Introduction
Bladder cancer, the majority of which is urothelial
carcinoma (UC), is a common malignancy worldwide. The prognosis of
patients with UC is poor when the disease includes muscle invasion.
Metastatic UC is almost uniformly fatal (1). However, progress in systemic therapies
for muscle-invasive bladder carcinoma (MIBC) has been stagnant for
decades, with few new systemic therapies being evaluated, until
recently (2). Therefore, there is an
urgent requirement for new potential therapeutic targets in UC.
Better knowledge of the changes in gene expression
that occur during carcinogenesis may lead to improvements in
diagnosis, treatment and prevention of cancer. Genes encoding
transmembrane/secretory proteins expressed specifically in certain
cancers may be ideal biomarkers for cancer diagnosis, and may
represent therapeutic targets. Escherichia coli ampicillin
secretion trap (CAST), a signal sequence trap method developed by
Ferguson et al (3), is a
unique large-scale analysis method that is useful to identify genes
encoding transmembrane/secretory proteins. Using the CAST method,
overexpression of the transmembrane protein bone marrow stromal
cell antigen-2 (BST-2) was detected in gastrointestinal cancer
(4). Furthermore, knockdown of the
BST2 gene inhibits gastric cancer cell growth, suggesting
that BST-2 could be a useful therapeutic target for gastric cancer
(4). BST-2 is a lipid raft-associated
type II transmembrane glycoprotein that is overexpressed on
multiple myeloma cells (5,6). Immunotherapy with a monoclonal antibody
against BST-2 reduces tumor size and improves survival in a
multiple myeloma mouse model (7).
Such monoclonal antibody against BST-2 induces antibody-dependent
cellular cytotoxicity, suggesting that it may be effective for a
wide range of human malignancies (7).
In addition to gastrointestinal cancer, high levels of BST-2 have
been reported in neoplastic B cells (6), and in ovarian (8), breast (9),
endometrial (10) and lung cancer
(11). However, the expression of
BST-2 has not been investigated in UC to date.
In the present study, the expression and
distribution of BST-2 was examined in UC by immunohistochemistry,
and potential correlations with clinicopathological factors were
analyzed. In addition, the effects of BST2 knockdown on cell
growth activity were evaluated using RNA interference (RNAi) or
forced expression of BST2 in UC cell lines.
Materials and methods
Tissue samples
Using a retrospective study design, 77 primary
tumors were collected from patients diagnosed with UC, who
underwent surgery between April 2003 and March 2007 at Hiroshima
University Hospital (Hiroshima, Japan). All patient samples were
obtained with consent, and the present study was approved by the
Ethical Committee for Human Genome Research of Hiroshima University
(Hiroshima, Japan). All patients underwent curative resection. Only
patients without preoperative radiotherapy or chemotherapy and
without clinical evidence of distant metastasis were enrolled in
the study. Operative mortality was defined as mortality within 30
days of patients leaving the hospital, and these patients were
removed from the analysis. Postoperative follow-up was scheduled
every 1, 2 or 3 months during the first 2 years after surgery, and
every 6 months thereafter, unless more frequent follow-ups were
deemed necessary. Chest X-ray, chest computed tomography scan and
serum chemistries were performed at every follow-up visit.
Follow-ups of the patients were conducted by the physician until
mortality or until the date of the last documented contact. Tumor
staging was performed according to the tumor-node-metastasis
classification system (12).
For reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), 8 UC samples were used. The samples were
frozen immediately in liquid nitrogen and stored at −80°C until
use. A total of 14 types of normal tissue samples [namely heart
(catalog no. 636532), lung (catalog no. 636524), stomach (catalog
no. 636578), small intestine (catalog no. 636539), colon (catalog
no. 636553), liver (catalog no. 636531), pancreas (catalog no.
636577), kidney (catalog no. 636529), bone marrow (catalog no.
636591), leukocytes (catalog no. 636592), spleen (catalog no.
636525), skeletal muscle (catalog no. 636547), brain (catalog no.
636530) and spinal cord (catalog no. 636554)] were purchased from
Clontech Laboratories, Inc. (Mountainview, CA, USA).
For immunohistochemical analysis, archival
formalin-fixed, paraffin-embedded tissues from 69 patients who had
undergone surgical excision for UC were used. All 69 patients with
UC were treated by cystectomy between April 2003 and March 2007 at
the Hiroshima University Hospital (Hiroshima, Japan).
RT-qPCR analysis
Total RNA was extracted with an RNeasy Mini kit
(Qiagen, Inc., Valencia, CA, USA), and 1 µg total RNA was converted
to complementary DNA (cDNA) using a First Strand cDNA Synthesis kit
(GE Healthcare Life Sciences, Chalfont, UK). Quantitation of
BST2 messenger RNA (mRNA) levels was performed by
quantitative fluorescence detection as described previously
(13). PCR was conducted using a
SYBR-Green PCR Core Reagents kit (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Real-time detection of the
emission intensity of SYBR Green bound to double-stranded DNA was
performed with the ABI PRISM 7700 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.).
β-Actin-specific PCR products were amplified from the same RNA
samples and served as an internal control. Quantitation of
ACTB mRNA levels was performed by quantitative fluorescence
detection as described previously (13). RT-qPCR reactions were performed in
triplicate for each sample primer set, and the mean of the three
experiments was used as the relative quantification value (14). Primer sequences for BST2 were
forward, 5′-CAGAAGGGCTTTCAGGATGT-3′ and reverse,
5′-TTCTCAGTCGCTCCACCTCT-3′. Primer sequences for ACTB were
forward, 5′-TCACCGAGCGCGGCT-3′ and reverse,
5′-TAATGTCACGCACGATTTCCC-3′. The thermocycling conditions were used
as described previously (13).
Immunohistochemistry
Immunohistochemical analysis was performed with the
EnVison+ Rabbit Peroxidase Detection System (Dako; Agilent
Technologies GmbH, Waldbronn, Germany) as described previously
(15). As the primary antibody, a
rabbit polyclonal anti-BST-2 antibody was used (dilution, 1:50;
catalog no. HPA017060, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) (4). A result was considered
positive if ≥10% of cancer cells were stained. When <10% of
cancer cells were stained, the immunostaining was considered
negative.
Cell lines
Two cell lines derived from human UC (T24 and KMBC2)
were used. Both cell lines were purchased from the Japanese
Collection of Research Bioresources Cell Bank (Osaka, Japan), and
were maintained in RPMI 1640 medium (Nissui Pharmaceutical Co.,
Ltd., Tokyo, Japan) containing 10% fetal bovine serum
(BioWhittaker; Lonza, Basel, Switzerland) at 37°C in a humidified
atmosphere with 5% CO2.
RNAi, expression vector and cell
growth assay
Small interfering RNA (siRNA) oligonucleotides
targeting BST2 and a negative control siRNA were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). Two independent
BST2 siRNA oligonucleotide sequences were used (catalog nos.
251993co3 and 251993co5). Transfection was performed using
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.)
as described previously (16).
Briefly, 60 pmol siRNA and 10 µl Lipofectamine RNAiMAX were mixed
in 1 ml RPMI 1640 medium (10 nmol/l final concentration). After 20
min of incubation, the mixture was added to the cells (100,000
cells/ml), and then the cells were plated in culture dishes. At 48
h post-transfection, the cells were analyzed.
For constitutive expression of the BST2 gene,
cDNA was amplified by PCR and then subcloned into the pcDNA3.1
vector (Invitrogen; Thermo Fisher Scientific, Inc.). The resultant
pcDNA-BST2 expression vector was transfected into KMBC2
cells with FuGENE6 (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's protocol. KMBC2 cells were selected
as the cell line express low levels of BST-2.
To examine cell growth, MTT assays were performed.
The cells were seeded at a density of 2,000 cells per well in
96-well plates. Cell growth was examined after 1, 2 and 4 days.
Three independent experiments were performed. The mean and standard
error were calculated for each experiment.
Western blot analysis
Cells were lysed as described previously (17). The lysates (40 µg protein) were
solubilized in Laemmli sample buffer (catalog no. S3401,
Sigma-Aldrich; Merck KGaA) by boiling and then subjected to 10%
SDS-PAGE, followed by electrotransfer onto a nitrocellulose
membrane. An anti-BST-2 monoclonal antibody (catalog no.
H00000684-B02P) was purchased from Abnova (Taipei, Taiwan)
(4). Peroxidase-conjugated anti-mouse
immunoglobulin G was used as the secondary antibody (4). Immunocomplexes were visualized with the
Amersham ECL Western Blotting Detection kit (GE Healthcare Life
Sciences). β-Actin (catalog no. A5441; Sigma-Aldrich; Merck KGaA)
was also stained as a loading control (4).
Statistical methods
Associations between clinicopathological parameters
and BST-2 expression were analyzed by the Fisher's exact test.
Kaplan-Meier survival curves were constructed for BST-2-positive
and -negative patients. Survival rates were compared between
BST-2-positive and -negative groups. Differences between survival
curves were evaluated for statistical significance by the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference. SPSS version 8.0 software was used for
these analyses (SPSS Inc., Chicago, IL, USA).
Results
Expression of BST2 in UC
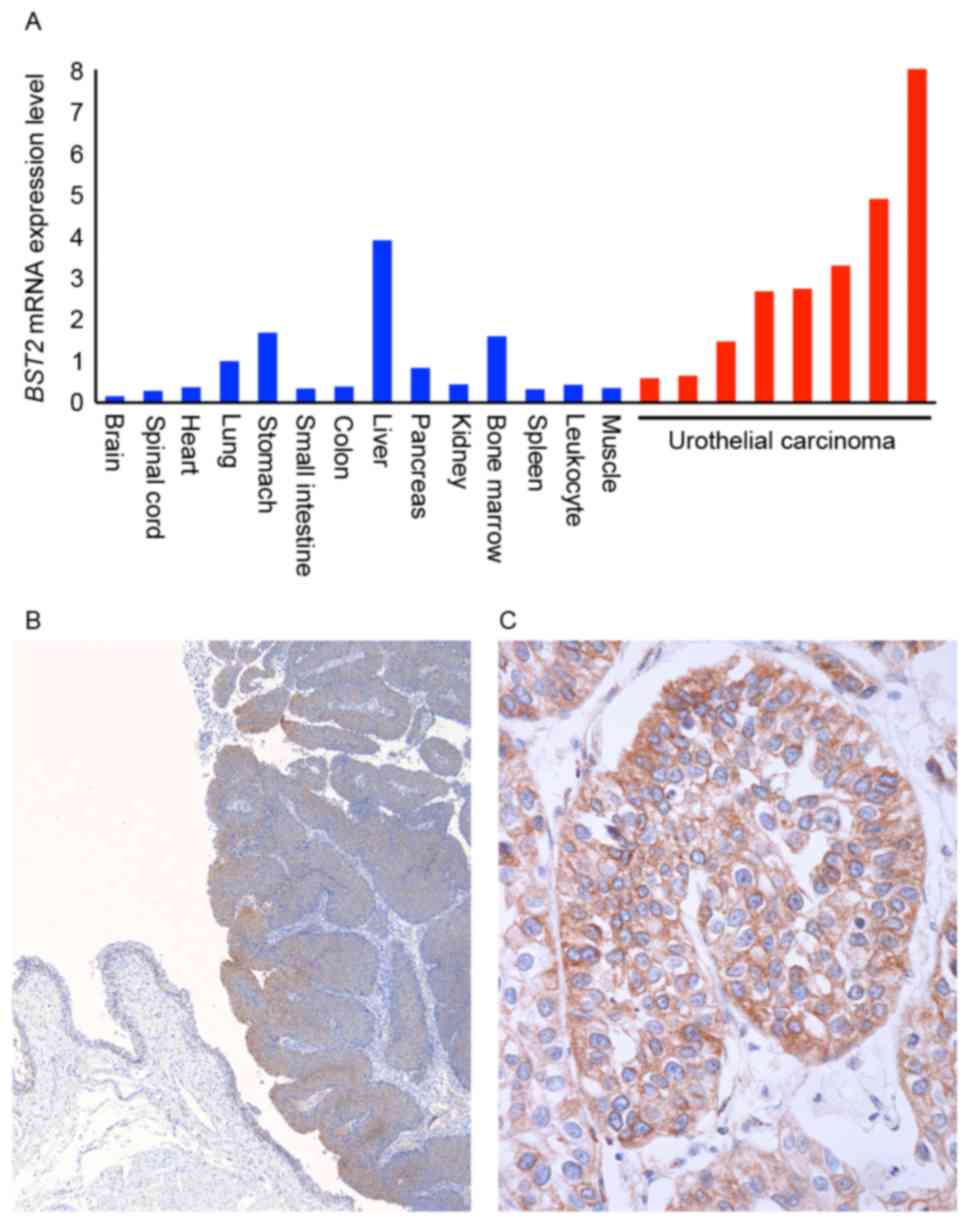
RT-qPCR analysis of BST2 was first performed
in 14 types of normal tissue samples and 8 UC tissue samples
(Fig. 1A). All 8 UC tissue samples
were randomly selected. Among the various normal tissue samples,
abundant BST2 mRNA expression was detected in normal lung,
stomach, liver and bone marrow samples. The expression of
BST2 in these normal tissue samples was highest in the
liver. However, expression of BST2 in UC tissue samples was
higher than in normal liver.
Next, immunohistochemistry was performed on 69 UC
tissue samples. In non-neoplastic mucosa, weak or no staining of
BST-2 was observed in urothelial and stromal cells, whereas UC
tissue exhibited stronger and more extensive staining compared with
non-neoplastic mucosa (Fig. 1B).
BST-2 staining was observed mainly on UC cell membranes (Fig. 1C). Numerous UC cases displayed
heterogeneity of BST-2 staining, and the percentage of
BST-2-stained UC cells ranged from 0 to 70%. A tendency for
upregulation of BST-2 was not observed at the invasive front. In
total, 28 (41%) of 69 UC cases were positive for BST-2
expression.
Next, the association of BST-2 staining with
clinicopathological characteristics was examined (Table I). UC cases positive for BST-2 were
more frequently T2/3/4 cases (so-called MIBC) than Ta/is/1 cases
(P=0.0001). However, Kaplan-Meier analysis demonstrated no
association between BST-2 expression and survival (P=0.4602) (data
not shown).
 | Table I.Association between BST-2 expression
and clinicopathological characteristics in bladder cancer. |
Table I.
Association between BST-2 expression
and clinicopathological characteristics in bladder cancer.
|
| BST-2 expression, n
(%) |
|
|---|
|
|
|
|
|---|
| Characteristic | Positive | Negative | P-value |
|---|
| Age, years |
|
|
|
| ≤70 | 16 (40) | 24 (60) | 0.9083 |
|
>70 | 12 (41) | 17 (59) |
|
| Sex |
|
|
|
| Male | 23 (40) | 34 (60) | 0.9327 |
|
Female | 5 (42) | 7 (58) |
|
| T classification |
|
|
|
|
Ta/is/1 | 6 (18) | 28 (82) | 0.0001 |
|
T2/3/4 | 22 (63) | 13 (37) |
|
| Cellular atypism
classification |
|
|
|
| Low
grade | 9 (30) | 21 (70) | 0.1164 |
| High
grade | 19 (49) | 20 (51) |
|
| Lymphatic
invasion |
|
|
|
|
Positive | 13 (46) | 15 (54) | 0.8470 |
|
Negative | 13 (35) | 24 (65) |
|
| Vascular
invasion |
|
|
|
|
Positive | 9 (60) | 6 (40) | 0.0714 |
|
Negative | 17 (34) | 33 (66) |
|
Effect of BST2 inhibition on cell
growth
Previously, it was demonstrated that inhibition of
BST2 by siRNA reduces cell growth, and that the levels of
phosphorylated epidermal growth factor receptor (EGFR), Akt and
extracellular signal-regulated kinase (Erk) are lower in
BST2 siRNA-transfected gastric cancer cells than in control
cells (4). However, the biological
function of BST-2 has not been investigated in UC cells thus far.
Therefore, the present study examined the effect of BST2
inhibition on cell growth using two different siRNA sequences
(catalog nos. 251993co3 and 251993co5; Invitrogen; Thermo Fisher
Scientific, Inc.). T24 UC cells were selected, which express
detectable levels of BST-2 protein (Fig.
2A). BST-2 expression was substantially suppressed by treatment
with siRNA1 and siRNA2, as shown by western blotting (Fig. 2B). Next, cell growth was analyzed by
MTT assays, which revealed that BST2 siRNA1- and
siRNA2-transfected T24 cells exhibited significantly reduced cell
growth relative to negative control siRNA-transfected T24 cells
(Fig. 2B).
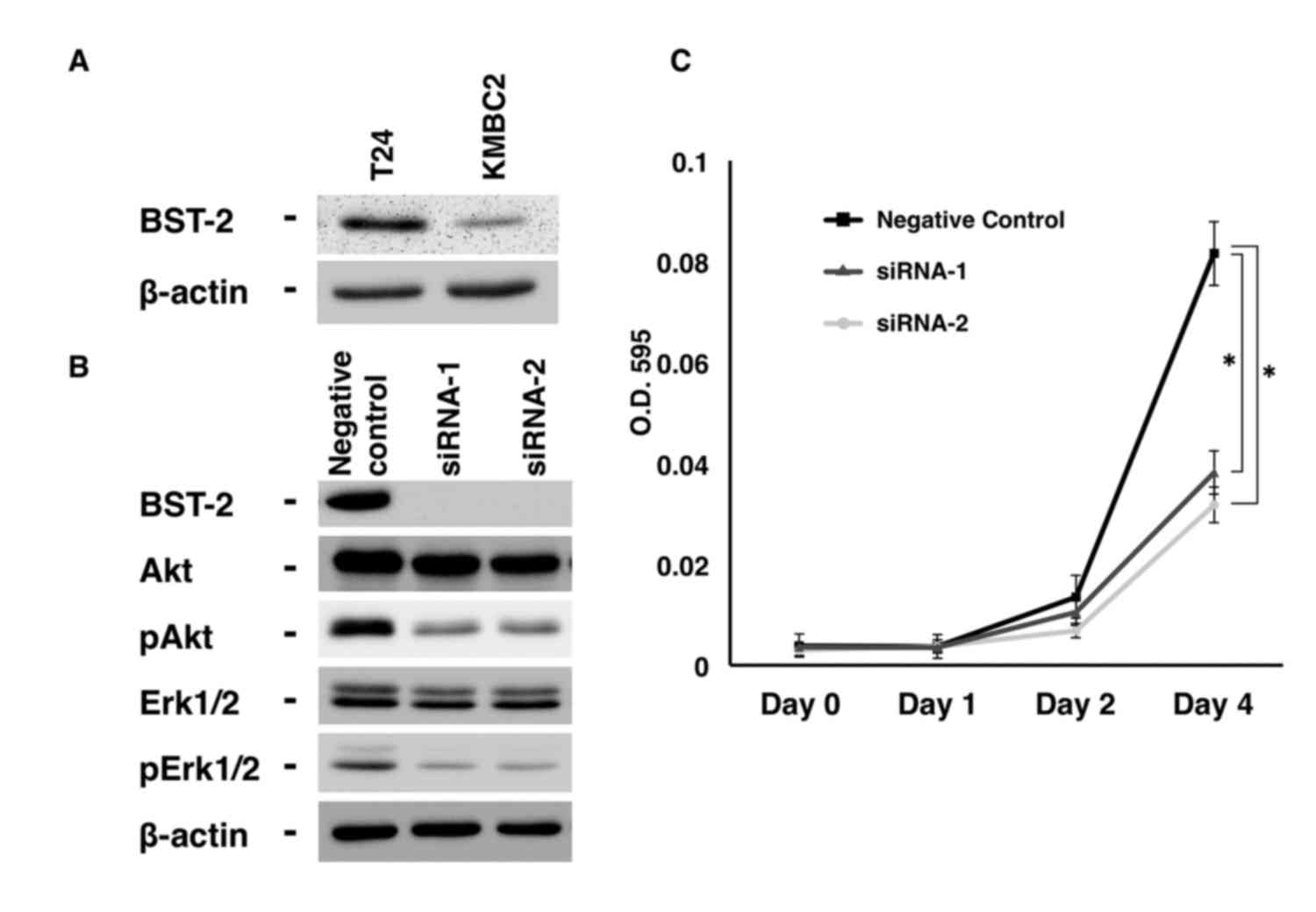 | Figure 2.Effect of BST2 inhibition in T24
urothelial carcinoma cells. (A) Western blot analysis of BST-2,
Akt, pAkt, Erk1/2 and pErk1/2 in lysates of T24 cells transfected
with BST2 siRNA or negative control siRNA. β-Actin was used as a
loading control. (B) Effect of BST2 knockdown on the growth of T24
cells. Cell viability was assessed by MTT assays at days 1, 2 and 4
after seeding on 96-well plates. Bars and error bars indicate the
mean and standard error, respectively, of three independent
experiments. siRNA, small interfering RNA; p, phosphorylated; Erk,
extracellular signal-regulated kinase; BST2, bone marrow stromal
cell antigen 2; O.D., optical density. *P<0.001. |
Since EGFR activates the Ras-mitogen-activated
protein kinase kinase-Erk and
Akt-phosphatidylinositol-4,5-bisphosphate 3-kinase signaling
pathways, thus leading to cancer cell proliferation and survival
(18), the effect of BST2
inhibition on EGFR signaling was analyzed in the present study. The
results indicated that the levels of phosphorylated Akt and Erk
were lower in BST2 siRNA1- and siRNA2-transfected T24 cells
than in control cells (Fig. 2A).
Effect of forced expression of BST2 on
cell growth
To further investigate the biological significance
of BST-2, the KMBC2 UC cell line was stably transfected with a
vector expressing BST2. KMBC2 cells were selected due to
their low expression levels of BST-2 compared with T24 cells
(Fig. 2A). Clones were selected with
G418 and examined for BST-2 expression by western blotting. Two
clones, KMBC2-BST2-1 and KMBC2-BST2-2, expressed BST-2 protein at
significantly higher levels than KMBC2 cells transfected with the
empty vector (Fig. 3A). To determine
the effect of BST-2 on cell growth, MTT assays were performed.
BST2-transfected KMBC2 cells exhibited significantly
increased cell growth relative to empty vector-transfected KMBC2
cells (Fig. 3B). The effect of
BST2 overexpression on EGFR signaling was also analyzed. The
levels of phosphorylated Akt and Erk were higher in
BST2-transfected KMBC2 cells compared with empty
vector-transfected KMBC2 cells (Fig.
3A).
 | Figure 3.Effect of BST2 overexpression in KMBC2
urothelial carcinoma cells. (A) Western blot analysis of BST-2,
Akt, pAkt, Erk1/2 and pErk1/2 in lysates of BST2-transfected and
empty vector-transfected KMBC2 cells. β-Actin was used as a loading
control. (B) Effect of BST-2 on the growth of KMBC2 cells. Cell
viability was assessed by MTT assays at days 1, 2 and 4 after
seeding on 96-well plates. Bars and error bars indicate the mean
and standard error, respectively, of three independent experiments.
Erk, extracellular signal-regulated kinase; p, phosphorylated;
BST2, bone marrow stromal cell antigen 2; O.D., optical density.
*P<0.001. |
Discussion
Platinum-based chemotherapy is currently the
standard treatment in previously untreated patients with metastatic
UC and is associated with a median overall survival of 15 months
(19). The prognosis for patients who
relapse following platinum-based chemotherapy is poor, with median
survival times ranging from 5 to 7 months, and no known
life-prolonging treatments are available (20). Therefore, a novel therapeutic target
is required for UC. In the present study, the expression of BST-2,
a lipid raft-associated type II transmembrane glycoprotein, was
analyzed in UC. Among the various normal tissue samples examined,
expression of BST2 was the highest in the liver. However,
expression of BST2 in UC tissue samples was higher than in
normal liver, suggesting that BST-2 is a therapeutic target with
fewer adverse effects compared with other anticancer drugs for
various cancer types, including UC. Immunohistochemical analysis
revealed BST-2 expression on the cell membrane, and 41% of UC cases
were positive for BST-2. UC cases positive for BST-2 were more
frequently T2/3/4 cases than Ta/is/1 cases. Taken together, these
results suggest that BST-2 serves an important role in the
pathogenesis of UC and is a good therapeutic target for T2/3/4 UC
(also known as MIBC) cases.
Previously, our group reported that 36% of gastric
cancer cases, 46% of colorectal cancer cases and 27% of esophageal
cancer cases were positive for BST-2 (4). Furthermore, high levels of BST-2 have
been reported in ovarian cancer (8),
neoplastic B cells (6), breast cancer
(9), endometrial cancer (10) and lung cancer (11), indicating that BST-2 expression is a
common event in human malignancies. Ozaki et al (7) reported that a monoclonal antibody
against BST-2 induces antibody-dependent cellular cytotoxicity, and
that immunotherapy using this anti-BST-2 antibody reduces tumor
size and improves survival in a multiple myeloma mouse model.
Therefore, immunotherapy using a similar anti-BST-2 antibody may
also improve the survival of UC patients.
In the present study, BST2 siRNA-transfected
T24 UC cells exhibited significantly reduced cell growth relative
to negative control siRNA-transfected T24 UC cells in MTT assays.
Furthermore, BST2-transfected KMBC2 UC cells displayed
significantly increased cell growth relative to empty
vector-transfected KMBC2 UC cells in MTT assays. The levels of
phosphorylated Akt and Erk were lower in BST2
siRNA-transfected T24 UC cells than in control cells. It was also
demonstrated that the levels of phosphorylated Akt and Erk were
higher in BST2-transfected KMBC2 UC cells than in empty
vector-transfected KMBC2 UC cells. Since the phosphorylation of Akt
and Erk inhibits apoptosis (21),
these results suggest that apoptosis could be induced in
BST2-transfected KMBC2 UC cells. EGFR overexpression in UC
is correlated with high tumor grade, muscle invasiveness, tumor
recurrence and overall survival (22). Although the underlying mechanisms
remain unclear, it is possible that a monoclonal antibody against
BST-2 could inhibit EGFR signaling and induce apoptosis in UC
cells. The safety and efficacy of a humanized anti-BST-2 antibody
has been investigated in a phase I/II clinical study on patients
with relapsed or refractory multiple myeloma and has shown mild and
manageable side effects (23). Thus,
the efficacy of this humanized anti-BST-2 antibody should be
examined in a clinical study on patients with metastatic UC or
those who relapse following platinum-based chemotherapy.
In summary, the present study demonstrated
overexpression of BST-2 in UC, particularly in T2/3/4 UC (MIBC)
cases. Since a humanized anti-BST-2 antibody is currently
available, the efficacy of such antibody should be examined in a
clinical study. Although lower levels of phosphorylated Akt and Erk
were detected in BST2 siRNA-transfected UC cells than in
control cells, the underlying mechanisms remain unclear.
Identification of BST-2 signaling will further improve the
understanding of the basic biology of BST-2.
Acknowledgements
The authors thank Mr. Shinichi Norimura (Hiroshima
University, Hiroshima, Japan) for his excellent technical
assistance and advice. The present study was supported by
Grants-in-Aid for Scientific Research (B-15H04713) and Challenging
Exploratory Research (grant nos. 26670175 and 16K15247) from the
Japan Society for the Promotion of Science. The present study was
also supported by the Takeda Science Foundation.
References
|
1
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knollman H, Godwin JL, Jain R, Wong YN,
Plimack ER and Geynisman DM: Muscle-invasive urothelial bladder
cancer: An update on systemic therapy. Ther Adv Urol. 7:312–330.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson DA, Muenster MR, Zang Q, Spencer
JA, Schageman JJ, Lian Y, Garner HR, Gaynor RB, Huff JW,
Pertsemlidis A, et al: Selective identification of secreted and
transmembrane breast cancer markers using Escherichia coli
ampicillin secretion trap. Cancer Res. 65:8209–8217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukai S, Oue N, Oshima T, Mukai R,
Tatsumoto Y, Sakamoto N, Sentani K, Tanabe K, Egi H, Hinoi T, et
al: Overexpression of Transmembrane Protein BST2 is Associated with
Poor Survival of Patients with Esophageal, Gastric, or Colorectal
Cancer. Ann Surg Oncol. 24:594–602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kupzig S, Korolchuk V, Rollason R, Sugden
A, Wilde A and Banting G: Bst-2/HM1.24 is a raft-associated apical
membrane protein with an unusual topology. Traffic. 4:694–709.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goto T, Kennel SJ, Abe M, Takishita M,
Kosaka M, Solomon A and Saito S: A novel membrane antigen
selectively expressed on terminally differentiated human B cells.
Blood. 84:1922–1930. 1994.PubMed/NCBI
|
|
7
|
Ozaki S, Kosaka M, Wakatsuki S, Abe M,
Koishihara Y and Matsumoto T: Immunotherapy of multiple myeloma
with a monoclonal antibody directed against a plasma cell-specific
antigen, HM1.24. Blood. 90:3179–3186. 1997.PubMed/NCBI
|
|
8
|
Walter-Yohrling J, Cao X, Callahan M,
Weber W, Morgenbesser S, Madden SL, Wang C and Teicher BA:
Identification of genes expressed in malignant cells that promote
invasion. Cancer Res. 63:8939–8947. 2003.PubMed/NCBI
|
|
9
|
Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W
and Yuan Z: Up-regulation of bone marrow stromal protein 2 (BST2)
in breast cancer with bone metastasis. BMC Cancer. 9:1022009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yokoyama T, Enomoto T, Serada S, Morimoto
A, Matsuzaki S, Ueda Y, Yoshino K, Fujita M, Kyo S, Iwahori K, et
al: Plasma membrane proteomics identifies bone marrow stromal
antigen 2 as a potential therapeutic target in endometrial cancer.
Int J Cancer. 132:472–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Nishioka Y, Ozaki S, Jalili A, Abe
S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T and Sone S:
HM1.24 (CD317) is a novel target against lung cancer for
immunotherapy using anti-HM1.24 antibody. Cancer Immunol
Immunother. 58:967–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM classification of malignant tumours. 7th. New York:
Wiley-Liss; pp. 262–265. 2009
|
|
13
|
Kondo T, Oue N, Yoshida K, Mitani Y, Naka
K, Nakayama H and Yasui W: Expression of POT1 is associated with
tumor stage and telomere length in gastric carcinoma. Cancer Res.
64:523–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oue N, Naito Y, Hayashi T, Takigahira M,
Kawano-Nagatsuma A, Sentani K, Sakamoto N, Oo H Zarni, Uraoka N,
Yanagihara K, et al: Signal peptidase complex 18, encoded by
SEC11A, contributes to progression via TGF-α secretion in gastric
cancer. Oncogene. 33:3918–3926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto N, Oue N, Sentani K, Anami K,
Uraoka N, Naito Y, Oo HZ, Hinoi T, Ohdan H, Yanagihara K, et al:
Liver-intestine cadherin induction by epidermal growth factor
receptor is associated with intestinal differentiation of gastric
cancer. Cancer Sci. 103:1744–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasui W, Ayhan A, Kitadai Y, Nishimura K,
Yokozaki H, Ito H and Tahara E: Increased expression of p34cdc2 and
its kinase activity in human gastric and colonic carcinomas. Int J
Cancer. 53:36–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi K and Ito F: EGF receptor in
relation to tumor development: Molecular basis of responsiveness of
cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J.
277:316–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhury NJ, Campanile A, Antic T, Yap
KL, Fitzpatrick CA, Wade JL III, Karrison T, Stadler WM, Nakamura Y
and O'Donnell PH: Afatinib activity in platinum-refractory
metastatic urothelial carcinoma in patients With ERBB alterations.
J Clin Oncol. 34:2165–2171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada T and Ozaki S: Targeted therapy for
HM1.24 (CD317) on multiple myeloma cells. Biomed Res Int.
2014:9653842014. View Article : Google Scholar : PubMed/NCBI
|