Introduction
Worldwide, the incidence and mortality rate of
cancer are on the increase, with an increasing number of young
cancer patients. Early detection, early diagnosis and early
treatment is the key to reducing the mortality rate. Application of
many new technologies significantly increases the accuracy of early
detection and diagnosis of lesions (1). Currently, exploration of the cause,
development mechanisms and laws of the tumor has been elevated to
the molecular and genetic level. Oncology imaging study has
developed from simple anatomic description to functional imaging,
molecular biology and pathophysiological development, and combined
with biological markers or gene technology, can detect and
determine the characteristics of the disease at the cell and
molecular level (2).
Luteinizing hormone-releasing hormone (LHRH) is a
decapeptide hormone, which is secreted by the hypothalamus arcuate
nucleus neurons and transported by axoplasm flow of
nodule-pituitary axis to nerve endings at the uplift, and then by
hypophysical portal veins, it flows into the anterior pituitary
gland with blood to stimulate the pituitary cells to secrete
luteinizing hormone and follicle-stimulating hormone for regulating
anterior pituitary gonadotropin secretion, which in turn stimulates
the secretion of luteinizing hormone and follicle-stimulating
hormone to regulate the level of gender hormones, in order to act
on the reproductive axis and regulate the formation of gametes and
gonadal endocrine (3,4). It must be combined with high affinity
transmembrane receptors to play its role, and these receptors
belong to seven transmembrane receptor families (5–8). LHRH and
its receptor are also found in the central nervous system (CNS) and
peripheral tissues, indicating that in addition to the release of
gonadotropin, this kind of decapeptide hormone also has other
functions. It has been previously reported that LHRH is generated
outside the hypothalamic-pituitary axis and plays specific
biological effects outside the hypothalamic-pituitary axis
(9). Previous findings have shown
LNRH binding sites on tumor cells of ovary, breast, and
endometrium, prostate and pituitary, and a high expression of
LHRH-R on the tumor cells of the pancreas, lung and liver (10–17).
Expression of LHRH-R in the corresponding normal tissue is
relatively low. Emons et al first proved that approximately
80% of ovarian cancer tissues possess binding sites of specificity
LHRH, LHRH agonist or antagonist analogues, which indicated the
existence of the high affinity LHRH receptor in human ovarian
cancer (7). Some LHRH agonists have
been used in the treatment of ovarian cancer. Eidne et al
first proved the existence of LHRH binding sites on human breast
cancer cells (18). Subsequent
literature reported the MTX mouse breast cancer cell nuclei has
LHRH-R distribution (19). Human
breast cancer cell lines MDA2MB2231 and ZR27521 cell extracts
cultured in vitro showed LHRH immunoreactive (20). Rusiecki et al observed LHRH
receptor expression in breast cancer and tissues adjacent to cancer
and the relationship between LHRH receptors, estrogen (ER), and
progesterone receptor (PgR). The study selected pathological tissue
of 90 cases of breast cancer patients, and randomly selected 40
cases of normal tissue adjacent to normal tissue (45 vs. 39%), the
results showed that LHRH-R expression is related to tumor tissue
with positive PgR (21). In addition,
LHRH-R expression of premenopausal breast cancer patients without
menstruation is higher (56 vs. 32%, the latter is the value of
normal humans) (22). Although LHRH
analogues for the treatment of endometrial cancer has a long
history, the study on LHRH and its receptor expression in
endometrial cancer tissue has just started in recent years, and
study of its therapeutic mechanism has been a hotspot over the past
2–3 years. Imai et al found that LHRH stimulation can induce
apoptosis in some human reproductive tract tumor cells mediated by
Fas ligand. The incidence of primary liver cancer has a significant
gender difference (male:female = 9:1 to 7:1), and experimental
study and clinical observations have suggested that liver cancer
may be a hormone-dependent tumor (23). Recently, LHRH-R gene in human
hepatocellular carcinoma (HCC) has been successfully cloned, with
investigators confirming that the complementary DNA libraries of
LHRH and its receptor produced by PCR reaction and human placenta
and pituitary gland LHRH receptor are identical through DNA blot
analysis with endogenous oligonucleotide primers as a probe
(24). This shows that, LHRH and
LHRH-R non-germline mRNAs are widely distributed in normal or
malignant tissues that do not belong to reproductive system
(25,26). The adjacent piece immunohistochemical
double labeling method also shows LHRH in HCC may regulate the
growth and differentiation of liver cancer cells in autocrine
manner (OS). Katsuno et al found the expression of LHRH on
hypophysis growth hormone cell tumors by means of
immunohistochemistry and in situ hybridization (27). Some researchers have applied LHRH to
the clinical treatment of pituitary adenomas, and found that after
a long-term treatment (6 months), pituitary tumor was significantly
reduced. This suggests that LHRH can inhibit the growth of
pituitary tumor cells (28). The
above facts show that normal human pituitary and pituitary tumor
cells can express LHRH and its receptor, and that LHRH in pituitary
tumor cells may act as an autocrine or paracrine regulator to
fulfill its function (28,29). Among tumors derived from non-gonadal
axis, pancreatic cancer is a malignant tumor relatively closely
connected to LHRH. It has been demonstrated that there are LHRH-R
distribution on hamster pancreatic cancer tissue induced by
N-dinitroso dioxopropyl two amines (BOP) and human pancreatic
tissue through autopsy; and no LHRH binding sites was detected on
normal hamster and human pancreatic cancer tissue (30). Szepeshazi et al (31) applied bombesin gastrin-releasing
peptide (GRP) agonist RC23095, growth hormone releasing
somatostatin analogues RC2160 or LHRH agonists cetrorelix to
hamsters induced by nitrite, 8 weeks later, they discovered that
epidermal growth factor (EGF) expression in carcinoma tissue
decreased by 71 and 69%, respectively. This indicates that EGF may
be involved in the adjustment of inhibitory effect LHRH exerts on
pancreatic tumor cell growth (32,33).
The identification of LHRH analogs (LHRH-a),
activator (LHRHag), and antagonist (LHRHanta) has promoted the
study on physiological function of LHRH and its receptor. LHRH is a
neurotransmitter in the CNS and the sympathetic nervous system; a
paracrine regulator in the gonads and placenta; an autocrine
regulator in some tumor cells (19,34,35). Human
LHRH-R is composed of 328 amino acids and 7 transmembrane domains,
which belongs to G protein-coupled receptors. Junction station of
LHRH-R and LHRH in mammalian pituitary gonadal cells has high
specificity (36). Photoaffinity
labeling, and chromatographical electrophoresis have proved that
LHRH-R is a glycoprotein of M-6000, and has 3 N-glycosylation
sites, the possible interaction between acidic amino acids at 90,
98 and 29 and arginine in LHRH enables LHRH to play its physiologic
adjustment function (37). LHRH-R can
also be generated and expressed in hypothalamic-pituitary axis, for
example, LHRH-R mRNA expression can be found on human ovarian
granulosa, luteal cells, testicular interstitial cells, breast
tissue, prostate and rat gastrointestinal epithelium and glandular
epithelial cells, suggesting that these LHRH target organs can
synthesize LHRH-R by themselves. The presence of LHRH or analogue
activates LHRH-R to perform different functions by G
protein-coupled phosphoinositide pathway and mediated calcium
mobilization, for instance, LHRH in the digestive tract and
circular blood may be a sort of gastrointestinal hormone, which can
not only play an indirect regulation through the vagus nerve on
functions of gastrointestinal system, but also plays a direct role
in the regulation of the digestive system through the binding with
LHRH-R on mucosal epithelium and epithelial cells (38–40).
Studies over the past decade also found that, LHRH
and its receptors are involved in the occurrence and development of
some tumors, especially certain tumors with the non-gonadal axis
organ origin. For example, LHRH-R also exist in lung cancer, kidney
cancer and liver cancer cells. Previous findings showed that LHRH-R
expression in gland cancer cells may be a common phenomenon
(41). Additionally, it was
identified that, LHRH and its receptor inhibits proliferation of
cancer cells, whose expression is closely related to
differentiation degree of cancer tissue, i.e., the higher the
differentiation degree, the higher the expression of LHRH and its
receptors, whereas, the poorer the differentiation degree, the
lower the LHRH and its receptor expression (42).
There is evidence showing that LHRH can directly
inhibit the proliferation of certain hormone-sensitive tumors, and
although it is known that such physiological stimuli leading to
cell death is firstly achieved by apoptosis, its antitumor
molecular mechanism remains unclear. Some scholars believe, LHRH
inhibitory effect on tumor is realized by a mechanism independent
of the release of pituitary gonadotropin. Nevertheless, antitumor
effect and its targeted therapeutic value of LHRH and its analogues
have been widely demonstrated in clinical applications. Studies
have reported that the treatment of malignant tumors with high
expression of corresponding receptors by combining LHRH targeting
with biological toxins or nanoparticle technology has been used in
clinical application (32,33).
High expression of a variety of aforementioned
pathological conditions provides a basis for using LHRH receptor
for imaging. However, studies on LHRH receptor imaging applied to
cancer diagnosis are scarce. There is some literature on the
characteristics of labeling method and labeled product of
radionuclide-labeled LHRH of 68Ga, 123I,
18F, 99mTc, some labeled products have
entered the pharmacological evaluation stage; there is other
literature examining the possibility of the corresponding labeled
product serving as developing agent in SPECT, PET imaging, some
findings suggested that LHRH would have a broad prospecting
receptor imaging of malignant tumors with high corresponding
receptor expression (34). However,
most such studies still remain at the stage of labeling conditions
and involve no further study of malignant tumor cells with high
corresponding receptor expression.
Application basis of LHRH receptor imaging in cancer
diagnosis lies in the number of expression of tumor cell LHRH-R, as
well as specificity level of its combination with LHRH. This study
employs immunohistochemical method to detect LHRH-R expression
level in tumor cells and normal tissues; pre-tinning to directly
label LHRH, measuring and calculating labeling rate and
radiochemical purity of labeled product, observing its stability
in vivo and in vitro, and selecting labeling method
and its optimal condition with higher radiochemical purity and
sound stability through comparative analysis. Normal mice were used
for live animal experiment to detect the expression levels in
tissues and organs of normal live animals as well as metabolism of
radio-labeled LHRH in vivo of live animals. The value of
LHRH-R expression in tumor cells and LHRH receptor imaging in
targeting diagnosis of tumors provided theoretical and experimental
basis for further study.
Materials and methods
LHRH-R expression in tumor and normal
tissue
Specimen collection
Paraffin specimen of related tumors were collected.
Criteria of inclusion were as follows: i) Tissue blocks were fixed
in 10% formalin and embedded in paraffin in a routine manner, and
were sliced into several sections at the thickness of 4 µm; ii)
none of the patients had received preoperative radiotherapy and
chemotherapy; iii) surgical treatment were radical resection and
tumor-free margins exist; and iv) the pathological diagnosis
complied with classification criteria of WHO lung cancer histology
in 1998, which did not include small cell lung cancer, carcinoid
tumors and metastatic cancer. Qualified cancer tissue samples in
the group were 93 cases, including 10 cases of prostate cancer, 20
cases of breast cancer, 20 cases of endometrial cancer, 23 cases of
liver cancer and 20 cases of lung cancer.
Treatment of specimens
Treatment of slides
Fresh slides were immersed in cleaning solution for
24 h, rinsed with clean water for 2 h, washed 3 times with
distilled water, and place in oven at 37°C after clean rinsing,
soaked in 1:10 poly-L-lysine for approximately 10 sec, drained and
placed in oven at 37°C. All the specimens were confirmed by
pathology.
Treatment of specimen slices
The specimens were fixed in 10% formalin and
embedded in paraffin, and were sliced into several pieces at 4 µm,
and placed at 37°C incubator for 2 h for standby application.
Control setting
With phosphate-buffered saline (PBS) substituting
primary antibody as negative control, the remaining steps were
unchanged.
Immunohistochemical staining procedure
Paraffin sections were dewaxed in xylene and
hydrated with graded alcohol, and then were washed with PBS (pH
7.4) 3 times, each time for 3 min. The sections were placed in
freshly-prepared boiled (pH 6.0) citrate buffer for antigen
retrieval. To each slice was added a drop of 3% hydrogen peroxide
solution, and incubated at room temperature for 15 min, so as to
block endogenous peroxidase and was washed with PBS 3 times, each
time for 3 min.
After tossing PBS solution, to each slice was added
with a drop of primary antibody (1:200 dilution), overnight at 4°C.
Blank control used primary antibody in place of PBS. The slice was
washed with PBS 3 times, each time for 5 min. After removing PBS
solution, to each slice was added a drop of polymer enhancer
(reagent A), and incubated at room temperature for 20 min. PBS
rinsing followed 3 times, each time for 3 min.
After removing PBS solution, each slice was added a
drop of anti-rabbit HRP polymer (reagent B), and incubated at room
temperature for 30 min. PBS rinsing was 3 times, each time for 3
min.
After removing PBS solution, each slice was added
with a drop of freshly-prepared DAB coloration liquid, and observed
under the microscope for 3 to 5 min, positive coloration is brown,
using tap water for rinsing to terminate the coloration.
The slices were rinsed with distilled water and
redyed with hematoxylin using 0.1% hydrochloric acid to
differentiate and tap water to rinse the slices, and then stained
blue. Employing graded alcohol to dehydrate, xylene for
transparency, and mounting with neutral gum. Elivision reagent kit
contained the reagents A and B.
Immunohistochemical results
LHRH-R positive coloration occurred mainly on cancer
cell cytoplasm taking on brown particles. Immunohistochemistry
using semi-quantitative scoring method for determination, i.e., the
overall score was calculated according to staining intensity and
the percentage of number of positive cells in total number of tumor
cells.
Rating according to the degree of positive staining:
0, no coloring, consistent with the background color; 1, pale
yellow, slightly higher than the background color; 2, claybank,
significantly higher than the background color; and 3, brown.
Rating according to the percentage of positive
cells: 0, negative; 1, <10%; 2, 11–50%; 3, 51–75%; and 4,
>75%.
The product of the two was calculated to determine
the positive result: 0–2 indicated negative (−); 3–4 indicated weak
positive (+); 5–8 indicated moderately positive (++); and 9–12
indicated strong positive (+++).
Using pre-tinning 99mTc to
directly label LHRH
Labeling method
After adding 0.1 ml of sodium gluconate (0.3 mol/l,
dissolved by PBS buffer), 0.05 ml of stannous chloride that was
dissolved by concentrated hydrochloric acid (40 mg/ml), 0.1 ml of
99mTcO4− physiological saline
eluent, and 0.1 ml of PBS buffer into the reaction tube, it was
placed at room temperature for 10 min after being mixed in vortex
mixer. Na2OH was used to set pH at 3.0, and 10 µg LHRH
was added. Placing it in a constant temperature water bath at 40°C
for 1 h. The labeling was completed.
Changing labeling conditions
i) Labeling under the circumstances of using sodium
gluconate as the complexing agent and without the complexing agent,
respectively; ii) dosage of stannous chloride and the pH of the
reaction system were changed, respectively, changing, dosage of
SnCl2 increased from 20 to 60 mg/ml, pH of the reaction
increased from 2.0 to 4.0; and iii) the reaction temperature
increased from 25 to 50°C.
Stability in vitro
99mTc-LHRH was added to fresh saline and
normal human plasma (37°C), respectively, and mixed, and then
radiochemical purity was measured at 30 min, 1 h; 90 min, 2, 3 and
4 h.
Determination of radiochemical purity
No. 1 Xinhua chromatography paper was used as
stationary phase, and saline as mobile phase to measure
radiochemical purity (development system I); no. 1 Xinhua
chromatography paper soaked in 2.5% bovine serum albumin (BSA) was
adopted as stationary phase, and ethanol developing
solvent:ammonia:water = 2:1:5 to measure colloid (development
system II), and GC-300γ counter was employed to measure
radioactivity.
Result criteria
In development system I, the free 99mTc
went to the leading edge with the expansion of solvent (Rf = 1.0),
while the colloid and marker remained at the original point (Rf =
0); in eluent II, the colloid was maintained at the origin (Rf =
0), while the free 99mTc and markers migrated to the
forefront along with the solvent (Rf = 1.0); eluent I and II system
could completely distinguish 99mTc-LHRH, colloids and
free 99mTc in this study. The finally obtained
radiochemical purity of 99mTc-LHRH exceeded 90%, and
colloid content was <10% indicating successful labeling.
99mTc-LHRH receptor binding
in vitro
Preparation of rat pituitary cell membrane
protein
The whole experiment process was set in an ice bath.
Pituitary was quickly removed after the sacrifice of Sprague-Dawley
(SD) rats, and rinsed in an ice bath of pH 7.4 10 mmol/l Tris-HCl
(containing 0.5 mmol/l PMSF, adding PMSF, 1.2 mmol/l
MgCl2, 0.01 mmol/l EDTA-Na2 before using)
twice to remove blood.
The pituitary was set inside the electric glass
homogenizer for homogenizing after the rinsing (2,000 rpm, 10 sec ×
3 times), and centrifuged at 4°C (2,000 × g, 5 min). After
discarding the sediment, the supernatant was centrifuged again at
4°C (20,000 × g, 20 min), then supernatant was discarded and the
sediment was rinsed 10 times the volume of Tris-HCl twice (20,000 ×
g, 20 min), thus, the rat pituitary cell membrane protein was
attained. A small amount of Tris-HCl suspension was added. BCA
protein assay kit was employed to measure absorbance values at 562
nm on a microplate reader. According to the standard curve scheme
membrane protein concentration, protein concentration was adjusted
to 2 mg/ml, and placed at −70°C for cryopreservation
separately.
Receptor assay
The 12×75 mm test tube was taken and prepared with
1% BSA (Tris-HCl, pH 7.4 preparation) before using (BSA was added
to the tube, and kept overnight at 4°C, and then BSA was emptied,
and the tube was inverted for standby application).
All the tubes were added with 50 µl of rat pituitary
membrane proteins (100 µg), including sub-unit binding tube (TB)
and nonspecific binding (NSB) tubes, then followed by 2, 5, 10, 20,
30, 40 and 50 µl of 99mTc-LHRH. NSB tubes were added
with 2 µg gonadorelin (prepared with Tris-HCl to ensure there were
much more gonadorelin than 99mTc-LHRH in NSB tube), and
finally the reaction volume was complemented to 150 µl with
Tris-HCl (this buffer was made by adding 0.2% BSA to
protein-extraction buffer, pH 7.4).
The tubes were incubated at 37°C set oscillator for
1 h and were added 1 ml precooled Tris-HCl at 4°C after the
removal. The reaction was quenched by mixing. The left reactant was
collected on glass fiber filter paper 49 with multi-point cell
harvester (ZT-II type), and the reaction tube was added Tris-HCl
and washed 3 times (1 ml/time), and finally 1 ml 5% TCA filter
paper was placed in the test tubes. Measurement of radioactivity of
each tube was counted with γ tube immune counter, as well as TB and
NSB.
Measured results employed SB/FREE as the vertical
axis, the amount of specific binding SB as the abscissa, and
Scatchard plot, equilibrium dissociation constant KD was attained,
SB (dpm) = TB (dpm) - NSB (dpm), unbound 99mTc-LHRH F
(dpm) = added 99mTc-LHRH T (dpm) - total binding TB
(dpm).
LHRH metabolisms in vivo
Preparation of 125I-LHRH
Chloramine-T method to label LHRH
4 mCi 125I/0.4 µl, LHRH 10 µg/10 µl, and
50 µg chloramine-T were added into Eppendorf (EP) tube
successively. The mixed solution was left to react for 90 sec
(during the reaction, the solution was shaken on the vortex mixer),
then 100 µg Na2S2O5 was added and
the solution was mixed on the vortex mixer, finally the reaction
was terminated. The labeling was completed.
Production of Sepahdex G-25 gel column
After the G-25 gel particles were immersed into pure
water for 24 h, they were added into glass acid buret containing
glass fiber at the bottom. After the air was exhausted, using 10
column volumes of PBS buffer to rinse slowly, then 10 ml 1%. The
BSA saturated gel column was added.
Separation and purification of
125I-LHRH
125I-LHRH was added into Sepahdex G-25
gel column, and the reaction tube was washed with PBS 2 times, each
time 100 µl, gel column was also added, and was rinsed slowly with
PBS. Dripping speed was adjusted to 6 drops/min, and leaching
liquid solution was collected (1 ml/tube). Employing paper
chromatography to measure its radiochemical purity, respectively
(no. 1 Xinhua paper chromatography paper was used as stationary
phase and the normal saline as the mobile phase, γ counter was used
to measure radioactivity count). The highest radiochemical purity
was taken for further experiments.
Animal experiments
Forty-five healthy male mice aged ~23 weeks and
weighing 20±2 g were divided into 9 groups according to random
number table, with each group including 5. The 54.1 mCi/100 µl
125I-LHRH was injected into tail vein. A group of mice
was taken, respectively, 15 and 30 min, 1, 2, 4, 6, 24, 48 and 72 h
after the injection. Blood was collected from picked eyeballs.
Using stem dislocation to sacrifice the mice and then they were
dissected, major organs (heart, lungs, liver, spleen, kidney,
muscle, bone, brain, small intestine and stomach) were taken and
weighed. γ counter was used to measure radioactivity count of blood
and organs, and unit mass of tissue percentage radioactivity uptake
dose rate (%, ID/g) was calculated (per gram of tissue
radioactivity count/total radioactivity injected into mice count ×
100%).
Statistical analysis
Data are presented as mean ± standard deviation
(mean ± SD). SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for Fisher's exact test. P<0.05 was considered
to indicate a statistically significant difference.
Results
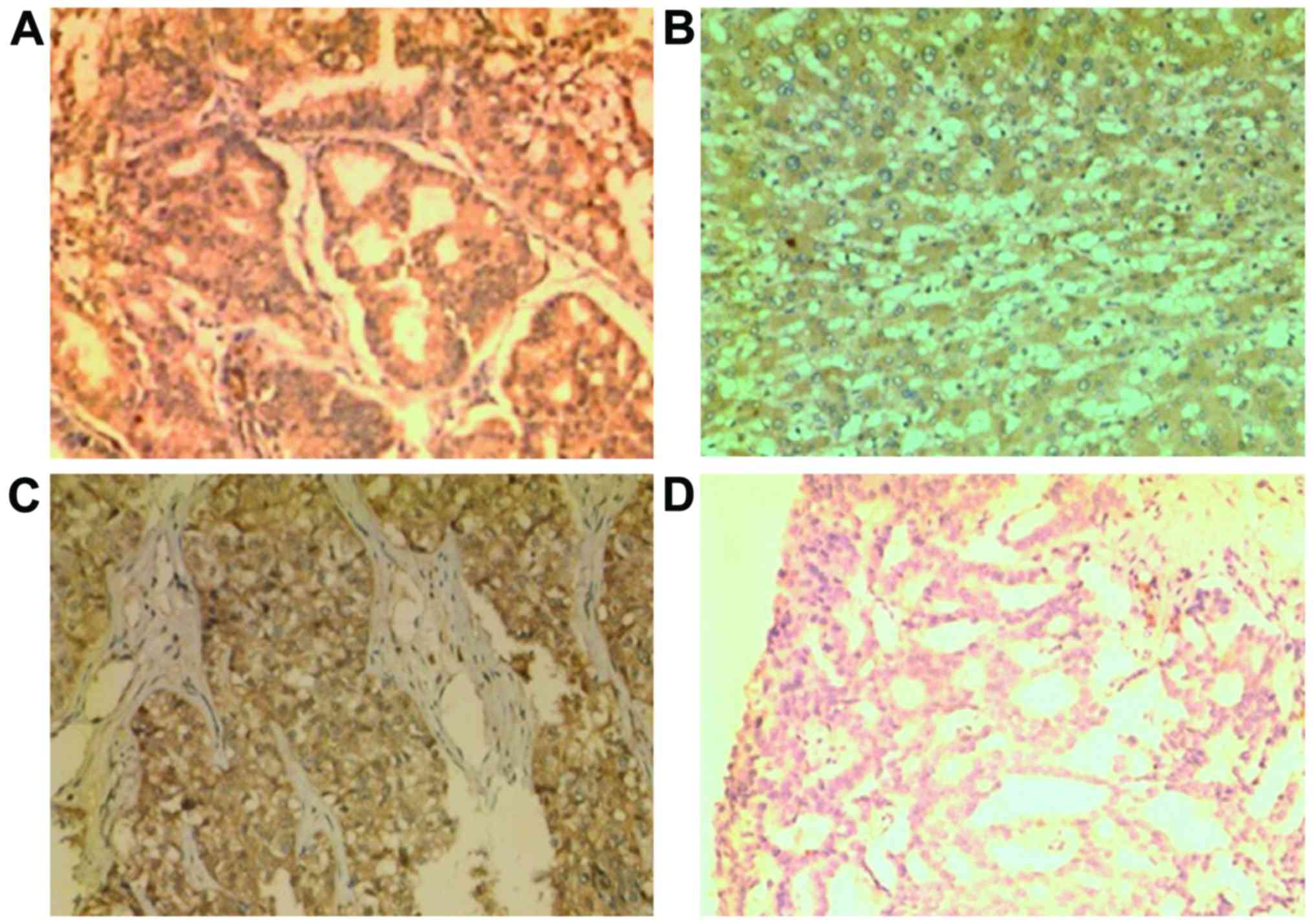
Immunohistochemical staining
Immunohistochemistry of specimens had good stain
specificity. Pale background or blank background. Positive product
of immunoreaction was brown. As contrast was obvious, it was easy
to confirm. Immunohistochemical staining results of LHRH in
different tissues are shown in Table
I and Fig. 1A-H.
 | Table I.The expression of LHRH in
tissues. |
Table I.
The expression of LHRH in
tissues.
| Pattern of
tissues | n | No. of positive
cases | Positive rate
(%) | P-value |
|---|
| Lung cancer | 20 | 17 | 85 | P<0.05 |
| Peritumoral lung
tissue | 19 | 3 | 15.79 |
|
| HCC | 23 | 19 | 82.61 | P<0.05 |
| Adjacent liver
tissue | 20 | 3 | 15 |
|
| Breast cancer | 20 | 19 | 95 | P<0.05 |
| Peritumoral breast
tissue | 20 | 4 | 20 |
|
| Endometrial
cancer | 20 | 16 | 80 | P<0.05 |
| Peritumoral
endometrial tissue | 18 | 3 | 16.7 |
|
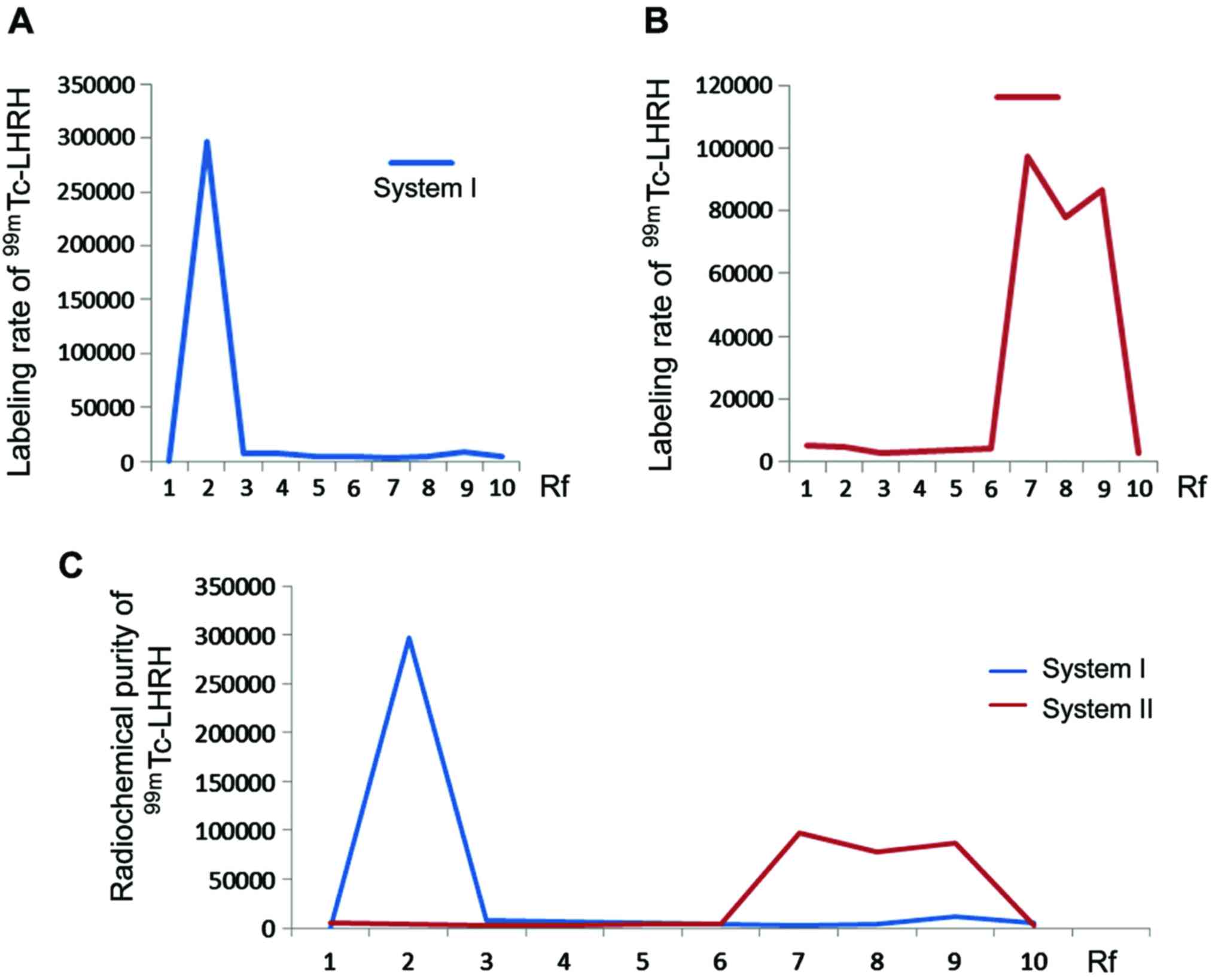
Labeling rate and radiochemical purity
of 99mTc-LHRH
In development system I, the free 99mTc
went to the leading edge with the expansion of solvent (Rf = 1.0),
while the colloid and marker remained at the original point (Rf =
0); in eluent II, the colloid was maintained at the origin (Rf =
0), while the free 99mTc and markers migrated to the
forefront along with the solvent (Rf = 1.0); eluent I and II system
could completely distinguish 99mTc-LHRH, colloids and
free 99mTc in this study. The highest LHRH labeling rate
by employing direct method of no. 1 Xinhua paper chromatographic
hit (98.7±0.93%); the highest radiochemical purity reached
95.2±1.02% (Fig. 2A-C).
Impact of complexing agent
In the case of no complexing agent was used, the
presence of white sediment in the reaction system signified
unsuccessful labeling. With 0.1 ml of sodium gluconate (0.3 mol/l)
as complexing agent, when pH value stood at 3.0, the radiochemical
purity was up to 95.2±1.02%, colloid content in the labeling
reaction was <5%.
Impact of
SnCl2·2H2O dosage and pH value on
radiochemical purity
In case that other labeling conditions (volume of
99mTc eluent, reaction temperature, peptide dosage)
remained the same, SnCl2·2H2O and pH value
were changed, resulting radiochemical purity is shown in Table II.
 | Table II.Impact of
SnCl2·2H2O dosage and pH value on
radiochemical purity (%). |
Table II.
Impact of
SnCl2·2H2O dosage and pH value on
radiochemical purity (%).
|
| pH value |
|---|
|
|
|
|---|
|
SnCl2·2H2O (µg) | 2 | 2.5 | 3 | 3.5 | 4 |
|---|
| 1,500 | 65.1±3.2 | 57.6±1.6 | 58.1±1.3 | 60.4±2.1 | 49.3±1.3 |
| 2,000 | 83.8±0.9 | 83.5±2.7 | 93.9±1.0 | 88.4±0.8 | 72.4±3.7 |
| 2,500 | 75.1±2.4 | 82.8±2.0 | 64.9±4.0 | 60.7±1.1 | # |
Effect of reaction temperature
In case that other labeling conditions (volume of
99mTc eluent, SnCl2·2H2O dosage,
pH value and peptide dosage) remained the same, the reaction
temperature was changed. At 25°C, the radiochemical purity was
32.6±1.9%, at 40°C, it was 91.6±3.5%, at 50°C, it was
57.8±1.9%.
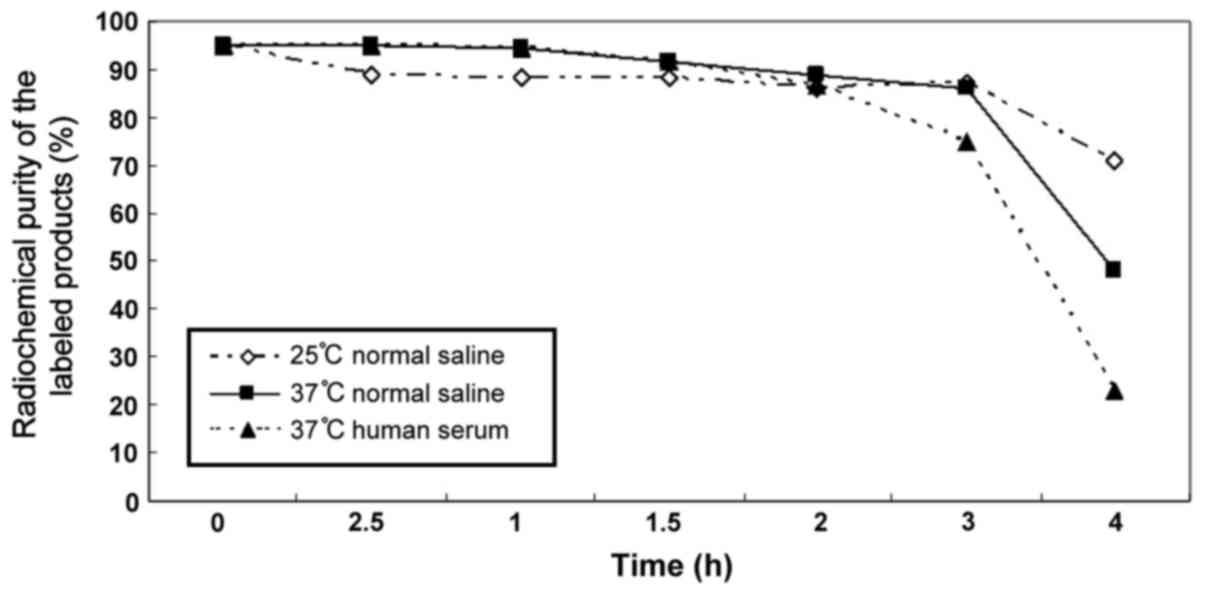
Stability in vitro of labeled
product
Within 3 h, reactants maintained a high
radiochemical purity and declined sharply after 3 h (Table III and Fig. 3).
 | Table III.Stability in vitro of labeled
product. |
Table III.
Stability in vitro of labeled
product.
| Degrees | 0 h | 0.5 h | 1 h | 1.5 h | 2 h | 3 h | 4 h |
|---|
| 25°C saline | 95.2±1.023 | 88.7±2.472 | 88.1±3.556 | 88.1±3.044 | 86.2±2.434 | 87.3±2.055 | 71.2±2.575 |
| 37°C saline | 95.2±1.023 | 95.0±1.466 | 94.2±1.563 | 91.7±0.634 | 89.0±2.677 | 85.8±10.537 | 48.2±2.423 |
| 37°C human
serum | 95.2±1.023 | 95.1±1.359 | 94.4±0.249 | 91.4±1.178 | 86.4±1.744 | 74.6±2.439 | 23.0±3.146 |
In vitro binding of
99mTc-LHRH receptor
Receptor saturation analysis showed that total
combination increased with 99mTc-LHRH and specific
binding rose quickly at the outset. When 99mTc-LHRH was
increased to a certain amount, the curve flattened and stopped
increasing, and showed saturation trend, indicating that the
receptor has been absorbed by 99mTc-LHRH. NSB increased
linearly with 99mTc-LHRH, showing no saturation trend
(Table IV and Fig. 4A). With B/F as the vertical axis, the
binding capacity as the abscissa, and figure was drawn by Scatchard
plot, RT = 23.2174 pmol, KD = 0.4348 nmol (Fig. 4B).
 | Table IV.Experiment data of in vitro
binding of 99mTc-LHRH receptor. |
Table IV.
Experiment data of in vitro
binding of 99mTc-LHRH receptor.
| Protein nos.
(µl) | Total count
(T) | Total binding count
(TB) | Count of
non-specific (NSB) | Count of specific
binding (SB) | Corresponding LHRH
amount of binding (pmol) | B/F |
|---|
| 2 | 12,742 | 1,088 | 587 | 501 | 3.9318 | 0.04299 |
| 5 | 31,855 | 1,795 | 714 | 1,081 | 8.484 | 0.03596 |
| 10 | 63,710 | 2,465 | 923 | 1,542 | 12.1016 | 0.02518 |
| 20 | 127,420 | 3,462 | 1,367 | 2,095 | 16.4416 | 0.0169 |
| 30 | 191,130 | 4,056 | 1,678 | 2,378 | 18.6625 | 0.01271 |
| 40 | 244,840 | 4,575 | 2,176 | 2,399 | 18.8274 | 0.009985 |
| 50 | 318,550 | 4,835 | 2,486 | 2,349 | 18.435 | 0.007488 |
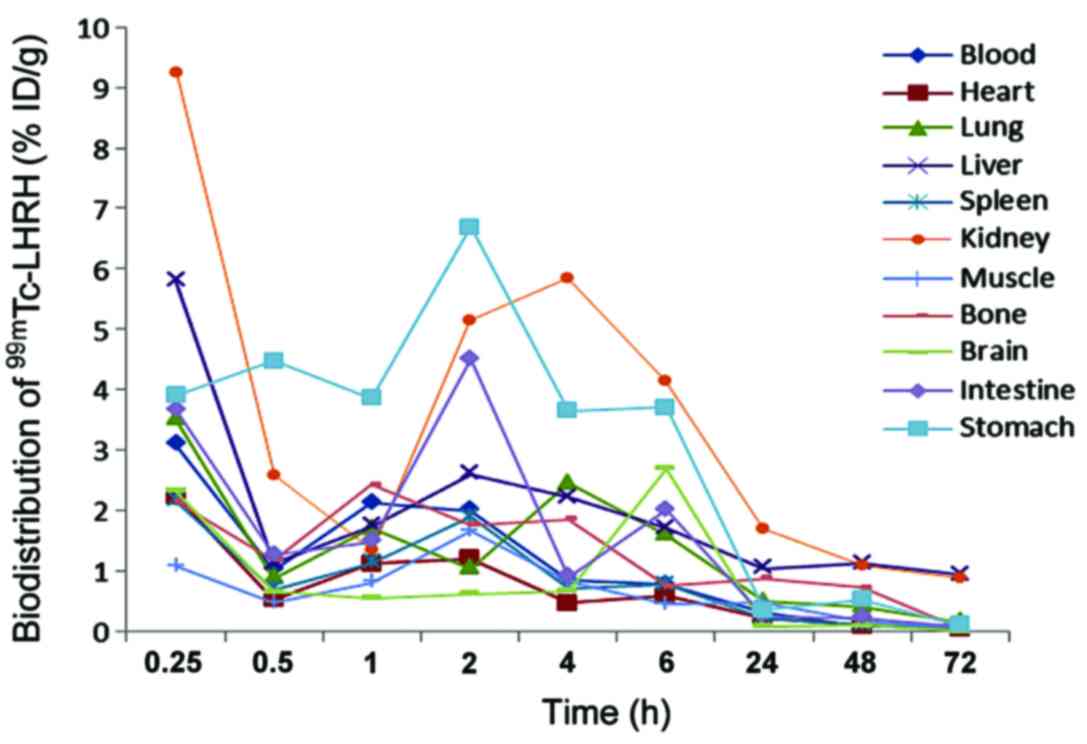
Distribution of 125I-LHRH
in normal mice
Distribution result in normal mice can be seen in
Fig. 5. 125I-LHRH was
injected into the mouse tail vein, 15 min later, blood
radioactivity was up to 3.09% ID/g, which was then quickly cleared,
and 4 h later, it reduced to <1% ID/g; radioactivity
distribution in kidneys was high, and peaked at 9.24% ID/g after 15
min, then decreased rapidly, and 1 h later, it decreased to 1.35%
ID/g, followed by a rapid rise to 5.85% ID/g again, and then
gradually cleared away; liver also had a high radioactivity uptake,
and 15 min later, it reached 5.79% ID/g, which may be associated
with blood perfusion and nonspecific uptake, and then was removed
quickly; gastrointestinal uptake was relatively high at the outset,
and peaked 2 h later at 6.69% ID/g and 4.50% ID/g, respectively.
Radioactivity in heart, lung, spleen, brain, bones and muscles
decreased over time.
Discussion
In this study, by using immunohistochemistry assay,
LHRH positive rate in 23 cases of HCC was 82.61%, and positive rate
of corresponding normal tissues was 15%; positive rate of 20 cases
of breast cancer reached 95%, and positive rate of the
corresponding normal tissues was 20%; positive rate of 10 cases of
prostate cancer, was 70%, while positive rate of corresponding
normal tissues was 40%; positive rate of 20 cases of lung cancer
was 85%, and positive rate of corresponding normal tissues reached
15.79%; positive rate of 20 cases of endometrial cancer was up to
80%, and positive rate of corresponding normal tissues was 16.67%.
Expression amount of LHRH-R in tumor cells constituted the
application basis of LHRH receptor imaging in cancer diagnosis,
while high expression of LHRH-R in these tumor tissues and
significant expression differences compared with their
corresponding normal tissues provided the foundation for using LHRH
body imaging.
Peptide radioactive agent has many advantages:
Ligand peptide is chemically synthesized, which overcome the
heterogeneity problem, and almost does not incur human
immunogenicity response; its low molecular weight makes it easy for
peptides to get through physiological barrier and penetrate
tissues, and it can reach target and clear away blood quickly,
which helps to reduce radioactive background and improve
tumor/non-tumor (T/NT) ratio (43),
therefore high-contrast tumor imaging can be obtained in a
relatively short time.
LHRH-R shows high expression in a variety of tumor
cells. Using appropriate radionuclide to label LHRH analogues is
conducive to early diagnosis and treatment of related cancer. There
are now a number of radionuclides for marking LHRH analogues
(44).
Chloramine-T methods most commonly used to label
LHRH with iodine. Radioactive iodine (such as
123I/125I/131I) is labeled on the
5th tyrosine of LHRH. The labeling process is simple with mild
reaction conditions (45). After the
labeling, separation and purification of free iodine and iodine
binding peptides must be conducted, which takes a long time, and
because the iodine connected to tyrosine is easy to fall off in the
body and 123I is expensive, the application of
iodine-labeled LHRH is rare (46,47).
18F labeled-LHRH mostly uses indirect
labeling method. 18F often causes deformation to LHRH
molecular structure, thereby affecting its biological activity, so
labeled prosthetic groups or the so-called bi-functional ligands
are widely used to connect radionuclides and biologically active
molecules (31). However, such
labeling methods often have various radioactive steps and long
duration (36).
Relative to other radionuclides, 99mTc
possesses moderate energy, and its gamma-ray energy is 140 keV,
which is readily available and of low cost. It can be easily
obtained through 99Mo-99mTc generator, which
is currently the most commonly used clinical radionuclide. Main
methods of using 99Mo-99mTc to label
polypeptides are as follows: i) Indirect labeling method carried
out by chelating agent; ii) direct reduction method; and iii) using
biotin and avidin system to label (48). Currently, modified Schwartz method and
pre-tinning method are frequently used (31). In this study, pre-tinning was adopted
to directly label LHRH, and adding sodium gluconate in the reaction
system as a complexing agent, in order to prevent the formation of
Sn-colloid, Tc-colloid and Sn-Tc colloid. By altering pH value of
reaction system, reaction temperature and the amount of reducing
agent, labeling rate and radiochemical purity of
99mTc-LHRH under different conditions were compared, the
resulting labeling rate of optimal labeling method was 97.9–100.0%,
radiochemical purity was up to 93.9–96.4%, and colloid content in
the labeling reaction was <5%. The method is simple with the
high radiochemical purity of the resulting product. Although 3 h
after being placed in 37°C human serum, the radiochemical purity
dropped to ~75%, but 2 h later, it was still nearly 90%. Given that
in the present clinical study, imaging often occurs 2 h after the
introducing imaging agent, 99mTc-LHRH obtained by this
method may become imaging agent for LHRH-R positive tumor
receptors.
Moreover, 99mTc-LHRH has receptor binding
characteristics, which is a basic condition for receptor imaging
agent. In receptor analysis, plenty of gonadorelin was added into
NSB, this peptide served as LHRH antagonist, three receptor
analysis revealed radioactivity counting measurement of each NSB
tube took on linear relationship with corresponding
99mTc-LHRH, and all exceeded 0.90, suggesting that
because at this time substantial amount of gonadorelin membrane
proteins occupied LHRH-R sites in pituitary, measurement of each
tube took on linear relation, which indicated that these counts
were combination of 99mTc-LHRH, miscellaneous proteins
in the cell membrane, and test wall rather than count formed by its
binding to the receptor, i.e., NSB. In TB tube, because there was
no LHRH, radioactive counts of each tube consisted of two parts,
one part of count was formed by 99mTc-LHRH binding to
its receptor (i.e., SB), and one part was the NSB count of
99mTc-LHRH, therefore SB was obtained through TB-NSB.
The count of each TB tube increased rapidly as the amount of
99mTc-LHRH increased, indicating that the receptor had a
surplus, then the count gradually increased, indicating that the
receptor binding was basically complete, and the count increased
simply because of the NSB.
The SB obtained showed similar curves with TB, and
although TB curve later rose slowly, it still increased as
99mTc-LHRH went up, but at certain time SB curve
flattened, showing that receptors were saturated. Usually, affinity
between receptor and ligand is bound by 10−9, KD
≥10−8 indicating the low affinity between receptor and
ligand, the bigger the KD, the lower the affinity, and KD
≤10−9 indicated high affinity between ligand and
receptor, which belongs to high affinity ligand, the smaller the
KD, the higher the affinity. In this study, by external cell
membrane protein receptor saturation binding experiment, it was
confirmed that 99mTc-LHRH could be suppressed by LHRH,
RT = 23.2174 pmol, KD = 0.4348 nmol, which indicated the labeled
99mTc-LHRH was the low-capacity and high-affinity ligand
of the receptor. Affinity was similar to 0.4792 nmol, KD value of
carcinoembryonic cell receptor obtained by predecessor who used
99mTc-LHRH, but lower than 10 nmol, KD value obtained by
Barda et al who analyzed the combination of external binding
between 99mTc-LHRH and receptor in rat pituitary cell
membrane (19). Probably differences
in tissue origin of receptor specimens and preparation methods, as
well as employed LHRH structure caused the figure discrepancy.
Receptor analysis demonstrated that 99mTc-LHRH was the
receptor's high affinity ligand, i.e., 99mTc-LHRH and
unlabeled LHRH had a high affinity for its receptor, and its
biological activity and receptor affinity was unaffected, which
also provided an experimental basis for 99mTc-LHRH as
receptor imaging agent for LHRH-R positive tumors.
In vivo metabolism experiment of
125I-LHRH in a live animal showed that after intravenous
injection of 125I-LHRH in the tail, blood radioactivity
of the mice reached a maximum of 3.09% ID/g after 15 min, then
quickly faded away, and 4 h later the figure was <1% ID/g, which
helped to reduce the background and increase the target/non-target
tissue radioactivity ratio; distribution of radioactivity in
kidneys was very high, and peaked at 9.24% ID/g after 15 min, but
followed by a rapid decrease, and after 1 h, the figure was 1.35%
ID/g, and then again rapidly increased to 5.85% ID/g, then
gradually faded away. The first peak indicated the high perfusion
of kidney tissue after the tail vein was injected with
125I-LHRH, and the second peak suggested that the
imaging agent was mainly excreted through the urinary system, which
was conducive to the detection of chest and abdominal tumor; liver
and lungs also had a higher uptake, which peaked at 15 min and may
be associated with blood perfusion and non-specific uptake,
followed by rapid clearance. Immunohistochemical results showed
normal liver and lung tissue has a certain amount of receptor
expression, but receptor expression in the tumor was much higher
than that in normal tissue, which was conducive to the detection of
liver and lung tumors; gastrointestinal radioactivity uptake was
relatively high at the outset, peaked at 2 h, which was 6.69% ID/g
and 4.50% ID/g, respectively. On one hand, it showed that there
were certain amounts of LHRH-R expression in gastrointestinal
tissue; on the other hand, it showed that part of marker was
excreted from the digestive tract, hence it was not suitable for
the detection of gastrointestinal cancer. 125I-LHRH in
heart and spleen mainly took on blood pool type distribution, and
its concentration decreased with clearance of the blood background.
Brain, bone and muscle radioactivity decreased over time.
In conclusion, positive rate of LHRH-R expression in
human liver, lung, breast, prostate, endometrial tumors is
significantly higher than that in adjacent normal tissue, and
difference in expression intensity compared with the normal control
group is statistically significant. Studies have shown that this
labeling method is fast and easy, and the resulting radiochemical
purity of the labeled product is relatively high, which does not
call for further purification and has good stability.
99mTc-LHRH labeled by this method has high affinity in
its binding with its receptor and its biological activity and
receptor affinity is not significantly affected. Radionuclide
labeled LHRH has an ideal kinetics performance in the body of
animal, which holds promise for an imaging agent of clinically
practical value of LHRH receptor.
References
|
1
|
Yuan B, Zhang J and Zhang YQ: Research
progress of relation between GnRH and its receptor and tumors. Prog
Anatomical Sci. 8:367–371. 2002.(In Chinese).
|
|
2
|
Jia PY and Wang YX: Application of
gonadotropin-releasing hormone and its receptor in cancer therapy.
J Int Pharm Res. 36:179–183. 2009.(In Chinese).
|
|
3
|
Yin H, Cheng KW, Hwa HL, Peng C, Auersperg
N and Leung PC: Expression of the messenger RNA for
gonadotropin-releasing hormone and its receptor in human cancer
cell lines. Life Sci. 62:2015–2023. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen S, Schwarz JR, Niculescu D, Dinu C,
Bauer CK, Hirdes W and Boehm U: Functional characterization of
genetically labeled gonadotropes. Endocrinology. 149:2701–2711.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie J: The current development and
prospect of tumor molecular nuclear medicine. Kawakita medical
report. 24:315–318. 2009.(In Chinese).
|
|
6
|
Ji Q, Zhou J and Huang W:
Immunohistochemical and in situ hybridization study of thyroid
gonadotropin-releasing hormone receptor in rats. Chinese J
Histochem Cytochem. 11:241–243. 2002.(In Chinese).
|
|
7
|
Emons G, Ortmann O, Becker M, Irmer G,
Springer B, Laun R, Hölzel F, Schulz KD and Schally AV: High
affinity binding and direct antiproliferative effects of LHRH
analogues in human ovarian cancer cell lines. Cancer Res.
53:5439–5446. 1993.PubMed/NCBI
|
|
8
|
Schottelius M, Berger S, Poethko T,
Schwaiger M and Wester HJ: Development of novel 68Ga- and
18F-labeled GnRH-I analogues with high GnRHR-targeting efficiency.
Bioconjug Chem. 19:1256–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mankoff DA, Link JM, Linden HM,
Sundararajan L and Krohn KA: Tumor receptor imaging. J Nucl Med. 49
Suppl 2:149S–163S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gründker C, Völker P and Emons G:
Antiproliferative signaling of luteinizing hormone-releasing
hormone in human endometrial and ovarian cancer cells through G
protein alpha(I)-mediated activation of phosphotyrosine
phosphatase. Endocrinology. 142:2369–2380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerber B, von Minckwitz G, Stehle H,
Reimer T, Felberbaum R, Maass N, Fischer D, Sommer HL, Conrad B,
Ortmann O, et al: Effect of luteinizing hormone-releasing hormone
agonist on ovarian function after modern adjuvant breast cancer
chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 29:2334–2341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emons G, Schröder B, Ortmann O, Westphalen
S, Schulz KD and Schally AV: High affinity binding and direct
antiproliferative effects of luteinizing hormone-releasing hormone
analogs in human endometrial cancer cell lines. J Clin Endocrinol
Metab. 77:1458–1464. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moreau JP, Delavault P and Blumberg J:
Luteinizing hormone-releasing hormone agonists in the treatment of
prostate cancer: a review of their discovery, development, and
place in therapy. Clin Ther. 28:1485–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rose A, Froment P, Perrot V, Quon MJ,
LeRoith D and Dupont J: The luteinizing hormone-releasing hormone
inhibits the anti-apoptotic activity of insulin-like growth
factor-1 in pituitary alphaT3 cells by protein kinase C
alpha-mediated negative regulation of Akt. J Biol Chem.
279:52500–52516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szende B, Srkalovic G, Schally AV, Lapis K
and Groot K: Inhibitory effects of analogs of luteinizing
hormone-releasing hormone and somatostatin on pancreatic cancers in
hamsters. Events that accompany tumor regression. Cancer.
65:2279–2290. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greco F and Vicent MJ: Combination
therapy: opportunities and challenges for polymer-drug conjugates
as anticancer nanomedicines. Adv Drug Deliv Rev. 61:1203–1213.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duncan R: Polymer conjugates as anticancer
nanomedicines. Nat Rev Cancer. 6:688–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eidne KA, Flanagan CA, Harris NS and
Millar RP: Gonadotropin-releasing hormone (GnRH)-binding sites in
human breast cancer cell lines and inhibitory effects of GnRH
antagonists. J Clin Endocrinol Metab. 64:425–432. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barda Y, Cohen N, Lev V, Ben-Aroya N, Koch
Y, Mishani E, Fridkin M and Gilon C: Backbone metal cyclization:
novel 99mTc labeled GnRH analog as potential SPECT molecular
imaging agent in cancer. Nucl Med Biol. 31:921–933. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seitz S, Buchholz S, Schally AV, Weber F,
Klinkhammer-Schalke M, Inwald EC, Perez R, Rick FG, Szalontay L,
Hohla F, et al: Triple negative breast cancers express receptors
for LHRH and are potential therapeutic targets for cytotoxic
LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer. 14:8472014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rusiecki JA, Holford TR, Zahm SH and Zheng
T: Breast cancer risk factors according to joint estrogen receptor
and progesterone receptor status. Cancer Detect Prev. 29:419–426.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Den Bossche B and Van de Wiele C:
Receptor imaging in oncology by means of nuclear medicine: current
status. J Clin Oncol. 22:3593–3607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imai A, Takagi A, Horibe S, Takagi H and
Tamaya T: Fas and Fas ligand system may mediate antiproliferative
activity of gonadotropin-releasing hormone receptor in endometrial
cancer cells. Int J Oncol. 13:97–100. 1998.PubMed/NCBI
|
|
24
|
Cai W and Chen X: Multimodality imaging of
vascular endothelial growth factor and vascular endothelial growth
factor receptor expression. Front Biosci. 12:4267–4279. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Virgolini I, Traub T, Novotny C, Leimer M,
Füger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M, et
al: Experience with indium-111 and yttrium-90-labeled somatostatin
analogs. Curs Pharm Des. 8:1781–1807. 2002. View Article : Google Scholar
|
|
26
|
Meko JB, Doherty GM, Siegel BA and Norton
JA: Evaluation of somatostatin-receptor scintigraphy for detecting
neuroendocrine tumors. Surgery. 120:975–983. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsuno M, Adachi H, Doyu M, Minamiyama M,
Sang C, Kobayashi Y, Inukai A and Sobue G: Leuprorelin rescues
polyglutamine-dependent phenotypes in a transgenic mouse model of
spinal and bulbar muscular atrophy. Nat Med. 9:768–773. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reisinger I, Bohuslavitzki KH, Brenner W,
Braune S, Dittrich I, Geide A, Kettner B, Otto HJ, Schmidt S and
Munz DL: Somatostatin receptor scintigraphy in small-cell lung
cancer: results of a multicenter study. J Nucl Med. 39:224–227.
1998.PubMed/NCBI
|
|
29
|
Thakur ML, Marcus CS, Saeed S, Pallela V,
Minami C, Diggles L, Le Pham H, Ahdoot R and Kalinowski EA:
99mTc-labeled vasoactive intestinal peptide analog for rapid
localization of tumors in humans. J Nucl Med. 41:107–110.
2000.PubMed/NCBI
|
|
30
|
Fekete M, Zalatnai A, Comaru-Schally AM
and Schally AV: Membrane receptors for peptides in experimental and
human pancreatic cancers. Pancreas. 4:521–528. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szepeshazi K, Schally AV, Groot K and
Halmos G: Effect of bombesin, gastrin-releasing peptide
(GRP)(14–27) and bombesin/GRP receptor antagonist RC-3095 on growth
of nitrosamine-induced pancreatic cancers in hamsters. Int J
Cancer. 54:282–289. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peremans K, Cornelissen B, Van Den Bossche
B, Audenaert K and Van de Wiele C: A review of small animal imaging
planar and pinhole spect Gamma camera imaging. Vet Radiol
Ultrasound. 46:162–170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friess H, Berberat P, Schilling M, Kunz J,
Korc M and Büchler MW: Pancreatic cancer: the potential clinical
relevance of alterations in growth factors and their receptors. J
Mol Med (Berl). 74:35–42. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schottelius M, Berger S, Poethko T,
Schwaiger M and Wester HJ: Development of novel 68Ga- and
18F-labeled GnRH-I analogues with high GnRHR-targeting efficiency.
Bioconjug Chem. 19:1256–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reubi JC and Maecke HR: Peptide-based
probes for cancer imaging. J Nucl Med. 49:1735–1738. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medina RA and Owen GI: Glucose
transporters: expression, regulation and cancer. Biol Res. 35:9–26.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang R: Clinical outlook for
radio-immunity imaging. Int J Radiat Med Nucl Med Volume. 13:40–42.
1989.
|
|
38
|
Wang R, Zhang C, Yu L and Guo Y: Clinical
application of radioimmunoimaging with 99mTc-BDI-1 in the diagnosis
of bladder cancer. Chin Med J (Engl). 113:396–399. 2000.PubMed/NCBI
|
|
39
|
Vriesendorp HM and Vriesendorp FJ: A
review of the intravenous administration of radiolabeled
immunoglobulin G to cancer patients. High or low protein dose?
Cancer Biother Radiopharm. l8:35–46. 2003. View Article : Google Scholar
|
|
40
|
Kang JH and Chung JK: Molecular-genetic
imaging based on reporter gene expression. J Nucl Med. 49 Suppl
2:164S–179S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai W and Chen X: Multimodality molecular
imaging of tumor angiogenesis. J Nucl Med. 49 Suppl 2:113S–128S.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Jong M, Breeman WA, Bernard BF, Bakker
WH, Schaar M, van Gameren A, Bugaj JE, Erion J, Schmidt M,
Srinivasan A, et al: [177Lu-DOTA0,Tyr3]octreotate for somatostatin
receptor-targeted radionuclide therapy. Int J Cancer. 92:628–633.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan TS, Sundin A, Juhlin C, Långström B,
Bergström M and Eriksson B: 11C-metomidate PET imaging of
adrenocortical cancer. Eur J Nucl Med Mol Imaging. 30:403–410.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dietz DW, Dehdashti F, Grigsby PW, Malyapa
RS, Myerson RJ, Picus J, Ritter J, Lewis JS, Welch MJ and Siegel
BA: Tumor hypoxia detected by positron emission tomography with
60Cu-ATSM as a predictor of response and survival in patients
undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a
pilot study. Dis Colon Rectum. 51:1641–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chao KS, Bosch WR, Mutic S, Lewis JS,
Dehdashti F, Mintun MA, Dempsey JF, Perez CA, Purdy JA and Welch
MJ: A novel approach to overcome hypoxic tumor resistance:
Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat
Oncol Biol Phys. 49:117l–1182. 2001. View Article : Google Scholar
|
|
46
|
Dehdashti F, Grigsby PW, Mintun MA, Lewis
JS, Siegel BA and Welch MJ: Assessing tumor hypoxia in cervical
cancer by positron emission tomography with 60Cu-ATSM: relationship
to therapeutic response-a preliminary report. Int J Radiat Oncol
Biol Phys. 55:1233–1238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Suzuki T, Nakamura K, Kawase T and Kubo A:
Monitoring of response to radiation therapy for human tumor
xenografts using 99mTc-HL91
(4,9-diaza-3,3,10,10-tetramethyldodecan-2,11-dione dioxime). Ann
Nucl Med. 17:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mochizuki T, Kuge Y, Zhao S, Tsukamoto E,
Hosokawa M, Strauss HW, Blankenberg FG, Tait JF and Tamaki N:
Detection of apoptotic tumor response in vivo after a single dose
of chemotherapy with 99mTc-Annexin V. J Nucl Med. 44:92–97.
2003.PubMed/NCBI
|