Introduction
Primary intracranial germ-cell tumors (GCT) are rare
tumors. They account for ~0.5–3% of all pediatric primary central
nervous system (CNS) tumors in Western regions, but are observed at
higher frequencies among pediatric CNS tumors in Asia, accounting
for up to 10% (1). The incidence in
the Far East area of Japan is 0.1–0.17 per 100,000 per year,
according to the Brain Tumor Registry of Japan (2), slightly higher than the incidence rate
of 0.1 per 100,000 per year in the United States (3). Primary intracranial GCT typically occurs
in children or young adults, with the majority of patients (60–70%)
aged under 20 (4). The peak incidence
of intracranial germ-cell tumors is in the early pubertal period
with a median age of diagnosis at 10–12 years (5). The disease occurs primarily in males,
with the ratio of male to female between 2:1 and 3:1 (6). The World Health Organization had
classified intracranial germ-cell tumors into three groups as
follows: Germinomas, non-germinomatous germ-cell tumors (NGGCTs)
and mixed germ-cell tumors (7). The
diseases are heterogeneous in terms of histology, tumor
characteristics, treatment response and tumor marker secretion.
Among NGGCTs, choriocarcinoma secretes β-human chorionic
gonadotropin (β-HCG) into the serum and/or the cerebrospinal fluid
(CSF), with high levels detected, while yolk sac tumors secrete
α-fetoprotein (AFP). Elevation of AFP levels, combined with
characteristic magnetic resonance imaging (MRI) results, is
diagnostic for NGGCT (8). In pure
germinomas, AFP is never elevated, but certain germinomas may
secrete β-HCG at levels seldom >50 IU/l (9).
Due to of the rarity of this disease, reports of
adult intracranial GCTs are scarce. The clinical features of GCT in
adult patients are likely to be different from those in children.
The present study described a rare case of β-hCG secreting primary
intracranial GCT in a 38 year-old man involving the pineal gland,
the stalk and posterior pituitary gland, the right basal ganglion,
the hypothalamus, the corpus callosum and posterior hippocampus,
with presentations of hypogonadotropic hypergonadism,
panhypopituitarism, diabetes insipidus (DI) and psychological
symptoms.
Case report
The present study was approved by the Ethics
Committee of Taipei City Hospital and written informed consent was
obtained from the patient.
A 38-year-old male patient with alcoholic liver
disease and chronic hepatitis B presented with general weakness and
body weight loss of 10 kg in 2 years was admitted to Department of
Medicine, Taipei City Hospital Ren-Ai Branch (Taipei, Taiwan) in
March 2012. The patient suffered poor work performance, emotional
instability, lack of energy, insomnia and decreased libido for 1
year. Polydipsia was also noted during this period.
A physical examination revealed a man of
chronically-ill appearance with normal development of the testis,
pubic hair and axillary hair without gynecomastia. The daily urine
output of the patient was >10,000 ml, which did not decrease
following fluid restriction. Low urine density and mild
hypernatremia suggested a diagnosis of DI, but it was not possible
to perform a water deprivation test due to the agitated mood and
behavior of the patient.
Laboratory evaluation of an 8AM sample on admission
revealed the presence of adrenocorticotropic hormone [14.3 pg/ml
(10–65 pg/ml)], cortisol [1.34 µg/dl (5–14 µg/dl)], thyroxine
stimulating hormone [0.12 µIU/ml (0.1–4.5 µIU/ml)], free thyroxine
[0.68 ng/dl (0.7–1.75 ng/dl)], follicle-stimulating hormone (FSH;
<0.1 mIU/ml), luteinizing hormone (LH; 0.17 mIU/ml),
testosterone [1,480 ng/dl (70–620 ng/dl)], prolactin (56 ng/ml),
insulin-like growth factor-1 [52.4 ng/ml (109.0–284.0 ng/ml)].
Panhypopituitarism-associated central hypoadrenalism, central
hypothyroidism and growth hormone deficiency were also noted.
However, it was not possible to explain the hypogonadotropic
hypergonadism.
A brain MRI scan revealed the presence of a
heterogeneous lesion involved in the right basal ganglion,
hypothalamus, and the extension to the corpus callosum and to the
posterior hippocampus. The pineal gland, stalk and posterior
pituitary gland were also involved (Fig
1). A lumber puncture was performed and CSF cytology revealed
no malignant cells, but CSF β-hCG levels of 1,936 IU/l and AFP
levels <0.24 ng/ml. The serum AFP level was 3.28 ng/ml, and the
β-hCG level was 178 IU/l, with a CSF:serum β-hCG ratio >2:1.
Neck to pelvis computed tomography excluded the presence of
metastatic lesions. It was not possible to obtain a pathological
diagnosis due to the deep location of the tumors. However, a β-hCG
secreting suprasella GCT was suspected due to imaging and tumor
markers.
The patient underwent hormone replacement therapy
consisting of 10 mcg of minirin nasal spray, 100 µg thyroxin and 5
mg prednisolone per day from March 2012. First cycle of
chemotherapy with BEP regimen (30 mg bleomycin in 0.9% saline as a
total of 100 ml on day 1, 8 and 15; 100 mg/m2 etoposide
per day for days 1–5 as a 4-h infusion in 0.9% sodium chloride in a
total of 500 ml; 20 mg/m2 cisplatin per day for days 1–5
as a 4-h infusion with 0.9% sodium chloride in a total of 500 ml)
was performed at Taipei City Hospital Ren-Ai Branch (Taipei,
Taiwan). After 2 cycles of this chemotherapy from April to May
2012, the serum β-hCG levels of the patient decreased to 22.2 IU/l
in May 2012. Intensity modulation radiation therapy was performed
with 25.2 Gy to the whole ventricle and a radiation treatment boost
to administer an overall total of 45 Gy to the GCT site from June
to August 2012.
Following 6 cycles of chemotherapy and radiotherapy,
a brain MRI scan revealed that the tumors markedly decreased in
size (Fig 2). The serum β-hCG levels
of the patient fell to 0.3 IU/l and hyperandrogenemia subsided,
with testosterone levels recorded as 84 ng/dl. However, the
psychological symptoms of the patient, including irritability and
emotional instability, only improved a little.
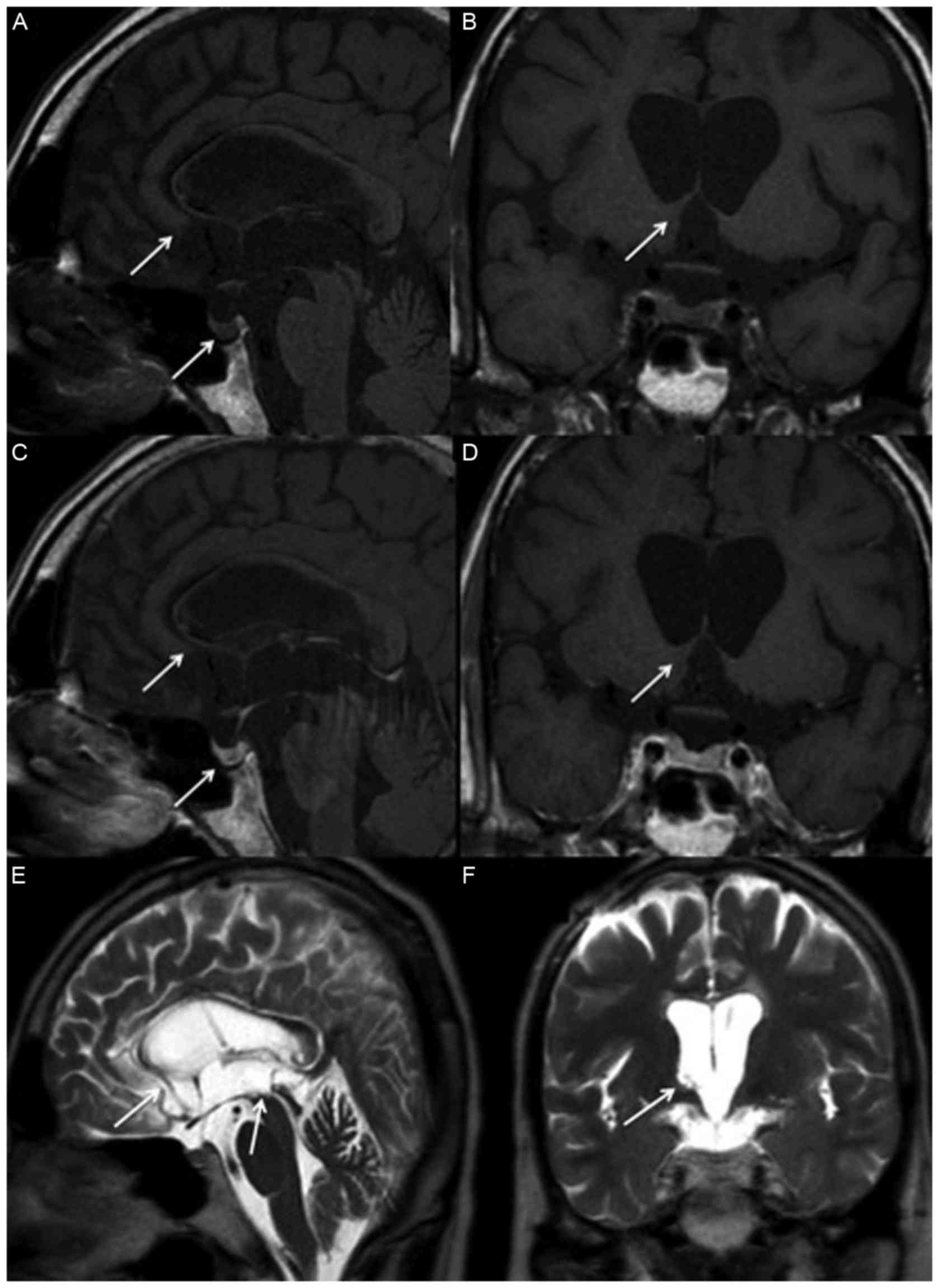 | Figure 2.Magnetic resonance imaging of
suprasella two years following treatment. The tumors over the basal
ganglion, hypothalamus, corpus callosum, posterior hippocampus,
pineal gland, stalk and posterior pituitary gland have disappeared,
as indicated by arrows, in (A) non-contrast sagittal, (B) coronal,
(C) contrast sagittal and (D) coronal T1 weighted images, as well
as (E) T2 weighted sagittal and (F) coronal images. |
Discussion
β-hCG-secreting intracranial GCTs are primarily
diagnosed by young adolescence. The majority of patients are
diagnosed with presentation of precocious puberty due to excess
testosterone, which permits physicians to detect the disease
earlier (10). Reports of adult β-hCG
secreting intracranial GCTs are rare, the clinical characteristics
are likely to be nonspecific and differ from those observed in
children. To the best of our knowledge, there are no prior
published case reports concerning patients older than 30 years that
have been diagnosed with β-hCG secreting intracranial GCTs.
Therefore, the present study will be the first to report a patient
with this later age of onset.
GCTs are a varied group of neoplasms derived from
the primordial germ cells, and they are classified as extragonadal,
if there is no evidence of a primary tumor in either the testes or
the ovaries. In adults, intracranial GCTs are the most well-known
extragonadal GCT. However, primary intracranial GCT are still rare,
accounting for 2% of all primary intracranial neoplasms in the USA
and Europe, and 3–10% of brain tumors in children from Asian
countries (1). Males are affected
more than females, at a ratio of 2:1 to 3:1. The majority of
patients (60–70%) are <20 years old, and 53% of patients are
between 10 and 19 years old at the point of diagnosis. The disease
is rare in patients >35 years old (4).
Intracranial GCTs are heterogeneous with respect to
histology, biological profile, response to treatment and secretion
of AFP and β-HCG into the serum and/or CSF (11). For the majority of patients with
presumed intracranial GCTs, clinical manifestation and neurological
image results are not specific enough to provide a definitive
diagnosis. Clinical presentation depends upon the size and the
localization of the tumor. Intracranial GCTs may be situated over
the pineal and suprasellar regions and spread along the neuroaxis,
with ~15% demonstrating the involvement of multiple sites (12). The majority of GCTs are located at the
pineal region, followed by the suprasellar region (12). Patients with pineal germinoma may
present with insomnia, but interference with pituitary function is
rarely observed (13). Pineal tumors
often manifest with symptoms of obstructive hydrocephalus (14) and Parinaud's syndrome, characterized
by the paralysis of upward gaze and convergence, may occur in 50%
of patients (15). Suprasellar tumors
are often characterized as endocrinopathies due to the disruption
of the hypothalamic-pituitary axis (16). Hypothalamic-pituitary dysfunction may
include DI, delayed pubertal development, isolated growth hormone
deficiency, hypogonadotropic hypogonadism and any aspect of
hypopituitarism, including central hypothyroidism and adrenal
insufficiency. DI is the most common and is often the first
presentation. Ophthalmic abnormalities, including bilateral
hemianopsia, may also develop due to chiasmic or optic nerve
compression (13). Delays in
diagnosis are common and may exceed 12 months, in particular when
patients present with symptoms associated with endocrinopathy, and
this results in higher incidences of disseminated disease.
Histologically, germinomas are the most common
subtype of intracranial GCTs, accounting for 70–80% of all GCTs,
and they are histologically identical to testicular seminoma and
dysgerminoma of the ovary (7,17). Non-germinomatous GCTs account for
20–30% of intracranial GCTs, including embryonal carcinoma, yolk
sac tumors, choriocarcinoma and teratoma (7). Patients with pure germinoma may have
mildly elevated β-hCG in contrast to the marked elevation of β-hCG
observed in choriocarcinoma, but AFP is never elevated in
germinoma. The latter is secreted by yolk sac tumors. When β-hCG is
secreted by GCTs, it causes gonadotropin-independent hypergonadism
with low LH/FSH and high testosterone due to the stimulating effect
of β-hCG on the LH receptor in the testes. When this occurs in
young male patients, which make up the majority of cases,
precocious puberty will occur. In adult male patients, there are no
reports of clinical features of androgen excess associated with
β-hCG secreting intracranial GCTs. Fung et al (18) reported a 32-year old male with
testicular seminoma with β-hCG secretion and associated
hyperandrogenism. The patient presented with worsening in acne and
increased muscle bulk (18). Other
case reports of testosterone excess by seminoma in testes or
mediastinum shared clinical features with gynecomastia or male
infertility in adult male patients (19–21).
The present study concerns a well-developed adult
patient with two daughters. In contrast with the symptoms of
hyperandrogenemia, the symptoms of the patient were decreased
libido, motivation and vitality. The aggressive behavior and
irritable mood exhibited by the patient were initially attributed
to high testosterone levels, but these symptoms did not disappear
when testosterone levels decreased. Structural damage to brain
tumor tissue and late effects following radiotherapy may be the
explanation. A debate remains concerning late neurocognitive
dysfunction following radiotherapy. The neurocognitive function of
the patient deteriorated to mirror child-like behavior, but
improved half a year later. The previous symptoms, including
fatigue and weakness, improved following hormone replacement with
eltroxin and prednisolone.
Radiological diagnosis of intracranial GCT with MRI
or computed tomography is a useful tool, with MRI being the optimal
modality. MRI demonstrates soft tissue masses with isointense or
slightly hyperintense signals in T1 weighted images, which may be
accompanied with calcification or cyst formation in T2 weighted
images (22). Definitive diagnosis of
GCT is performed through the histopathology approach: The majority
of GCTs demonstrate immunohistochemical staining for placenta-like
alkaline phophatase and c-Kit, otherwise known as CD117, which is
an important mitogen for normal germ cells (11). Unfortunately, a biopsy is not possible
for patients where the tumor location is inaccessible. CSF β-hCG
assays reflect the intensity of intracranial β-hCG secretion and
are more sensitive than serum β-hCG levels (23). A β-hCG concentration in CSF >50
IU/l and a CSF/serum β-hCG ratio ≥2 has been suggested to be an
indicator of the presence of a CNS GCT (24), which was observed in the patient
enrolled in the present study.
Intracranial GCTs are sensitive to chemotherapy and
radiotherapy. Cranio-spinal irradiation or whole ventricular
radiotherapy to a dose of 25–35 Gy followed by a primary tumor
boost for a total dose of 45–50 Gy is associated with a superior
outcome, with a 5-year survival rate of 80–99.5% in retrospective
and prospective studies (25).
Chemotherapy agents including cyclophosphamide, ifosfamide,
etoposide, cisplatin, and carboplatin are also highly active in CNS
GCTs (26). The brain MRI of the
patient enrolled in the present study following treatment revealed
that the tumors markedly decreased in size, and β-hCG levels were
within normal range. The patient has maintained a stable disease
status since August 2015 in Taipei City Hospital Ren-Ai Branch.
In conclusion, the incidence of adult β-hCG
secreting intracranial GCT is low. Compared with the majority
patients, who are diagnosed in early pubertal years upon
presentation of precocious puberty, the symptoms in adult patients
are primarily associated with tumor size and location, with
pituitary hormone deficiency rather than symptoms associated with
testosterone excess.
References
|
1
|
Echevarría ME, Fangusaro J and Goldman S:
Pediatric central nervous system germ cell tumors: A review.
Oncologist. 13:690–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura H, Makino K, Yano S and Kuratsu
J; Kumamoto Brain Tumor Research Group, : Epidemiological study of
primary intracranial tumors: A regional survey in Kumamoto
prefecture in southern Japan-20-year study. Int J Clin Oncol.
16:314–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16
Suppl 4:iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCarthy BJ, Shibui S, Kayama T, Miyaoka
E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE,
et al: Primary CNS germ cell tumors in Japan and the United States:
An analysis of 4 tumor registries. Neuro Oncol. 14:1194–1200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jennings MT, Gelman R and Hochberg F:
Intracranial germ-cell tumors: Natural history and pathogenesis. J
Neurosurg. 63:155–167. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoffman HJ, Otsubo H, Hendrick EB,
Humphreys RP, Drake JM, Becker LE, Greenberg M and Jenkin D:
Intracranial germ-cell tumors in children. J Neurosurg. 74:545–551.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calaminus G, Bamberg M, Harms D, Jürgens
H, Kortmann RD, Sörensen N, Wiestler OD and Göbel U: AFP/beta-HCG
secreting CNS germ cell tumors: Long-term outcome with respect to
initial symptoms and primary tumor resection. Results of the
cooperative trial MAKEI 89. Neuropediatrics. 36:71–77. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogino H, Shibamoto Y, Takanaka T, Suzuki
K, Ishihara S, Yamada T, Sugie C, Nomoto Y and Mimura M: CNS
germinoma with elevated serum human chorionic gonadotropin level:
Clinical characteristics and treatment outcome. Int J Radiat Oncol
Biol Phys. 62:803–808. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marshall GA, McMahon SK, Nicholls W,
Pretorius CJ and Ungerer JP: Gonadotrophin-independent precocious
puberty in an eight-year-old boy due to ectopic human chorionic
gonadotrophin from the central nervous system. Ann Clin Biochem.
47:271–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bromberg JE, Baumert BG, De Vos F,
Gijtenbeek JM, Kurt E, Westermann AM and Wesseling P: Primary
intracranial germ-cell tumors in adults: A practical review. J
Neurooncol. 113:175–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsutani M, Sano K, Takakura K, Fujimaki
T, Nakamura O, Funata N and Seto T: Primary intracranial germ cell
tumors: A clinical analysis of 153 histologically verified cases. J
Neurosurg. 86:446–455. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loto MG, Danilowicz K, González Abbati S,
Torino R and Misiunas A: Germinoma with involvement of midline and
off-midline intracranial structures. Case Rep Endocrinol.
2014:9369372014.PubMed/NCBI
|
|
14
|
Sethi RV, Marino R, Niemierko A, Tarbell
NJ, Yock TI and MacDonald SM: Delayed diagnosis in children with
intracranial germ cell tumors. J Pediatr. 163:1448–1453. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao CK, Lee ST, Lin FJ, Tang SG and Leung
WM: A multivariate analysis of prognostic factors in management of
pineal tumor. Int J Radiat Oncol Biol Phys. 27:1185–1191. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crawford JR, Santi MR, Vezina G, Myseros
JS, Keating RF, LaFond DA, Rood BR, MacDonald TJ and Packer RJ: CNS
germ cell tumor (CNSGCT) of childhood: Presentation and delayed
diagnosis. Neurology. 68:1668–1673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamoshima Y and Sawamura Y: Update on
current standard treatments in central nervous system germ cell
tumors. Curr Opin Neurol. 23:571–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fung LC, Honey RJ and Gardiner GW:
Testicular seminoma presenting with features of androgen excess.
Urology. 44:927–929. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagi DK, Jones WG and Belchetz PE:
Gynecomastia caused by a primary mediastinal seminoma. Clin
Endocrinol (Oxf). 40:545–549. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lefebvre H, Laquerriere A, Cleret JM and
Kuhn JM: A hCG-secreting testicular seminoma revealed by male
infertility: Mechanism of hCG-evoked endocrine disturbances.
Andrologia. 25:283–287. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Djaladat H, Nichols C and Daneshmand S:
Androgen-producing testicular germ cell tumors. J Clin Oncol.
29:e634–e635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanagaki M, Miki Y, Takahashi JA,
Shibamoto Y, Takahashi T, Ueba T, Hashimoto N and Konishi J: MRI
and CT findings of neurohypophyseal germinoma. Eur J Radiol.
49:204–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian C, Shi Q, Pu C, Huang X, Yu S, Zhang
J, Huang D, Wang X and Liu R: Re-evaluation of the significance of
cerebrospinal fluid human chorionic gonadotropin in detecting
intracranial ectopic germinomas. J Clin Neurosci. 18:223–226. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katagami H, Hashida S, Yamaguchi H, Yazawa
S, Nakano S and Wakisaka S: Diagnosis and course of treatment of
CNS germinoma assessed by highly sensitive immune complex transfer
enzyme immunoassay to detect HCG. Horumon to Rinsho. 51:196–206.
2003.
|
|
25
|
Kortmann RD: Current concepts and future
strategies in the management of intracranial germinoma. Expert Rev
Anticancer Ther. 14:105–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim DH, Yoo KH, Lee NH, Lee SH, Sung KW,
Koo HH, Kim JH, Suh YL, Joung YS and Shin HJ: Intensive
chemotherapy followed by reduced-dose radiotherapy for
biopsy-proven CNS germinoma with elevated beta-human chorionic
gonadotropin. J Neurooncol. 117:279–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
















