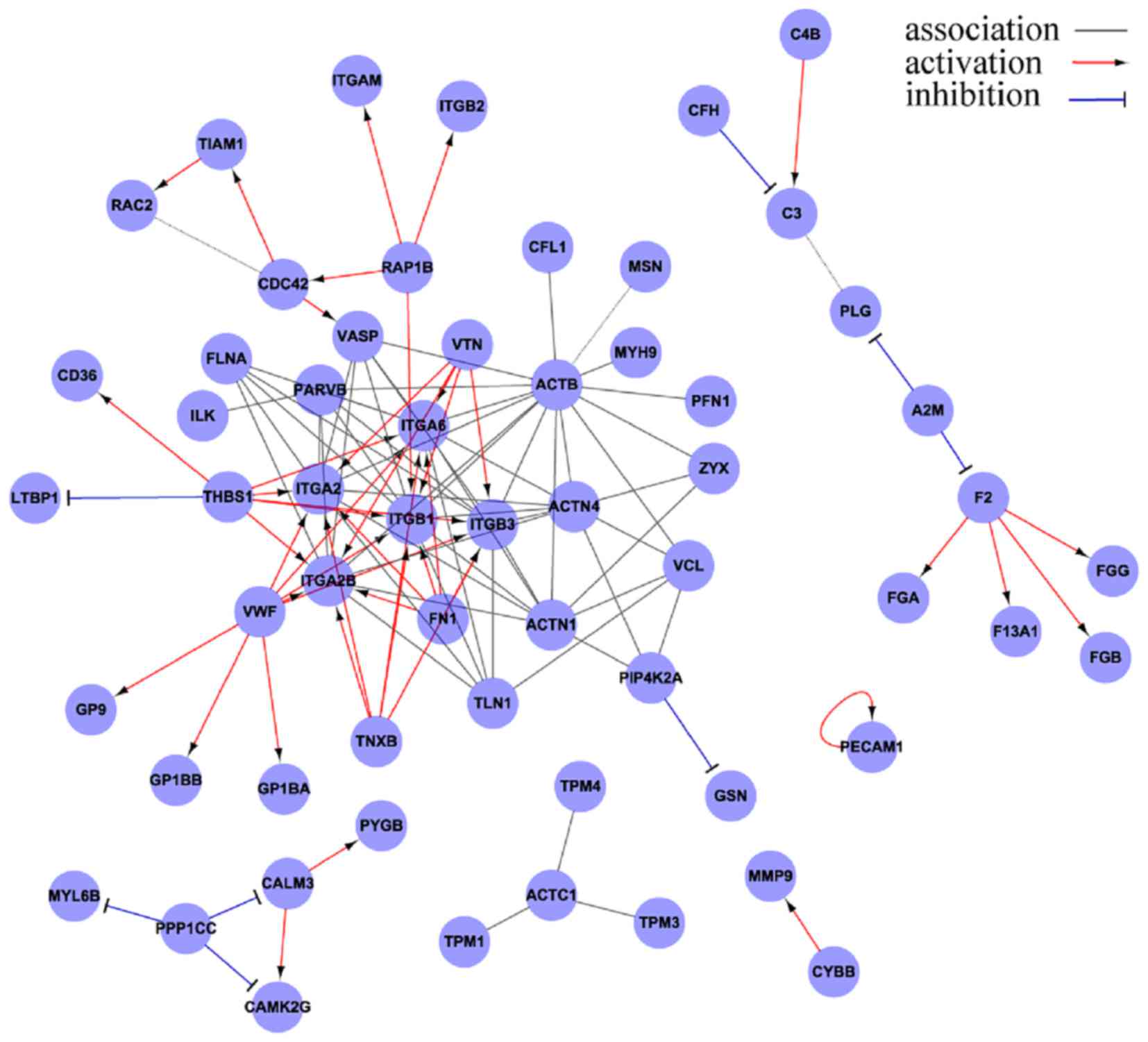
with acute PE has revealed that integrin subunits β1, β2 and β3 are
the core proteins of the emboli (Fig.
2).
Integrins are important members of the cell adhesion
molecule family, mediating adhesion between cells, and between
cells and the extracellular matrix (ECM), with involvement in
bidirectional signaling transduction between cells and the ECM.
Integrins can combine to their different ligands in various
cellular processes, namely the physical or pathological processes
of angiogenesis, invasion, metastasis, inflammation, wound healing
and coagulation (13).
Integrin is a transmembrane heterodimer composed of
subunits α and β at a ratio of 1:1. To date (Fig. 3A), a total of 18 α subunits and 8 β
subunits have been identified, and these can form 24 functional
heterodimers, which may be classified into 8 groups (β1-β8) based
on the β subunit. In the same group, the β subunit is identical,
but the α subunit is distinct. At rest, the α subunit is covered by
the β subunit and thus the integrin is unable to bind to ligands.
Following activation, the extension of the β subunit exposes the α
subunit. The α subunit mainly mediates the specific and reversible
binding between integrins and their ligands (Fig. 3B), and the β subunit dominates the
signal transduction and regulation of the affinity of integrins
(14–16).
Localization of core proteins in an acute
venous thrombus at a cellular level
The β1 subunit exists mainly on lymphocytes and
platelets, and its ligands include laminin, collagen,
thrombospondin, fibronetin and vascular cell adhesion molecule-1.
The β2 subunit is mainly distributed on neutrophils and monocytes,
and its ligands include fibrinogen, intracellular adhesion molecule
(ICAM), factorX and ic3b. The β3 subunit is mainly observed on
platelets, and its ligands include fibrinogen, fibronetin,
vitronectin, von Willebrand factor (vWF) and thrombospondin
(17–19).
Wang et al (11) collected thrombi through a catheter
attached to the pulmonary artery of patients with acute PE.
Immunohistochemistry revealed that dark-brown integrin β1 was
expressed on the lymphocytes, but that no expression of laminin,
fibronectin, collagen-I or collagen-II was observed on the
lymphocytes. Dark-brown integrin β2 was expressed on the
neutrophils, which bound to fibrinogen. ICAM, factor X and iC3b
were expressed on the neutrophils, and dark-brown integrin β3 was
expressed on the platelets, which aggregated to become a thrombotic
skeleton with a coral-like structure; these platelets bound
fibrinogen to create a mesh-like structure (Fig. 4A), which is similar to artificial vena
cava filters and was termed an intravenous biological filter in the
study. No expression of fibronectin, vitronectin or vWF was
observed on the platelets, and dark-brown factor Xa was distributed
on the mesh-like structure, which was composed of
fibrin/fibrinogen.
 | Figure 4.(A) Immunohistochemistry demonstrating
that platelets, which aggregate to become a thrombotic skeleton,
bind fibrinogen to construct a mesh-like structure (anti-fibrinogen
antibody; 1:100 dilution; magnification, ×1,000), which is similar
to artificial vena cava filters and known as an intravenous
biological filter. (B) (Left) Mode pattern of artificial nest-like
inferior vena cava filter. (Inset) Mesh-like structure is a
nest-like biological filter in veins under the microscope
(magnification, ×400, Masson staining). (C) (Left) Mode pattern of
artificial nest-like inferior vena cava filter filled with thrombi.
(Inset) Nest-like biological filters formed inside a venous
thrombus under the microscope, in which red blood cells are the
dominant blood cells found (magnification, ×400, Masson staining).
Inset images reproduced with permission (11). |
Another study reported that the expression of
subunits β1, β2 and β3 increased significantly in patients with VTE
(20). ROC curve analysis was used to
assess the diagnostic performance of these proteins in 120 VTE
patients. The area under the curve (AUC) of integrins β1, β2 and β3
in the VTE patients was 0.870, 0.821 and 0.731, respectively.
Optimum cutoffs of integrins β1, β2 and β3 calculated according to
Youden's index were 10.29, 91.10 and 10.35 pg/ml, respectively.
With these optimum cutoffs, the sensitivity, specificity, and
positive and negative predictive values were as follows: For
integrin β1, 80.3, 83.7, 71.1 and 89.3%, respectively; for integrin
β2, 78.6, 73.7, 59.4 and 87.6%, respectively; and for integrin β3,
68.4, 71.2, 54.3 and 81.8% respectively. The combined AUC of the
three integrins was 0.916, and the sensitivity, specificity, and
positive and negative predictive values were 84.6, 90.8, 81.7 and
92.0%, respectively. A clinical study confirmed that the VTE
patients with significantly increased expression of integrins β1,
β2 and β3 also exhibited relatively high specificity and
sensitivity (20).
Establishing biological filters during acute
venous thrombosis
Different types of artificial nest-like inferior
vena cava filters have been used in clinics for 30 years, the
mechanism of which prevents the genesis of PE by blocking the deep
venous thrombi flowing back to the pulmonary arteries through the
filter (Fig. 4A). Two core proteins
of thrombi, integrins β2 and β3, bind their ligand fibrinogen to
construct a mesh-like structure, which becomes a nest-like
biological filter in the veins (Fig. 4B
and C) (11).
As a precise and perfect life entity, the human body
is constantly regulating itself towards balance and stability. The
production of intravenous biological filters is a result of the
self-regulation of the human body.
Xiong et al (16) identified biological filters in the
veins of resected sigmoid colon adenocarcinoma tissues (Fig. 5A). Malignant cancer cells were
identified in biological filters, which impeded the hematogenous
metastasis of the cancer cells (Fig.
5B).
Inevitability of activation of an
intravenous physical defense line
The defense system of the human body is the immune
system. Simply speaking, it is the function of the immune system to
remove all foreign agents inside the human body, including external
pathogenic microorganisms, implants, invasive foreign bodies and
toxins from wounds, and internally generated senile and malignant
cells (10).
The genesis of malignancy indicates the loss of
innate and adaptive immune cell balancing functions. In other
words, the occurrence of cancer means a collapse of the immune cell
balancing function, which means a loss of the function of immune
clearance of malignant cells. When the immune cell balancing
function collapses, it is a basic principle to start using reserved
immune functions. Thus, an alternative defensive barrier is
activated (10).
The proliferation speed of cancer cells usually
exceeds that of small vessels (veins and arteries), which could
easily cause ischemic necrosis, and thus increased permeability of
vessels and the destruction of small vessels. In addition, the
proliferation of malignant tumor cells and invasion of surrounding
small vessels can also cause the destruction of small vessels
(10). Wang et al (10) identified a biological filter in the
veins of resected sigmoid colon adenocarcinoma tissue (Fig. 5A). Malignant cancer cells were
revealed in the biological filter, which impeded the hematogenous
metastasis of the cancer cells (Fig.
5B). It was found that (10)
fibrinogen formed a mesh-like structure in the peripheral small
veins of a resected sigmoid colon adenocarcinoma, which was filled
with cancer cells, thus preventing their translocation. When cells
escaped from the mesh-like structure, this presented as metastasis
of the tumor cells. The formation of a biological filter in the
surrounding veins of malignant tumor tissue is a replacement or
supplement to the loss of immune function to prevent hematogenous
metastasis. The occurrence of VTE in patients with malignancy is
the first stage of physical immune defense. Patients with malignant
tumors bleed easily. Wang et al (10) also identified exudation of a large
number of red blood cells out of vessels (Fig. 6) and the accumulation of large amounts
of fibrinogen in necrotic regions of poorly-differentiated gastric
carcinoma, indicating destruction of small vessels and/or the
increased permeability of vessels.
According to a study on the biopsies of malignant
tumor patients, 50% of such individuals were affected by VTE prior
to mortality (21). From the genesis
and development of malignant tumor cells, and the morphological
characteristics of the proliferation stage, the present study
speculated that almost every patient with a malignant tumor has the
possibility of having VTE, and that almost every patient with a
malignant tumor has the possibility of having bleeding, due to the
processes of tumor cell proliferation, the destruction of
peripheral tissues and vessels, and the establishment of a
defensive barrier, which are physical/pathological phenomena of the
human body. However, VTE and bleeding of the peripheral tissues
around cancer cells are hard to recognize at an early stage.
7. Increased integrin β1, β2 and β3 levels in
patients with malignant tumors
Song et al (22) reported that the levels of the integrin
β1, β2 and β3 core proteins of thrombi increased in patients with
malignant tumor cells, among which, integrins β1 and β3 increased
significantly. The relative risks of integrins β1, β2 and β3 in
malignant tumor patients were 1.655, 1.314 and 1.852 times that of
a control group, respectively, while the combined risk of increased
integrins β1, β2 and β3 in the malignant tumor group was 4.895
times that of a control group, indicating that integrins β1, β2 and
β3 are the molecular basis of the increased risk of VTE in patients
with cancer.
There are significant differences in the therapy,
risk of reoccurrence and survival period between cancerous and
non-cancerous VTEs. If patients with malignant tumors are able to
receive an early diagnosis due to early warning signals from VTE
occurrence, precious time may be saved for early treatment, which
could have a significant impact on the VTE and tumors. Based on the
aforementioned data, CG144 guidelines published by the National
Institute for Health and Clinical Excellence (NICE) in 2012
(https://www.nice.org.uk/guidance/cg144) have begun to
recommend that idiopathic VTE patients >40 years old should be
screened for tumors in order to exclude occult tumors (23); this has been declared to be a
milestone in the treatment and prevention of VTE by Shaboodien
et al (21).
Conclusion
Clinical tests to detect core proteins of venous
thrombi, presenting as increased levels of integrins β1, β2 and β3,
are useful not only in the diagnosis of VTE, but also in the early
recognition of occult malignant tumors in idiopathic VTE
patients.
References
|
1
|
Piazza G and Goldhaber SZ: Physician
alerts to prevent venous thromboembolism. J Thromb Thrombolysis.
30:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardiovascular Disease Educational and
Research Trust, ; Cyprus Cardiovascular Disease Educational and
Research Trust, ; European Venous Forum, ; International Surgical
Thrombosis Forum, ; International Union of Angiology, ; Union
Internationale de Phlébologie, . Prevention and treatment of venous
thromboembolism. International Consensus Statement (guidelines
according to scientific evidence). Int Angiol. 25:101–161.
2006.
|
|
3
|
Heit JA: The epidemiology of venous
thromboembolism in the community. Arterioscler Thromb Vasc Biol.
28:370–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guyatt GH, Akl EA, Crowther M, Gutterman
DD and Schuunemann HJ: American College of Chest Physicians
Antithrombotic Therapy and Prevention of Thrombosis Panel:
Executive summary: Antithrombotic Therapy and Prevention of
Thrombosis, 9th ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 141 2
Suppl:7S–47S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falanga A and Russo L: Epidemiology, risk
and outcomes of venous thromboembolism in cancer. Hamostaseologie.
32:115–125. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seddighzadeh A, Shetty R and Goldhaber SZ:
Venous thromboembolism in patients with active cancer. Thromb
Haemost. 98:656–661. 2007.PubMed/NCBI
|
|
7
|
Khorana AA, Ahrendt SA, Ryan CK, Francis
CW, Hruban RH, Hu YC, Hostetter G, Harvey J and Taubman MB: Tissue
factor expression, angiogenesis, and thrombosis in pancreatic
cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakkar AK: Cancer-associated thrombosis.
Br J Cancer. 102 Suppl 1:S12010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldhaber SZ and Bounameaux H: Pulmonary
embolism and deep vein thrombosis. Lancet. 379:1835–1846. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LM, Duan QL, Yi XH, Zeng Y, Gong Z
and Yang F: Venous thromboembolism is a product in proliferation of
cancer cells. Int J Clin Exp Med. 7:1319–1323. 2014.PubMed/NCBI
|
|
11
|
Wang LM, Duan QL, Yang F, Yi XH, Zeng Y,
Tian HY, Lv W and Jin Y: Activation of circulated immune cells and
inflammatory immune adherence are involved in the whole process of
acute venous thrombosis. Int J Clin Exp Med. 7:566–572.
2014.PubMed/NCBI
|
|
12
|
Wang L, Gong Z, Jiang J, Xu W, Duan Q, Liu
J and Qin C: Confusion of wide thrombolytic time window for acute
pulmonary embolism: Mass spectrographic analysis for thrombus
proteins. Am J Respir Crit Care Med. 184:145–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong JP, Stehle T, Diefenbach B, Zhang R,
Dunker R, Scott DL, Joachimiak A, Goodman SL and Arnaout MA:
Crystal structure of the extracellular segment of integrin alpha
Vbeta3. Science. 294:339–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerber DJ, Pereira P, Huang SY, Pelletier
C and Tonegawa S: Expression of alpha v and beta 3 integrin chains
on murine lymphocytes. Proc Natl Acad Sci USA. 93:pp. 14698–14703.
1996; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lityńska A, Przybyło M, Ksiazek D and
Laidler P: Differences of alpha3beta1 integrin glycans from
different human bladder cell lines. Acta Biochim Pol. 47:427–434.
2000.PubMed/NCBI
|
|
19
|
Solovjov DA, Pluskota E and Plow EF:
Distinct roles for the alpha and beta subunits in the functions of
integrin alphaMbeta2. J Biol Chem. 280:1336–1345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song Y, Yang F, Wang L, Duan Q, Jin Y and
Gong Z: Increased expressions of integrin subunit β1, β2 and β3 in
patients with venous thromboembolism: New markers for venous
thromboembolism. Int J Clin Exp Med. 7:2578–2584. 2014.PubMed/NCBI
|
|
21
|
Shaboodien R, Stansby G, Hunt BJ and
Agarwal R: Unprovoked venous thromboembolism: Assess for cancer.
Lancet Oncol. 13:973–974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Y, Wang L, Yang F, Li G, Duan Q and
Gong Z: Increased expressions of integrin subunit β1, β2 and β3 in
patients with cancer-correlation analysis between risk factors of
VTE and expression of core proteins. Int J Clin Exp Med.
8:2772–2777. 2015.PubMed/NCBI
|
|
23
|
Chong LY, Fenu E, Stansby G and Hodgkinson
S; Guideline Development Group, : Management of venous
thromboembolic diseases and the role of thrombophilia testing:
Summary of NICE guidance. BMJ. 344:e39792012. View Article : Google Scholar : PubMed/NCBI
|