Introduction
Cholangiocarcinoma (CCA) is a tumor consisting of
bile duct epithelial cells, which is associated in Thailand with
liver fluke disease (caused by Opisthorchis viverrini
infection), and an important direct risk factor of this disease is
inhabitation of the Opisthorchiasis endemic area in the
northeastern part of the country (1).
CCA is the major health problem in northeastern Khon Kaen province
of Thailand, which has the highest incidence of liver fluke-induced
CCA in the world (2–4). The prognosis of patients with CCA is
poor, and the 3- and 5-year survival rate of patients with
intrahepatic CCA following surgery is 21.7 and 11.2%, respectively
(5). This tumor demonstrates highly
aggressive behavior, and is difficult to diagnose and to
effectively predict its prognosis (6). Although serum carbohydrate antigen 19-9
(CA19-9) is currently the typical serum biomarker used for
diagnosis in combination with other modalities, its sensitivity and
specificity is low (7). In addition,
it is not predictive of disease progression or prognosis. A novel
serum marker as a prognostic marker is of increasing interest to be
applied in CCA management.
As in other solid tumors including prostate
(8), breast (9), non-small cell lung (10) and pancreatic cancer (11), the interaction between CCA cells and
stromal cells in certain cancer-associated fibroblasts contribute
to tumor development and progression of the disease. The
fibroblasts isolated from fresh CCA tissues expressed alpha-smooth
muscle cells, indicating that these fibroblasts were activated. The
degree to which these fibroblasts are activated may be predictive
of patient prognosis (12). These
CCA-associated fibroblasts were demonstrated to have an abnormal
gene expression profile compared with that of normal liver
fibroblasts using a complementary DNA microarray (13). In a previous study, secreted
tumor-promoting proteins were investigated, and it was identified
that CCA-associated fibroblasts exhibited the capability of
producing periostin (PN), an extracellular matrix protein, and the
tissue level of PN was observed to be associated with patient
survival (13). As PN has been
demonstrated to be a multifunctional protein involved in the
processes of carcinogenesis and progression (14,15),
previous in vitro CCA studies have suggested that PN has an
important impact in cancer aggressiveness, as it induces a variety
of tumorigenic properties, including cell proliferation, growth and
invasion, partly via the integrin receptor α5β1 through the
phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT signaling
pathway (13,16).
PN has been identified in numerous cancer tissues,
and it is produced primarily from cancer cells (17), stromal fibroblasts (13,18) or
both cell types (19–23). Human epidermal growth factor receptor
2-positive and triple negative breast cancer tissue proteins were
analyzed in a previous study, and the results demonstrated that PN
was one of the proteins associated with tumor treatment response
(17). In CCA, PN is expressed
exclusively in tumor stromal fibroblasts (13,18).
Utispan et al detected expression of PN in fibroblasts but
not in CCA or immune cells (13). By
contrast, in hepatocellular carcinoma (HCC), a closely associated
tumor to CCA, PN was observed at negative-to-low expression
(13). Correlation analysis of PN
levels and clinicopathological data suggested that tissue PN was an
independent marker of poor prognosis in patients with CCA (13). PN was detected in epithelial cancer
cells and stromal fibroblasts with a high incidence of PN-positive
fibroblasts in 70% of total cases, compared with a lower
PN-positive rate in cancer cells (20). The predominant expression of PN in
epithelial cells was established in HCC, and was significantly
associated with higher tumor grade and hepatitis B virus incidence,
but not with overall survival rate (20). PN was associated with angiogenesis,
and therefore, the combination of PN overexpression and
microvascular invasion may be a marker of poor prognosis in
patients with HCC (24,25). However, a significant impact of PN
level in tissues on the overall survival time of patients with CCA
was observed (13).
Using laser capture microdissection and reverse
transcription-polymerase chain reaction, a previous study
demonstrated that cancerous and stromal cells may be the source of
PN production in non-small cell lung cancer (21). In prostate cancer,
immunohistochemistry (IHC) analysis exhibited increased PN
expression in carcinoma cells, which was associated with a high
Gleason score and advanced tumor stage (19). Additionally, increased PN expression
levels in tumor stroma were identified in the majority of primary
and metastatic prostate cancer samples, leading to the conclusion
that PN upregulation was associated with tumor aggressiveness in
prostate cancer, and that it may be a potential target for
therapeutic intervention (19). The
medium and high level of stromal PN expression, compared with
epithelial expression, independently predicted unfavorable
prognosis in colorectal cancer (23).
Additionally, the stromal PN expression increased consecutively
from adjacent mucosa to primary tumor mass and metastatic
colorectal cancer tissues (23).
Taken together, these findings suggest that tissue PN is a
potential molecular marker for diagnosis and prediction of
prognosis, and a novel therapeutic target in numerous types of
cancer, including CCA (26).
Serum PN level has been suggested as a diagnostic or
prognostic marker in certain types of cancer, including non-small
cell lung (27), colorectal (28), early-stage breast (29), breast with bone metastasis (30), ovarian (31), prostate (32), pancreatic (33), and head and neck squamous cell cancer
(34). PN has been reported as a
potential serodiagnostic marker in patients with CCA (35). In a previous study, a limited number
of CCA cases were investigated, and serum PN, which was detected
using an in-house established PN sandwich ELISA, which had been
modified from a previous study (35)
with the addition of a different antibody clone, was observed to be
increased and able to distinguish patients with CCA from other
associated conditions, particularly cirrhosis and HCC (35). The application of serum PN measurement
to a large sample size of patients with CCA, however, remains to be
investigated. In the present study, the use of an in-house
established human PN ELISA to measure the sera PN in a large sample
size of Thai patients with intrahepatic CCA was investigated, and
compared with healthy controls and other associated and/or common
malignancies. The serum and tissue PN levels and
clinicopathological parameters were analyzed to indicate the
potential of using circulating PN in serum for the diagnosis and
prediction of prognosis in patients with CCA.
Materials and methods
Patients and tissues
In total 68 patients with CCA were enrolled on the
study together with the patients from the control groups: 6
patients with benign liver diseases, including liver fibrosis,
granulomatous liver, cholecystitis, obstructive bile ducts, biliary
cystadenoma and cavernous hemangioma; 2 patients with HCC; 21
patients with breast cancer; and 50 control individuals (normal).
All patients, with the exception of breast cancer patients,
underwent surgery at the Faculty of Medicine, Khon Kaen University
(Khon Kaen, Thailand) for standard treatment. The patients with
breast cancer were admitted for treatment at Faculty of Medicine,
Siriraj Hospital, Mahidol University (Bangkok, Thailand). Serum
collection was approved by Khon Kaen Ethics Committee (EC no.
490143). The liver surgical specimen diagnoses were confirmed by
pathologists according to the standard clinical guidelines.
Patients with breast cancer had sera collected whilst they
underwent surgery at the Faculty of Medicine Siriraj Hospital,
Mahidol University (Bangkok, Thailand), under the protocol approval
by Siriraj Institute Revision Board (si520/2010). Each patient was
informed, and written informed consent was obtained. The sera were
obtained prior to tumor mass removal and kept at −80°C until used
for experiments. The blood chemistry results of 50 normal controls
that visited to the hospital for their yearly health check-up were
used as the normal control in the present study. All 68 patients
with CCA were used in experimental analyses regarding serum PN,
whereas tissue PN was analyzed in only 66 cases, as there was an
insufficient amount of tissues from 2 patients.
ELISA for PN expression levels
Sera PN were measured using an established human PN
sandwich ELISA as previously described (36–39).
Briefly, 2 µg/ml rat SS18A IgG1 from the ‘Periostin ELISA Kit
(Human)’ (Shino-Test Corporation, Tokyo, Japan) was incubated
overnight at 25°C on ELISA plates (Loose MaxiSorp®
Nunc-Immuno® Modules plates, Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The blocking buffer (0.5% casein in 1X
TBS, pH 8.0) was used to block non-specific binding by incubating
the plates with the buffer overnight at 4°C, and the tissue samples
were then washed 3 times with washing buffer (0.05% Tween-20 in 1X
PBS). The ELISA plates were incubated with samples or 0–2 ng/ml
recombinant PN standards derived from Drosophila S2 cells
(40) (Shino-Test Corporation, Tokyo,
Japan) for 18 h at 25°C, followed by five washes. The
peroxidase-labeled SS17B monoclonal antibody (50 ng/ml) sourced
from the ‘Periostin ELISA Kit (Human)’ was added to 0.05% casein in
TBS, followed by incubation for 90 min at 25°C. Following washing
of the ELISA plates with washing buffer, the reaction solution (0.8
mM 3,3′,5,5′-tetramethylbenzine, 2.5 mM H2O2)
was added, followed by incubation for 10 min at 25°C. The reactions
were stopped by adding the stop solution (0.7 N HCl) and incubating
the plates at room temperature for 10 min. The absorbance at 450 nm
was measured within 60 min after the addition of the stop solution.
The values were calculated simultaneously using the recombinant PN
protein by subtraction of the absorbance at 550 nm from that of 450
nm (Δ optical density) measured in the microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each sample was performed
in triplicate and the mean value was used.
IHC of PN in CCA tissues
In total, 66/68 cases of patients with CCA used for
serum PN measurements were processed for paraffin embedding for PN
detection using IHC. The tissue sections of 1–3 mm were fixed in
10% formaldehyde at room temperature for 1–3 h. The tissues were
obtained from patients who underwent a hepatectomy using the
protocol approved by the Human Research Ethics Committee of Khon
Kaen University (EC no. 490143). The age, sex, tumor size,
histological type and staging data were obtained from the medical
and pathological records. Briefly, antigens were retrieved by
incubating the paraffin-embedded tissues in 10 mM citrate buffer
(pH 6.0) at 95°C for 40 min, and endogenous peroxidase was blocked
by incubation with 3% H2O2 for 5 min.
Following blocking of non-specific binding with 2% bovine serum
albumin (BSA, VWR International, Leicestershire, UK) for 20 min at
room temperature, an in-house-developed rabbit anti-human PN
(39) was applied to the sections at
room temperature overnight at 1:10,000 dilution. The slides were
then washed with 1X TBST (0.01% Tween 20) washing buffer 3 times
for 10 min followed by incubation with anti-rabbit EnVision+ System
horseradish-peroxidase-labeled polymer (cat. no. K4003-11;
dilution, ready to use; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 30 min at room temperature. The slides were
then washed with 1X TBST (0.01% Tween 20) washing buffer for 10 min
3 times. The immunoreactive signals were developed using
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The tissues were washed with tap water for 5 min and
counterstained with hematoxylin. The images were captured using a
light microscope with magnification, ×100.
PN expression in the fibroblasts was
semi-quantitatively scored on the basis of PN-positive fibroblasts
percentages and immunostaining intensity, as previously described
(13). The interpretation of PN
expression was performed by multiplying the scores of the
percentage of positive cells (0–3) with the scores of staining
intensity (1–3) to reach a total IHC score of 0–9. The
results were categorized as follows: Low expression, IHC score of
≤4; and high expression, IHC score of >4. All tissue samples
were anonymously and independently scored by two medical doctors
(Dr Peti Thuwajit and Dr Chanitra Thuwajit). In case of
disagreement, the slides were reexamined to obtain a consensus.
Statistical analysis
Data analysis was performed using the SPSS 18.0
statistical software package (SPSS, Inc., Chicago, IL, USA). The
continuous data of serum PN levels were expressed as the median,
including the first and third quartiles. Receiver operating
characteristic (ROC) analyses were used to determine the threshold
value of serum PN levels for distinguishing patients with CCA from
patients with other diseases. The significance of the threshold
value was evaluated for diagnostic performance using the
sensitivity, specificity, accuracy, positive predictive value (PPV)
and negative predictive value (NPV). The Mann-Whitney U test was
performed to compare the PN level between different groups of
patients. A survival analysis curve was calculated according to the
Kaplan-Meier log rank test. Multivariate analysis for survival
parameters was performed using the Cox proportional hazard
regression model. The Fisher's exact test was used to analyze the
correlation between parameters. P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum PN is increased in patients with
CCA
Among the enrolled patients with CCA, 68% were male
and 32% were female, equating to a ratio of 2:1 male: female
(Table I). The median age for all
patients in the present study was 56.5 years (range, 38–76 years).
All the CCA cases were diagnosed as intrahepatic CCA, and the
majority were pathologically classified as well-differentiated
(37%) or papillary (57%) types.
 | Table I.Univariate analysis of serum and
tissue PN with clinicopathological and blood chemistry
parameters. |
Table I.
Univariate analysis of serum and
tissue PN with clinicopathological and blood chemistry
parameters.
|
|
| Serum PN
(ng/ml) |
|
| Tissue PN (IHC
score) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristic | No. (n=68) | Low (≤94) | High (>94) | P-value | No. (n=66) | Low (≤4) | High (>4) | P-value |
|---|
| Age, years |
|
|
| 0.146 |
|
|
| 0.087 |
|
≤56 | 34 | 24 | 10 |
| 38 | 22 | 16 |
|
|
>56 | 34 | 18 | 16 |
| 28 | 10 | 18 |
|
| Sex |
|
|
| 1.000 |
|
|
| 0.188 |
|
Female | 22 | 14 | 8 |
| 21 | 13 | 8 |
|
|
Male | 46 | 28 | 18 |
| 45 | 19 | 26 |
|
| Histological
type |
|
|
|
|
|
|
|
|
|
Well-differentiated | 25 | 10 | 15 | 0.009 | 24 | 9 | 15 | 0.208 |
|
Moderately-differentiated | 3 | 1 | 2 | 0.553 | 3 | 0 | 3 | 0.239 |
|
Poorly-differentiated | 1 | 1 | 0 | 1.000 | 1 | 0 | 1 | 1.000 |
|
Papillary | 39 | 30 | 9 | 0.005 | 38 | 23 | 15 | 0.027 |
| Soft tissue/hilar
invasion |
|
|
| 0.190 |
|
|
| 0.797 |
|
Absence | 23 | 17 | 6 |
| 23 | 12 | 11 |
|
|
Presence | 45 | 25 | 20 |
| 43 | 20 | 23 |
|
| Lymph node
metastasis |
|
|
| 0.585 |
|
|
| 0.185 |
|
Absence | 48 | 31 | 17 |
| 46 | 25 | 21 |
|
|
Presence | 20 | 11 | 9 |
| 20 | 7 | 13 |
|
| Vascular
invasion |
|
|
| 0.737 |
|
|
| 0.513 |
|
Absence | 57 | 36 | 21 |
| 55 | 28 | 27 |
|
|
Presence | 11 | 6 | 5 |
| 11 | 4 | 7 |
|
| Alanine
transaminase, U/l |
|
|
| 0.804 |
|
|
| 0.616 |
|
≤40 | 29 | 17 | 12 |
| 29 | 15 | 14 |
|
|
>40 | 37 | 23 | 14 |
| 35 | 15 | 20 |
|
| Aspartate
transaminase, U/l |
|
|
| 0.137 |
|
|
| 1.000 |
|
≤40 | 28 | 20 | 8 |
| 28 | 13 | 15 |
|
|
>40 | 38 | 20 | 18 |
| 36 | 17 | 19 |
|
| Albumin, mg/dl |
|
|
| 1.000 |
|
|
| 1.000 |
|
≤4.5 | 60 | 36 | 24 |
| 58 | 27 | 31 |
|
|
>4.5 | 6 | 4 | 2 |
| 6 | 3 | 3 |
|
| Globulin,
mg/dl |
|
|
| 1.000 |
|
|
| 0.564 |
|
≤3.5 | 23 | 14 | 9 |
| 16 | 6 | 10 |
|
|
>3.5 | 43 | 26 | 17 |
| 48 | 24 | 24 |
|
| Prothrombin time,
min |
|
|
| 0.032 |
|
|
| 0.426 |
|
≤13 | 44 | 31 | 13 |
| 43 | 22 | 21 |
|
|
>13 | 22 | 9 | 13 |
| 21 | 8 | 13 |
|
Using a sandwich ELISA, recombinant human PN at a
range of 0–2 ng/ml in 100 µl diluent containing 50 mM Tris-HCl, pH
8.0, 100 mM NaCl, 0–9% NaN3 and 0.5% casein was used to
create a standard curve for estimation of the concentration of PN
in the patient sera (Fig. 1A). A
linear curve was observed at a concentration of PN ≤0.5 ng/ml. The
minimum detection limit of the ELISA was 20 pg/ml. The median of
serum PN was 52 ng/ml for patients with benign liver disease,
whereas PN level was increased to 100 ng/ml in patients with HCC
and 62 ng/ml in patients with breast cancer. Based on the Youden
index, the optimal threshold value of serum PN level was 94 ng/ml
(Fig. 1B). The ROC curve of serum PN
in the comparison between patients with CCA and normal controls
demonstrated that the area under the ROC curve was 0.608, with a
confidence interval (CI) of 0.509–0.706, and was statistically
significant (P=0.043; Fig. 1B). The
overall serum PN level of the normal control patients had a median
value of 59 ng/ml, which was lower compared with that of patients
with CCA (median, 79 ng/ml). The Mann-Whitney U test and Grubbs
outlier test demonstrated significantly higher levels of serum PN
in patients with CCA compared with those in normal controls
(P=0.041; Fig. 1C).
 | Figure 1.Serum PN measurement. (A) Standard
curve of recombinant human PN in ng/ml. (B) Receiver operating
characteristic curve for serum PN level to distinguish patients
with CCA from normal controls. The area under the curve was 0.608.
The optimal threshold value between patients and healthy controls
is 94 ng/ml (Youden's index, 0.266; P=0.043). (C) Serum PN levels
in normal volunteers and patients with benign liver disease, CCA
and other malignancies, including HCC and breast cancer. The
vertical bars indicate the range, while the horizontal boundaries
of the boxes represent the standard deviation. Horizontal lines in
the boxes represent median values. Statistical comparisons among
multiple groups were performed using the Mann-Whitney U test. *,
P=0.041 (D) Survival analysis of patients with CCA and high or low
levels of serum PN using Kaplan-Meier analysis. Using the 3-year
survival rate, the cumulative survival of patients with high serum
PN levels was significantly reduced compared with that of patients
with low serum PN levels, according to the Mann-Whitney U test
(P<0.001). PN, periostin; ΔOD, subtraction of the optical
density, or absorbance, at 550 nm from that of 450 nm; CCA,
cholangiocarcinoma; HCC, hepatocellular carcinoma. |
The diagnostic performances for distinguishing CCA
from other conditions, including benign liver diseases, HCC, breast
cancer and healthy controls, reached statistically significant
values for: Sensitivity, 0.38 (95% CI, 0.27–0.51); specificity,
0.89 (95% CI, 0.80–0.95); accuracy, 0.66 (95% CI, 0.57–0.73); PPV,
0.74 (95% CI, 0.57–0.88); and NPV, 0.63 (95% CI, 0.53–0.72) (all
P<0.001) (Table II). However,
univariate analysis did not identify significant differences in
serum PN or CA19-9, and therefore these may not be effective as
potential diagnostic markers to identify CCA from benign conditions
of the liver. The P-value of using 94 ng/ml serum PN as the
threshold, 37 U/ml serum CA19-9 as the threshold and 94 ng/ml serum
PN + 37 U/ml CA19-9 to distinguish CCA from benign liver diseases
were P=0.406, P=0.348 and P=1.000, respectively.
 | Table II.Diagnostic performance of serum
periostin levels for distinguishing cholangiocarcinoma from others
diseases, including patients with benign liver disease,
hepatocellular carcinoma, breast cancer and healthy controls. |
Table II.
Diagnostic performance of serum
periostin levels for distinguishing cholangiocarcinoma from others
diseases, including patients with benign liver disease,
hepatocellular carcinoma, breast cancer and healthy controls.
| CCA compared
to | TP | TN | FN | FP | Sensitivity (95%
CI) | Specificity (95%
CI) | Accuracy (95%
CI) | PPV (95% CI) | NPV (95% CI) | P-value |
|---|
| All other
conditions | 26 | 70 | 42 | 9 | 0.38
(0.27–0.51) | 0.90
(0.81–0.96) | 0.66
(0.58–0.74) | 0.76
(0.59–0.89) | 0.63
(0.53–0.72) | <0.001 |
| Healthy
controls | 26 | 44 | 42 | 6 | 0.38
(0.27–0.51) | 0.88
(0.76–0.95) | 0.59
(0.50–0.68) | 0.81
(0.64–0.93) | 0.51
(0.40–0.62) | 0.003 |
| Benign liver
disease | 26 | 5 | 42 | 1 | 0.38
(0.27–0.51) | 0.83
(0.36–1.00) | 0.42
(0.31–0.54) | 0.96
(0.81–1.00) | 0.11
(0.04–0.23) | 0.406 |
| Hepatocellular
carcinoma + Breast cancer | 26 | 21 | 42 | 2 | 0.38
(0.27–0.51) | 0.91
(0.72–0.99) | 0.52
(0.41–0.62) | 0.93
(0.76–0.99) | 0.33
(0.22–0.46) | 0.008 |
Serum PN is associated with survival
time of patients with CCA
There were no significant differences in serum PN
levels according to age, gender, soft tissue/hilar invasion, lymph
node metastasis or vascular invasion (Table I). The level of serum PN, however, was
associated with well-differentiated CCA and papillary CCA, with
P-values of P=0.009 and P=0.005, respectively. Notably, the present
study demonstrated a significant association between serum PN and
prothrombin time (PT) (P=0.032), whereas other blood chemistry
parameters that represent liver functions were not significantly
associated. In patients with CCA, serum with low PN was defined
with the level of ≤94 ng/ml, and >94 ng/ml was defined as high
PN. Multivariate analysis using Cox proportional hazard regression
revealed the potential of high serum PN level as a factor of poor
prognosis for CCA with statistical significance (P=0.001), whereas
other parameters, including age, histological type, lymph node
metastasis, vascular invasion and soft tissue/hilar invasion, were
not significantly different (Table
III). There were no cases of mortality in the current study
within 2 weeks following surgery, and therefore, all 68 enrolled
cases were used in the survival analysis. The results demonstrated
that patients with a high serum PN level (n=26) had a significant
reduced survival rate (221.5 days) compared with that of patients
with a low serum PN level (n=42; 612 days; P<0.001 (Fig. 1D). Using the 3-year survival time as
the threshold, the Kaplan-Meier log rank test demonstrated that
serum PN levels may be used as a prognostic biomarker for poor
survival rate in patients with CCA.
 | Table III.Multivariate analysis by Cox
proportional hazard regression model for the evaluation of
prognostic factors regarding serum and tissue PN levels. |
Table III.
Multivariate analysis by Cox
proportional hazard regression model for the evaluation of
prognostic factors regarding serum and tissue PN levels.
|
| Serum PN levels
(total n=68, mortalities n=51) | Tissue PN levels
(total n=66, mortalities n=51) |
|---|
|
|
|
|
|---|
| Variable | 3-year
survival | HR | 95% CI | P-value | 3-year
survival | HR | 95% CI | P-value |
|---|
| PN level |
| 3.197 | 1.572–6.502 | 0.001 |
| 1.645 | 0.863–3.136 | 0.131 |
| Low
(serum n=42, tissue n=32) | 26 |
|
|
| 21 |
|
|
|
| High
(serum n=26, tissue n=34) | 25 |
|
|
| 29 |
|
|
|
| Age, years |
| 0.899 | 0.512–1.581 | 0.713 |
| 1.038 | 0.572–1.881 | 0.903 |
| ≤56
(n=34) | 26 |
|
|
| 25 |
|
|
|
| >56
(n=34) | 25 |
|
|
| 25 |
|
|
|
| Histological
type |
| 0.974 | 0.407–2.327 | 0.952 |
| 0.882 | 0.712–1.093 | 0.253 |
|
Well-differentiated
(n=25) | 23 |
|
|
| 22 |
|
|
|
|
Moderately-differentiated
(n=3) | 3 |
|
|
| 3 |
|
|
|
|
Poorly-differentiate
(n=1) | 1 |
|
|
| 1 |
|
|
|
|
Papillary (n=39) | 24 |
|
|
| 24 |
|
|
|
| Soft tissue/hilar
invasion |
| 1.693 | 0.872–3.288 | 0.120 |
| 2.201 | 1.112–4.355 | 0.024 |
| Absence
(n=23) | 13 |
|
|
| 13 |
|
|
|
|
Presence (n=45) | 38 |
|
|
| 37 |
|
|
|
| Lymph node
metastasis |
| 0.975 | 0.775–1.227 | 0.831 |
| 1.036 | 0.474–2.265 | 0.929 |
| Absence
(n=48) | 34 |
|
|
| 33 |
|
|
|
|
Presence (n=20) | 17 |
|
|
| 17 |
|
|
|
| Vascular
invasion |
| 1.287 | 0.620–2.674 | 0.498 |
| 0.896 | 0.354–2.296 | 0.817 |
| Absence
(n=57) | 41 |
|
|
| 40 |
|
|
|
|
Presence (n=11) | 10 |
|
|
| 10 |
|
|
|
Level of serum PN is associated with
tissue PN expression
The IHC results demonstrated that fibroblasts were
the only source of PN expression in all histopathological types of
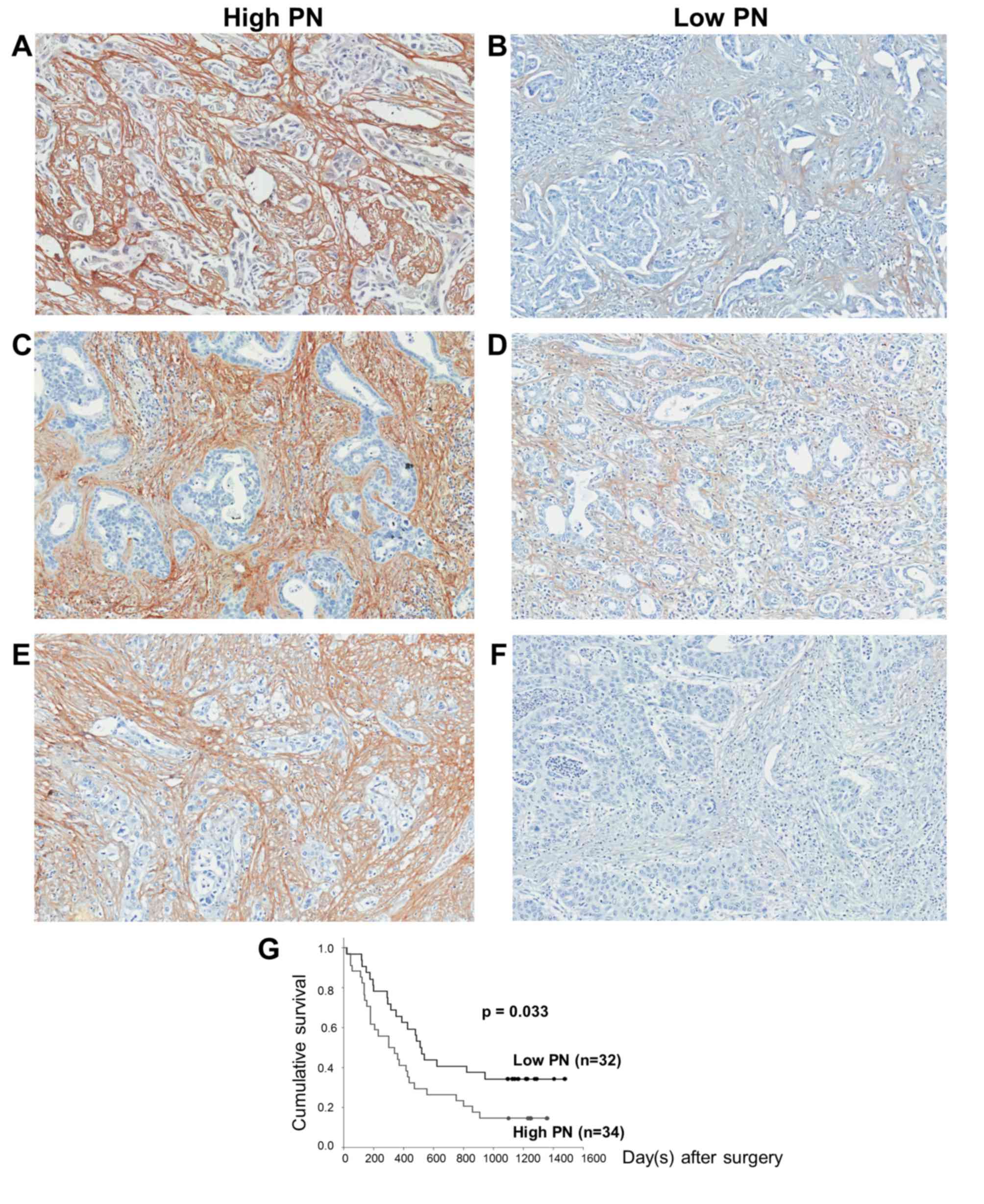
CCA tissues (Fig. 2). In each
histopathological type, PN was expressed at various levels, which
were grouped into high (Fig. 2A, C and
E) and low PN (Fig. 2B, D and F).
Regarding the IHC score, the tissues with a score of ≤4 were
classified in the low PN group, whereas those with a score of >4
were placed in the high PN group.
Tissue PN, as well as serum PN, was significantly
associated with the papillary histopathological type (P=0.027)
(Table I). There was no significant
association of tissue PN with other clinicopathological parameters
or blood chemistry parameters for liver function. Multivariate
analysis revealed a significant association between tissue PN and
soft tissue/hilar invasion (P=0.024) (Table III). Although the result identified
that tissue PN may not be effective as an independent prognostic
factor for poor survival, the Kaplan-Meier log rank test revealed a
significant association between tissue PN and cumulative survival
time (P=0.033) (Fig. 2G). These
results are concordant with patients with high tissue PN expression
(n=34) having reduced survival rates (304 days) compared with those
with low tissue PN expression (n=32; 514 days) (P=0.038). The
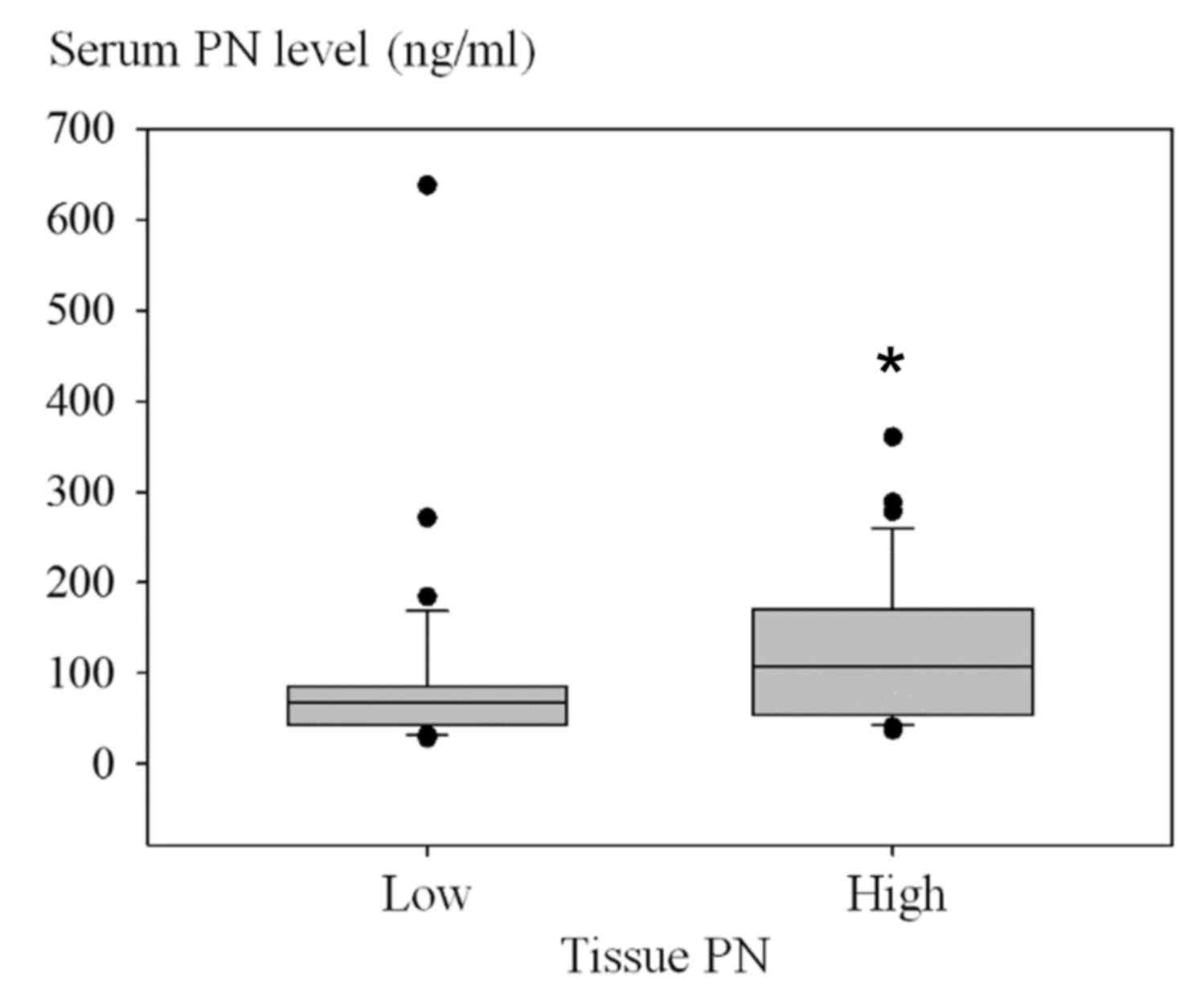
association of the number of low or high serum PN cases and the
level of tissue PN is presented in Fig.
3 (P=0.001). Using the Fisher's exact test, patients with high
serum PN also frequently exhibited high tissue PN (P=0.001)
(Table IV). Similarly, patients with
low serum PN were significantly associated with low tissue PN.
 | Table IV.Clustering of patients with high or
low PN levels. |
Table IV.
Clustering of patients with high or
low PN levels.
|
| Serum PN
expression, ng/ml |
|
|---|
|
|
|
|
|---|
| Variable | Low, ≤94
(n=40) | High, >94
(n=26) | P-value |
|---|
| Tissue PN, IHC
score |
|
| 0.001 |
| High,
>4 (n=34) | 14 | 20 |
|
| Low, ≤4
(n=32) | 26 | 6 |
|
Discussion
The potential of using serum PN as a prognostic
marker in patients with CCA was demonstrated in the present study.
In concordance with previous studies that demonstrated that PN was
exclusively located in CCA stromal fibroblasts in the patients'
tissues (13,20), PN was detected in patients' sera and
utilized to classify patients into high or low serum PN groups.
Notably, the high level of serum PN was significantly associated
with a reduced survival rate following surgery in patients with
CCA, which indicates the potential for serum PN to predict the
aggressiveness of the disease.
The impact of PN on cancer cells leading to tumor
progression has been established (15). A number of tumors have been identified
to express high levels of PN in clinical specimens (13,17,19,21).
The expression of PN has been demonstrated to be induced by
transforming growth factor-b (TGF-β) and bone morphogenetic protein
(BMP)-2 (41,42). In CCA, TGF-β was expressed in cancer
cells (43), and it is established
that activated fibroblasts in cancer tissues may produce TGF-β
(44). Therefore, it may be possible
that stromal fibroblasts are activated by cancer-derived or
fibroblast-derived TGF-β in a paracrine or autocrine manner, which
leads to the activation of PN production from stromal cells into
the tumor microenvironment. Although the reason for the observation
of negative-to-low expression of PN in CCA cells has yet to be
elucidated, it may be explained by the aberrant PN expression
control machinery, particularly, the abnormalities of certain
transcription factors (45–47) and microRNAs (miRNAs) (48,49). In
addition, the high PN-expressing CCA-associated fibroblasts were
identified to have low level of miR-1, whereas those with low PN
had high miR-1 expression (unpublished data from our group). These
results suggest the potential of miR-1 as a negative regulator of
PN production in CCA-associated fibroblasts via blocking GATA-6 and
leading to the down-regulation of BMP-4, which normally activates
PN expression (49). miR-1 has been
reported to be able to regulate GATA-6, its targeted gene, which
indirectly activates the expression of PN through the GATA-6 direct
transcription target BMP-4 (49).
Therefore, the reduction of miR-1 leads to the increased GATA-6
that was observed in cells with PN overexpression. These results
are in accordance with the previously described PN over-production
from CCA stromal fibroblasts (13).
PN binds to the membrane receptor integrin α5β1 on
CCA cells and activates cancer cell progression via the AKT
signaling pathway to increase tumor aggressive properties,
including cell proliferation, growth, migration and invasion
(13,16). Regarding PN as a secreted protein,
serum PN has been detected in various types of activated stromal
fibroblast-containing tumors, including non-small cell lung,
colorectal, metastatic breast and ovarian cancer (27,28,30,31).
Notably, the majority of these tumors demonstrated that serum PN
levels were associated with poor prognostic status of the diseases
(28,30). In CCA, serum PN was reported to be a
diagnostic marker (35); however, the
application of using serum PN as a prognostic marker has not been
investigated thus far. The median serum PN level in 8 Japanese
patients with CCA was 513 ng/ml (35), which is higher than that in the Thai
patients with CCA evaluated in the present study (79 ng/ml). As
serum PN levels in the blood samples in the above previous study
(35) were investigated using a
different ELISA kit (SS16A and SS17B; Shino-Test Corporation), the
results cannot be directly compared with the PN values obtained in
the present study. However, despite the modified ELISA kit (SS18A
and SS17B) used on the sera samples analyzed in the present study
that contained a different clone of the anti-PN antibody, the
median PN values in the tissue samples was 230 ng/ml (unpublished
data), which was higher than that detected in the present study.
This discrepancy may be attributed to differences in the genetic
background between Thai and Japanese populations, tumor size, tumor
invasiveness and/or the etiological causes of CCA. However, the
primary result is the identification of increased serum PN levels
in patients with CCA compared with those in patients with other
conditions.
Serum PN is positively associated with liver enzymes
and proteins (aspartate transaminase, alanine transaminase,
alkaline phosphatase and total albumin) and liver stiffness, but
negatively associated with serum albumin in patients with
post-operative biliary atresia (50).
This previous study concluded that serum PN was associated with
hepatic dysfunction, liver fibrosis and jaundice status (50). The univariate analysis conducted in
the present study demonstrated that serum PN was significantly
associated with PT. The majority of patients with CCA and normal PT
had low serum PN, whilst a large number of patients with high serum
PN had prolonged PT. This may be explained by the liver dysfunction
and PN overexpression observed in patients with CCA. Regarding the
histological type, papillary CCA has been established to be
associated with good prognosis, and concordant with this, the rate
of liver metastasis has been identified to be significantly higher
for intraductal CCA with a tubular growth pattern than for CCA with
a papillary growth pattern (51).
Therefore, the results of the current study indicated that the low
PN levels in serum and tissue were significantly associated with
papillary CCA and are of clinical importance. However, the
significant association of serum PN, unlike tissue PN, with
well-differentiated CCA may be statistically significant, but not
biologically significant.
Although IHC has previously demonstrated that breast
cancer exhibits an increased PN expression level (52), no significant increase in the level of
serum PN was observed in the current study. This may be due to the
area of cancer cells and/or stromal fibroblasts being identified as
the source of PN in breast cancer compared with that in CCA, which
is smaller and may not have this caveat. These previous data are
concordant with the results of the present study that the serum PN
levels in patients with breast cancer, of which none had metastatic
disease, were not significantly increased compared with those in
the normal controls. In the metastatic cases, breast cancer cells
that have settled in the bone marrow stroma may activate the
stromal and bone cells to support the growth of metastatic cancer
cells through bone-derived factors, including TGF-β and BMPs
(53). These secreted substances may
then activate cancer cells and/or stromal fibroblasts to produce PN
and release this into the blood circulation. In HCC tissues, the
fibrous capsule surrounding the cancer cells was mildly stained for
PN (13,35). In the present study, serum PN in
patients with HCC exhibited a higher level compared with that in
patients with CCA, which is opposed to the findings of Fujimoto
et al (35). However,
increased serum PN in patients with HCC compared with that in
normal controls and benign liver diseases was supported by Lv et
al (54). Serum markers,
including alpha-fetoprotein and CA19-9, may be used for early
detection of liver and bile duct cancer but only have moderate
sensitivity and specificity (55).
When using the 94 ng/ml threshold value to distinguish patients
with CCA from patients with other conditions, the results of the
present study demonstrated that the diagnostic performance was
statistically significant with high specificity, even though the
sensitivity was low. The specificity of serum PN in the present
study was higher compared with that reported for CA19-9 (7); however, the sensitivity of the two serum
markers was low. Therefore, it is not remarkable that using serum
PN to distinguish CCA from benign liver diseases was not
statistically significant. This result from the present study may
suggest that serum PN is specific to CCA, but using it to detect
early CCA may not be effective. This conclusion is similar to that
regarding serum CA19-9 that was summarized in a guideline, which
reported CA19-9 to be insufficient to establish the diagnosis of
CCA (56). In the current study, the
combination of serum PN and CA19-9 did not add value to the
diagnostic potential of differentiating CCA from benign liver
diseases. Novel biomarkers, including the soluble fragment of
cytokeratin 19 (CYFRA 21-1), mucins, pyruvate kinase and certain
metalloproteinases, including matrix metalloproteinase 7, have been
reported to aid in the diagnosis of CCA, and in certain cases, also
act to establish prognostic values (57). However, none of these biomarkers are
part of current routine clinical practice.
The increased PN level in the serum of patients with
CCA was associated with increased PN expression levels in
CCA-associated fibroblasts. The fibroblast-derived PN may not only
act on cancer cells locally to activate the tumorigenic properties,
leading to cancer aggressiveness and reduced survival rate
(13), but also may be secreted into
the blood circulation. Notably, the results of the present study
supported that the serum and tissue PN levels may be used to divide
patients with CCA into high and low PN groups. In addition to a
previous study that demonstrated that tissue PN level was
significantly associated with reduced patient survival time
(13), high serum PN in the current
study was associated with reduced cumulative survival of patients
with CCA. Therefore, serum PN may be applied as a marker of poor
prognosis to predict the aggressiveness of the disease. Serum
CA19-9 was analyzed in the present study, and did not exhibit an
association with survival rates (data not shown). This is in
contrast to the previously reported recommendation that CA19-9 may
be of prognostic significance in CCA (56). This may be explained by the varying
CA19-9 threshold level used in the previous studies (56,58) and in
the present study. Carcinoembryonic antigen (CEA) is another serum
tumor marker that is used in CCA management and has been
identified, together with lymph node metastasis, to be associated
with advanced stage tumors and negative prognostic factors in CCA
(59). Notably, the results of the
present study indicate that the statistical impact of serum PN as a
prognostic marker appears to be an improvement upon serum CEA.
In conclusion, the current study suggests that the
impact of serum PN exclusively produced in CCA cancer-associated
fibroblasts may be used as a validated predictive marker for
patient survival. Although certain stromal substances produced from
cancer cells or cancer-associated fibroblasts have been
investigated using IHC for their increased levels and proposed as a
prognostic marker in intrahepatic CCA, including PN (13), osteopontin, laminin and TGF-β2
(60), the current study has
demonstrated that PN is a substance that is detectable in patients'
serum and able to predict the aggressiveness of the disease.
Patients with high serum PN levels at the time of diagnosis may be
considered to require aggressive adjuvant treatment. Although a
well-designed study, in which extensive patient follow-up and
regular testing of blood samples from the patients, to investigate
patients with CCA following surgery is required to inform further,
it can be suggested that serum PN is an effective indicative marker
for treatment responsiveness and tumor recurrence.
Acknowledgements
The English editing of the present manuscript was
kindly performed by Professor James A. Will (University of
Wisconsin, Madison, WI, USA). The present study has been supported
by a research grant from Thailand Research Fund (grant no.
RSA5480003) awarded to Dr Chanitra Thuwajit, and a grant from the
Faculty of Medicine Siriraj Hospital, Mahidol University (Bangkok,
Thailand).
Professor Kenji Izuhara has received >2,000,000
Japanese Yen from Shino-Test Corporation (Kanagawa, Japan) as
research funding.
References
|
1
|
Sithithaworn P, Andrews RH, Nguyen VD,
Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ and
Sripa B: The current status of opisthorchiasis and clonorchiasis in
the Mekong Basin. Parasitol Int. 61:10–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin HR, Oh JK, Masuyer E, Curado MP,
Bouvard V, Fang YY, Wiangnon S, Sripa B and Hong ST: Epidemiology
of cholangiocarcinoma: An update focusing on risk factors. Cancer
Sci. 101:579–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vatanasapt V, Uttaravichien T, Mairiang
EO, Pairojkul C, Chartbanchachai W and Haswell-Elkins M:
Cholangiocarcinoma in north-east Thailand. Lancet. 335:116–117.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sripa B, Brindley PJ, Mulvenna J, Laha T,
Smout MJ, Mairiang E, Bethony JM and Loukas A: The tumorigenic
liver fluke Opisthorchis viverrini-multiple pathways to cancer.
Trends Parasitol. 28:395–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sriputtha S, Khuntikeo N, Promthet S and
Kamsa-Ard S: Survival rate of intrahepatic cholangiocarcinoma
patients after surgical treatment in Thailand. Asian Pac J Cancer
Prev. 14:1107–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA, Miras A, Pelling M and
Taylor-Robinson SD: Cholangiocarcinoma and its management. Gut.
56:1755–1756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franco OE and Hayward SW: Targeting the
tumor stroma as a novel therapeutic approach for prostate cancer.
Adv Pharmacol. 65:267–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasebe T: Tumor-stromal interactions in
breast tumor progression-significance of histological heterogeneity
of tumor-stromal fibroblasts. Expert Opin Ther Targets. 17:449–460.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bremnes RM, Donnem T, Al-Saad S, Al-Shibli
K, Andersen S, Sirera R, Camps C, Marinez I and Busund LT: The role
of tumor stroma in cancer progression and prognosis: Emphasis on
carcinoma-associated fibroblasts and non-small cell lung cancer. J
Thorac Oncol. 6:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spector I, Zilberstein Y, Lavy A, Nagler
A, Genin O and Pines M: Involvement of host stroma cells and tissue
fibrosis in pancreatic tumor development in transgenic mice. PLoS
One. 7:e418332012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuaysri C, Thuwajit P, Paupairoj A,
Chau-In S, Suthiphongchai T and Thuwajit C: Alpha-smooth muscle
actin-positive fibroblasts promote biliary cell proliferation and
correlate with poor survival in cholangiocarcinoma. Oncol Rep.
21:957–969. 2009.PubMed/NCBI
|
|
13
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma-derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ratajczak-Wielgomas K and Dziegiel P: The
role of periostin in neoplastic processes. Folia Histochem
Cytobiol. 53:120–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Utispan K, Sonongbua J, Thuwajit P,
Chau-In S, Pairojkul C, Wongkham S and Thuwajit C: Periostin
activates integrin α5β1 through a PI3K/AKT-dependent pathway in
invasion of cholangiocarcinoma. Int J Oncol. 41:1110–1118.
2012.PubMed/NCBI
|
|
17
|
He J, Whelan SA, Lu M, Shen D, Chung DU,
Saxton RE, Faull KF, Whitelegge JP and Chang HR: Proteomic-based
biosignatures in breast cancer classification and prediction of
therapeutic response. Int J Proteomics. 2011:8964762011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumur CI, Campbell DJ, DeWitt JL, Oyesanya
RA and Sirica AE: Differential gene expression profiling of
cultured neu-transformed versus spontaneously-transformed rat
cholangiocytes and of corresponding cholangiocarcinomas. Exp Mol
Pathol. 89:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tischler V, Fritzsche FR, Wild PJ, Stephan
C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J,
Schraml P, et al: Periostin is up-regulated in high grade and high
stage prostate cancer. BMC Cancer. 10:2732010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riener MO, Fritzsche FR, Soll C,
Pestalozzi BC, Probst-Hensch N, Clavien PA, Jochum W, Soltermann A,
Moch H and Kristiansen G: Expression of the extracellular matrix
protein periostin in liver tumours and bile duct carcinomas.
Histopathology. 56:600–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morra L, Rechsteiner M, Casagrande S, von
Teichman A, Schraml P, Moch H and Soltermann A: Characterization of
periostin isoform pattern in non-small cell lung cancer. Lung
Cancer. 76:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Wang Y and Jiang C: Stromal
protein periostin identified as a progression associated and
prognostic biomarker in glioma via inducing an invasive and
proliferative phenotype. Int J Oncol. 42:1716–1724. 2013.PubMed/NCBI
|
|
23
|
Xu X, Chang W, Yuan J, Han X, Tan X, Ding
Y, Luo Y, Cai H, Liu Y, Gao X, et al: Periostin expression in
intra-tumoral stromal cells is prognostic and predictive for
colorectal carcinoma via creating a cancer-supportive niche.
Oncotarget. 7:798–813. 2016.PubMed/NCBI
|
|
24
|
Lv Y, Wang W, Jia WD, Sun QK, Li JS, Ma
JL, Liu WB, Zhou HC, Ge YS, Yu JH, et al: High-level expression of
periostin is closely related to metastatic potential and poor
prognosis of hepatocellular carcinoma. Med Oncol. 30:3852013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jang SY, Park SY, Lee HW, Choi YK, Park
KG, Yoon GS, Tak WY, Kweon YO, Hur K and Lee WK: The Combination of
Periostin Overexpression and Microvascular Invasion Is Related to a
Poor Prognosis for Hepatocellular Carcinoma. Gut Liver. 10:948–954.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sirica AE, Almenara JA and Li C: Periostin
in intrahepatic cholangiocarcinoma: Pathobiological insights and
clinical implications. Exp Mol Pathol. 97:515–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F and
Yuan YZ: Circulating levels of periostin may help identify patients
with more aggressive colorectal cancer. Int J Oncol. 34:821–828.
2009.PubMed/NCBI
|
|
29
|
Nuzzo PV, Rubagotti A, Argellati F, Di
Meglio A, Zanardi E, Zinoli L, Comite P, Mussap M and Boccardo F:
Prognostic value of preoperative serum levels of periostin (PN) in
early breast cancer (BCa). Int J Mol Sci. 16:17181–17192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Contie S, Voorzanger-Rousselot N, Litvin
J, Clézardin P and Garnero P: Increased expression and serum levels
of the stromal cell-secreted protein periostin in breast cancer
bone metastases. Int J Cancer. 128:352–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abbott KL, Lim JM, Wells L, Benigno BB,
McDonald JF and Pierce M: Identification of candidate biomarkers
with cancer-specific glycosylation in the tissue and serum of
endometrioid ovarian cancer patients by glycoproteomic analysis.
Proteomics. 10:470–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capoun O, Soukup V, Kalousová M, Sobotka
R, Pešl M, Zima T and Hanuš T: Diagnostic importance of selected
protein serum markers in the primary diagnostics of prostate
cancer. Urol Int. 95:429–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y and Du L: Role of pancreatic
stellate cells and periostin in pancreatic cancer progression.
Tumour Biol. 36:3171–3177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kudo Y, Iizuka S, Yoshida M, Nguyen PT,
Siriwardena SB, Tsunematsu T, Ohbayashi M, Ando T, Hatakeyama D and
Shibata T: Periostin directly and indirectly promotes tumor
lymphangiogenesis of head and neck cancer. PLoS One. 7:e444882012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujimoto K, Kawaguchi T, Nakashima O, Ono
J, Ohta S, Kawaguchi A, Tonan T, Ohshima K, Yano H, Hayabuchi N, et
al: Periostin, a matrix protein, has potential as a novel
serodiagnostic marker for cholangiocarcinoma. Oncol Rep.
25:1211–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okamoto M, Hoshino T, Kitasato Y, Sakazaki
Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J,
et al: Periostin, a matrix protein, is a novel biomarker for
idiopathic interstitial pneumonias. Eur Respir J. 37:1119–1127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masuoka M, Shiraishi H, Ohta S, Suzuki S,
Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al:
Periostin promotes chronic allergic inflammation in response to Th2
cytokines. J Clin Invest. 122:2590–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanemitsu Y, Matsumoto H, Izuhara K, Tohda
Y, Kita H, Horiguchi T, Kuwabara K, Tomii K, Otsuka K, Fujimura M,
et al: Increased periostin associates with greater airflow
limitation in patients receiving inhaled corticosteroids. J Allergy
Clin Immunol. 132:305–12 e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamaguchi Y, Ono J, Masuoka M, Ohta S,
Izuhara K, Ikezawa Z, Aihara M and Takahashi K: Serum periostin
levels are correlated with progressive skin sclerosis in patients
with systemic sclerosis. Br J Dermatol. 168:717–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takayama G, Arima K, Kanaji T, Toda S,
Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T and Izuhara
K: Periostin: A novel component of subepithelial fibrosis of
bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy
Clin Immunol. 118:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inai K, Norris RA, Hoffman S, Markwald RR
and Sugi Y: BMP-2 induces cell migration and periostin expression
during atrioventricular valvulogenesis. Dev Biol. 315:383–396.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ohira S, Itatsu K, Sasaki M, Harada K,
Sato Y, Zen Y, Ishikawa A, Oda K, Nagasaka T, Nimura Y and Nakanuma
Y: Local balance of transforming growth factor-beta1 secreted from
cholangiocarcinoma cells and stromal-derived factor-1 secreted from
stromal fibroblasts is a factor involved in invasion of
cholangiocarcinoma. Pathol Int. 56:381–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu JP, Mao JQ, Li MS, Lu SL, Hu XQ, Zhu SN
and Nomura S: In situ detection of TGF betas, TGF beta receptor II
mRNA and telomerase activity in rat cholangiocarcinogenesis. World
J Gastroenterol. 9:590–594. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Puppin C, Passon N, Frasca F, Vigneri R,
Tomay F, Tomaciello S and Damante G: In thyroid cancer cell lines
expression of periostin gene is controlled by p73 and is not
related to epigenetic marks of active transcription. Cell Oncol
(Dordr). 34:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oshima A, Tanabe H, Yan T, Lowe GN,
Glackin CA and Kudo A: A novel mechanism for the regulation of
osteoblast differentiation: Transcription of periostin, a member of
the fasciclin I family, is regulated by the bHLH transcription
factor, twist. J Cell Biochem. 86:792–804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Romeo F, Falbo L, Di Sanzo M, Misaggi R,
Faniello MC, Barni T, Cuda G, Viglietto G, Santoro C, Quaresima B
and Costanzo F: Negative transcriptional regulation of the human
periostin gene by YingYang-1 transcription factor. Gene.
487:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zinn PO, Mahajan B, Sathyan P, Singh SK,
Majumder S, Jolesz FA and Colen RR: Radiogenomic mapping of
edema/cellular invasion MRI-phenotypes in glioblastoma multiforme.
PLoS One. 6:e254512011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Niu Z, Iyer D, Conway SJ, Martin JF, Ivey
K, Srivastava D, Nordheim A and Schwartz RJ: Serum response factor
orchestrates nascent sarcomerogenesis and silences the
biomineralization gene program in the heart. Proc Natl Acad Sci
USA. 105:pp. 17824–17829. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Honsawek S, Udomsinprasert W, Vejchapipat
P, Chongsrisawat V, Phavichitr N and Poovorawan Y: Elevated serum
periostin is associated with liver stiffness and clinical outcome
in biliary atresia. Biomarkers. 20:157–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsukahara T, Shimoyama Y, Ebata T,
Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nakamura S
and Nagino M: Cholangiocarcinoma with intraductal tubular growth
pattern versus intraductal papillary growth pattern. Mod Pathol.
29:293–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kharaishvili G, Cizkova M, Bouchalova K,
Mgebrishvili G, Kolar Z and Bouchal J: Collagen triple helix repeat
containing 1 protein, periostin and versican in primary and
metastatic breast cancer: An immunohistochemical study. J Clin
Pathol. 64:977–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clezardin P and Teti A: Bone metastasis:
Pathogenesis and therapeutic implications. Clin Exp Metastasis.
24:599–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Riener MO: Diagnosis of tumours of the
liver and the biliary tract: New tissue and serum markers.
Pathologe. 32 Suppl 2:S304–S309. 2011. View Article : Google Scholar
|
|
56
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Malaguarnera G, Paladina I, Giordano M,
Malaguarnera M, Bertino G and Berretta M: Serum markers of
intrahepatic cholangiocarcinoma. Dis Markers. 34:219–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tamandl D, Herberger B, Gruenberger B,
Puhalla H, Klinger M and Gruenberger T: Influence of hepatic
resection margin on recurrence and survival in intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 15:2787–2794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bjornsson E, Kilander A and Olsson R: CA
19-9 and CEA are unreliable markers for cholangiocarcinoma in
patients with primary sclerosing cholangitis. Liver. 19:501–508.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sulpice L, Rayar M, Desille M, Turlin B,
Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K,
Clément B and Coulouarn C: Molecular profiling of stroma identifies
osteopontin as an independent predictor of poor prognosis in
intrahepatic cholangiocarcinoma. Hepatology. 58:1992–2000. 2013.
View Article : Google Scholar : PubMed/NCBI
|