Introduction
The prognosis of patients with glioblastoma is poor
(1–3).
Gross total resection, radiotherapy and more recently, alternating
electric fields, improve the outcome (4–9).
Chemotherapy with temozolomide improves overall survival (OS) time
predominantly in patients with glioblastoma that harbor a
methylated O-6-methylguanine-DNA methyltransferase (MGMT) promoter
(10,11). Even in these patients, tumor
progression is inevitable; at first progression, median
progression-free survival (PFS) and median overall survival (OS)
times typically reach 2–3 and 8–9 months, respectively (12). At progression, gross total resection
can be achieved again in certain patients (13). Re-irradiation is also applied when
technically possible (14).
Temozolomide rechallenge and lomustine chemotherapy are frequently
used to treat recurrent glioblastoma (12,15).
Nonetheless, as demonstrated by low PFS and OS times, the efficacy
of all approaches at recurrence is limited (12).
The anti-vascular endothelial growth factor-A
antibody bevacizumab was approved for the treatment of patients
with recurrent glioblastoma in a number of countries, including the
United States (16). European
authorities refused approval in the same year on the grounds of
insufficient evidence for improved OS time (17). In 2014, the results of two randomized
phase III trials on the use of bevacizumab with first-line
treatment were published; these trials failed to show increased OS
times when standard therapy was combined with bevacizumab (18,19). In
recurrent glioblastoma, a randomized phase II trial indicated that
bevacizumab combined with lomustine improves survival rate compared
with lomustine alone (20).
Therefore, a phase III European Organization for Research and
Treatment of Cancer trial (designated BELOREC) (21) was conducted to compare lomustine
monotherapy with lomustine plus bevacizumab in patients with
glioblastoma at first recurrence. The results were presented at the
2015 Society for Neuro-oncology annual meeting. As in the
first-line trials, bevacizumab prolonged PFS time; however, OS time
was not improved (21).
Besides being approved in a number of countries,
bevacizumab may be reimbursed by health insurance companies in
certain countries that lack approval (17). Procedures for reimbursement are
heterogeneous in the EU. Whereas certain countries, including the
Netherlands, refuse reimbursement in general, others, including
France, have a more generous approach to access to bevacizumab for
patients with glioblastoma. In Germany, reimbursement must be
applied for; such applications are only promising if all treatment
options (e.g. surgery and radiotherapy) and approved drugs (e.g.
temozolomide and lomustine) have already been used for the specific
patient. Due to the aforementioned negative study results, the
reimbursement chance is likely to decrease in the future. Due to
the reimbursement issue, although bevacizumab failed to prolong OS
as a first-line treatment and at first recurrence after failure of
radiotherapy and chemotherapy with temozolomide, crossover was a
critical issue in the previously described trials (18–21). A
relevant number of patients had been treated with bevacizumab in
the control treatment arm.
Despite these negative trials, bevacizumab is widely
used for the treatment of patients with recurrent glioblastoma.
Randomized clinical trials comparing bevacizumab with best
supportive care or the investigator's choice of treatment following
the failure of radiotherapy, temozolomide and lomustine are
lacking. To the best of our knowledge, there are also no
retrospective studies on bevacizumab focusing on patients with
primary glioblastoma following the failure of radiotherapy,
temozolomide and lomustine. Therefore, in the present study, a
database was screened for patients with primary glioblastoma who
had been treated with bevacizumab for recurrent glioblastoma. The
key inclusion criterion was previous treatment with radiotherapy,
temozolomide and lomustine. The response rate, PFS and OS rate and
time, and the influence of response on PFS and OS rate and time
were evaluated.
Patients and methods
Patient selection
The database of the University Hospital Frankfurt
(Frankfurt, Germany) was screened for patients with recurrent
glioblastoma who were treated with bevacizumab between January 2009
and December 2016. Only patients with primary glioblastoma were
included. Patients with an isocitrate dehydrogenase (IDH) 1
mutation or a previous diagnosis of a lower grade glioma were
excluded. Only patients treated with radiotherapy, temozolomide and
lomustine prior to the initiation of bevacizumab treatment were
included, and there were no exclusion criteria. The general patient
characteristics, Karnofsky performance score (KPS) at the start of
bevacizumab treatment, MGMT promoter methylation status, prior
treatments, number of prior recurrences and details of bevacizumab
treatment were recorded. In order to evaluate response rate,
magnetic resonance imaging (MRI) scans from prior to and during
bevacizumab treatment were assessed, according to the Response
Assessment in Neuro-Oncology (RANO) criteria (22). To estimate progression-free survival
(PFS) and overall survival (OS) times, the interval from the
initiation of bevacizumab treatment to the time of progression
according to RANO criteria, and to the date of patient mortality
from any cause, were recorded, respectively.
A number of patients from the cohort were included
in previously published studies on the use of bevacizumab in
recurrent glioblastoma (23–25).
Ethics and approval
The present study was performed in accordance with
the ethical standards of the 1964 Declaration of Helsinki and its
later amendments. All patient data were anonymized prior to
statistical analysis. Ethics board approval (Ethics Committee,
University Hospital, Frankfurt, Germany) was obtained for this
retrospective study. No additional data was created or used aside
from the retrospective evaluation of the database.
Statistical analysis
Survival analysis was performed using the
Kaplan-Meier method and the log-rank test to compare the group of
non-responders with the group of responders using SPSS Statistics
version 20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate statistical significance.
Results
Patient characteristics
The recorded patient characteristics are included in
Table I. The median age of the
patient cohort was 53 years; approximately one-third (21/62) of the
patients were female. All patients had previously been diagnosed
with primary glioblastoma. A total of 54.8% of the patients
exhibited an unmethylated MGMT promoter and 27.4% exhibited a
methylated MGMT promoter; for the remaining 17.8%, promoter
methylation status was inconclusive or unknown. A total of 8.1% of
the patients underwent biopsy only as the means of initial
diagnosis, with no further surgical procedures, whereas 40.3%
underwent ≥2 resections prior to the start of bevacizumab
treatment. All patients received prior radiotherapy and 45.2% had
undergone at least a second course of radiotherapy prior to
treatment with bevacizumab. The median number of previous
chemotherapies was 2, and according to the inclusion criteria, all
patients had received prior temozolomide and lomustine
chemotherapy. The median number of prior recurrences was 3.
 | Table I.Patient characteristics and
pretreatments (n=62). |
Table I.
Patient characteristics and
pretreatments (n=62).
|
Characteristics | Value |
|---|
| General |
|
| Median
age (range), years | 53 (28–75) |
| Female,
% (n) | 33.9 (21) |
| Karnofsky
performance score |
|
| Median
(range), % | 80 (50–100) |
| 50–60%,
% (n) | 27.4 (17) |
| 70–80%,
% (n) | 45.2 (28) |
|
90–100%, % (n) | 27.4 (17) |
| Neuropathology, %
(n) |
|
| Primary
glioblastoma | 100.0 (62) |
|
Glioblastoma with
oligodendroglial component | 3.2 (2) |
|
O-6-methylguanine-DNA methyltransferase
promoter status, % (n) |
|
|
Unmethylated | 54.8 (34) |
|
Methylated | 27.4 (17) |
|
Inconclusive | 11.3 (7) |
|
Unknown | 6.5 (4) |
| Prior surgery, %
(n) |
|
| Biopsy
only | 8.1 (5) |
| One
resection | 51.6 (32) |
| Two
resections | 27.4 (17) |
| More
than two resections | 12.9 (8) |
| Prior radiotherapy,
% (n) |
|
| Prior
radiotherapy | 100.0 (62) |
| One
radiotherapy | 54.8 (34) |
| Two
radiotherapies | 41.9 (26) |
| More
than two radiotherapies | 3.2 (2) |
| Prior
chemotherapy |
|
| Median
number of chemotherapies (range) | 2 (2–6) |
| Two
chemotherapies, % (n) | 58.1 (36) |
| Three
chemotherapies, % (n) | 35.5 (22) |
| Prior
temozolomide treatment, % (n) | 100.0 (62) |
| Prior
lomustine treatment, % (n) | 100.0 (62) |
| Prior
recurrences |
|
| Median
number of recurrences (range) | 3 (2–5) |
| Two
recurrences, % (n) | 37.1 (23) |
| Three
recurrences, % (n) | 38.7 (24) |
| Four
recurrences, % (n) | 16.1 (10) |
Concomitant treatment and response
rate
The majority of patients (59.7%) were treated with
bevacizumab monotherapy, whereas 27.4% received irinotecan or
lomustine in combination with bevacizumab (Table II). The response to therapy was
evaluated according to RANO criteria by two experienced
neuroradiologists and results are included in Table II. A total of 35.5% of the patients
exhibited progressive disease at the first MRI follow-up scan,
whereas 9.7% exhibited stable disease. These groups were regarded
as non-responders. On the other hand, 51.6% of patients achieved
partial remission and 3.2% achieved complete remission. These
groups were regarded as responders; therefore, the response rate
was 54.8%.
 | Table II.Concomitant treatment and response
(n=62). |
Table II.
Concomitant treatment and response
(n=62).
| Characteristic | % (n) |
|---|
| Concomitant
therapy |
|
|
Bevacizumab monotherapy | 59.7 (37) |
|
Irinotecan | 16.1 (10) |
|
Lomustin | 11.3 (7) |
|
Radiotherapy | 6.5 (4) |
|
Other | 6.5 (4) |
|
Responsea |
|
Progressive disease | 35.5 (22) |
| Stable
disease | 9.7 (6) |
| Partial
remission | 51.6 (32) |
|
Complete remission | 3.2 (2) |
Survival
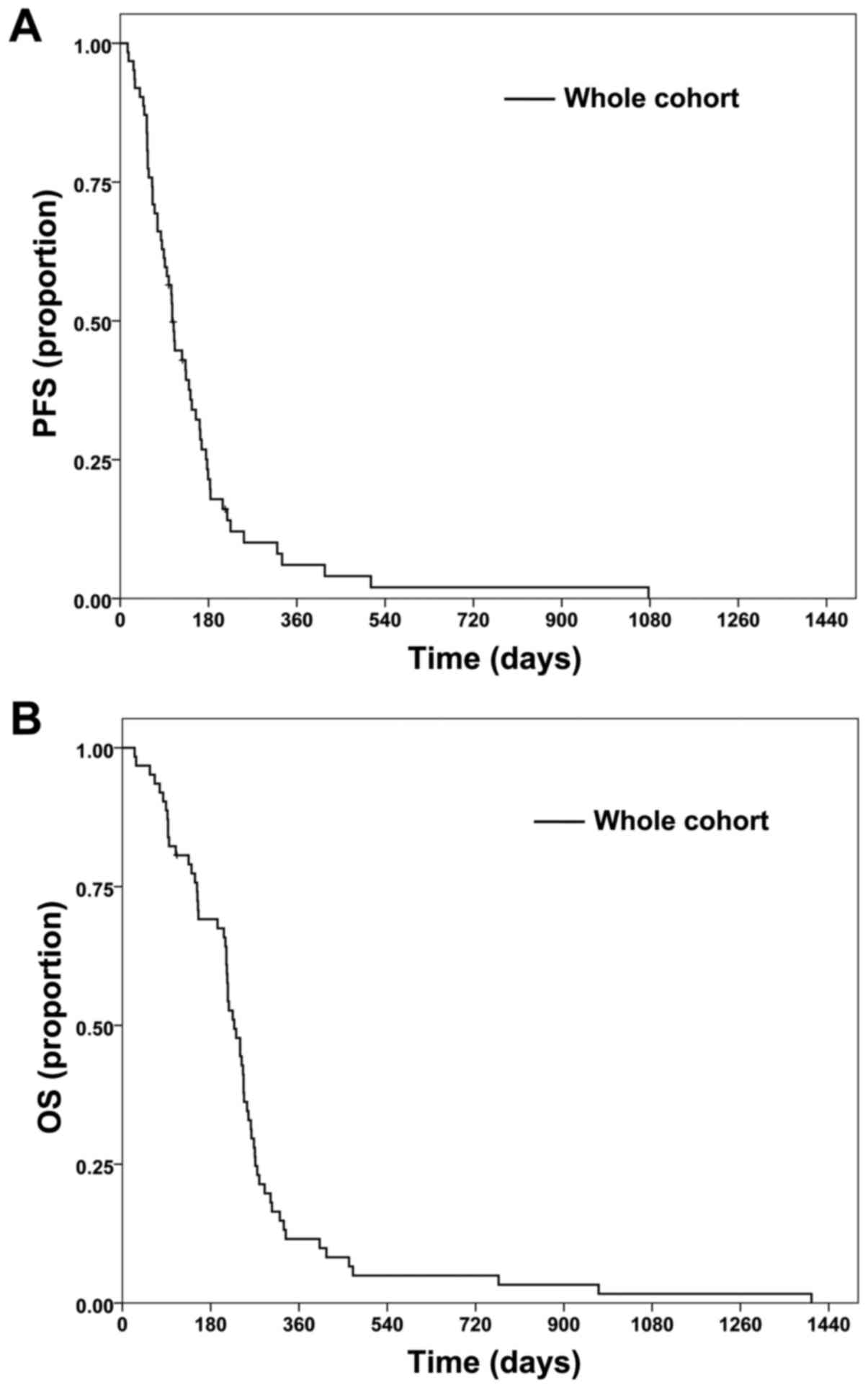
PFS and OS times for the cohort are shown in
Fig. 1. The median PFS time was 3.5
months (Fig. 1A) and the median OS
time was 7.5 months (Fig. 1B). PFS
rate at 6 months was 21.5%, while OS rate at 12 months was 11.5%.
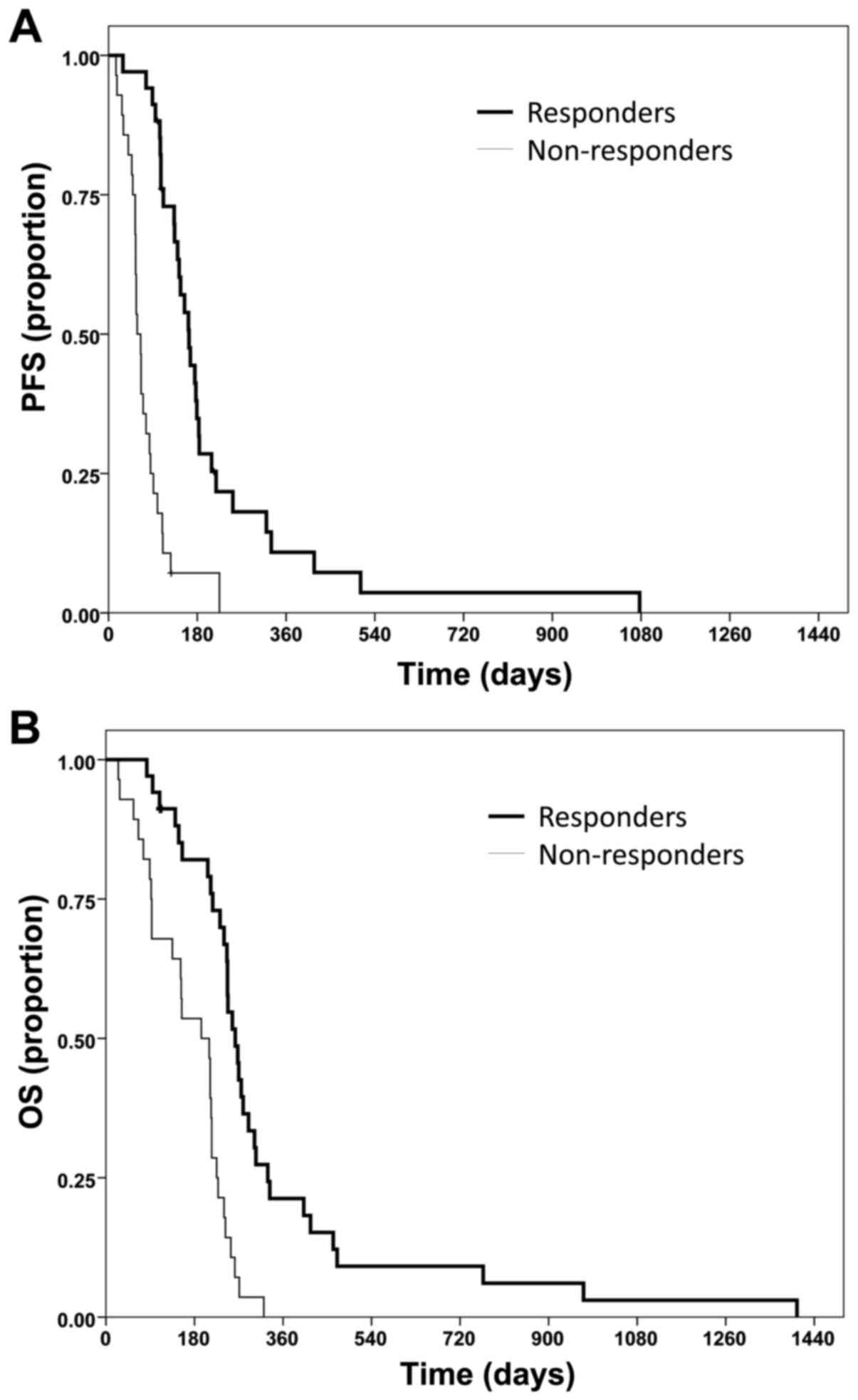
The median PFS time was 5.4 months for responders (Fig. 2A) compared with 1.9 months in
non-responding patients (Fig. 2B;
P<0.0001). The PFS rate at 6 months was also superior in
responders (34.9%) compared to non-responders (7.1%; P<0.0001).
The median OS time in responders was 8.6 months, whereas
non-responders only reached a median of 6.4 months (P<0.0001).
OS rate at 12 months was 21.3% for responders and 0% for
non-responders (P<0.0001).
MGMT promoter methylation status and patient age had
no significant association with OS time and rate (data not shown).
By contrast, patients with a KPS of 50 or 60% exhibited a decreased
median OS time (4.4 months) compared with patients with a score of
70 or 80% (8.0 months) and patients with a score of 90 or 100% (8.2
months) (data not shown).
Discussion
Bevacizumab has failed to prolong OS time when
combined with radiotherapy and temozolomide chemotherapy as a
first-line treatment, and at first recurrence when combined with
lomustine (18–21,26).
Randomized clinical trials at second or further recurrences
following the failure of radiotherapy, temozolomide and lomustine
are lacking. To the best of our knowledge, the present study
provides the first retrospective data for patients with primary
glioblastoma treated with bevacizumab following the failure of
radiotherapy, temozolomide and lomustine. In contrast to previous
retrospective studies, patients with lower grade gliomas or
secondary glioblastoma were not included. Moreover, previous
retrospective and prospective studies have included patients at the
first, second or further recurrence, with patients at the first
recurrence usually being the largest group of patients (16,27–34). The
present study only evaluated patients who had received
radiotherapy, temozolomide and lomustine prior to bevacizumab
treatment. The majority of patients had undergone first-line
treatment according to the Stupp protocol (1) and had received lomustine at the first
progression prior to treatment with bevacizumab at the second
progression. Consequently, the median number of prior
chemotherapies in the cohort was 2. Patients with >2
chemotherapies usually received another course of temozolomide. In
addition, >40% of the patients received a second radiotherapy;
the median number of recurrences prior to bevacizumab treatment was
3. In summary, bevacizumab usually was the last line of therapy for
the patients in the cohort of the present study.
Overall, >50% of patients in the cohort responded
to bevacizumab treatment, with 51.6% (n=32) exhibiting partial
remission and 3.2% (n=2) complete remission, according to RANO
criteria. These values match or exceed the response rates from
trials that predominantly enrolled patients at first recurrence
(16,21,22,27–34).
In line with several previous studies, this suggests that response
rates do not decrease when bevacizumab is used at later progression
(16,21,22,27–34).
The median PFS time of 3.5 months that was
identified in the present study is in the range of previous study
results. In the ‘BRAIN’ trial, median PFS time was 4.2 months for
bevacizumab monotherapy and 5.6 months for the combination with
irinotecan (16). The BELOREC trial
demonstrated a median PFS time of 1.54 months for lomustine and
4.17 months for lomustine plus bevacizumab (21). Other trials, which featured a
lomustine monotherapy arm at the first recurrence, exhibited a
median PFS time of 1.6 and 2.7 months (35,36).
Together with a PFS rate at 6 months of 21.5%, this suggests that
bevacizumab is associated with a favorable PFS outcome in patients
even subsequent to the failure of radiotherapy, temozolomide and
lomustine.
Median OS time in the present study was 7.5 months.
In the BELOREC trial, a time of 9.1 months was reported following
treatment at first recurrence for the lomustine plus bevacizumab
arm, and 8.6 months for the lomustine arm (21). Other trials reported a median OS of
8–9 months for patients treated at the first recurrence (16,20).
Bearing in mind that the patients of the present study were treated
with bevacizumab at a median of the third recurrence and that
bevacizumab was typically the last-line therapy, this suggests that
bevacizumab positively influenced the OS outcome.
Response rates for bevacizumab treatment have been
challenged since response may only reflect the influence of
bevacizumab on the contrast enhancement and edema without actually
affecting tumor growth (37).
Therefore, the present study also evaluated how the response to
bevacizumab was associated with PFS and OS time. Responders
exhibited a median PFS time of 5.4 months compared with 1.9 months
in non-responders. The median OS time in responders was 8.6 months,
whereas non-responders reached 6.4 months. OS rate at 12 months was
21.3% for responders and 0% for non-responders. This association
between response and PFS and OS rates and times suggests that
radiological response may be a surrogate marker for survival.
One explanation for this association is that
bevacizumab exhibits antitumor activity. This would fit with our
clinical impression that certain patients benefit from treatment
with bevacizumab. A detailed gene expression analysis of the cohort
from the AVAGlio study corroborates this impression; patients with
an IDH1 wild-type proneural glioblastoma exhibited an increase in
OS of 4.3 months (38). Our previous
studies in a relevant number of patients with glioblastoma
identified radiological biomarkers suggesting that bevacizumab may
exhibit antitumor activity (23,24). In
the first of these studies, ~60% of bevacizumab treated patients
developed tumor calcifications; survival statistics were superior
for these patients (23). In the
second study, 28% of the patients treated with bevacizumab
developed MRI lesions that were T1 hyperintense and exhibited
restricted diffusion. These patients exhibited superior median OS
times (13 vs. 6.6 months) and histological analysis of these
lesions revealed extensive calcified necrosis with no viable tumor
cells (24). Alternately, the
influence of bevacizumab on OS time could be conferred by the
reduction of edema or the incidental treatment of radiation
necrosis. The latter may be unlikely in the present study, as
patients were significantly pre-treated, and the time from initial
radiotherapy was, in general, >6 months.
The combination of bevacizumab with re-irradiation
is another promising concept for the therapy of glioblastoma. This
may not only reduce the risk of pseudoprogression, but also enhance
the efficacy of re-irradiation due to the initially improved
perfusion and oxygenation of the tumor. Prospective, randomized and
controlled trials to consider this combination are missing. Schnell
et al (39) recently reported
on promising results for this combination in a large retrospective
cohort: Patients that received re-irradiation and bevacizumab
showed a median OS of 13.1 months. The results were further
corroborated by a retrospective Italian study (40). Median OS was 11 months for patient
that received re-irradiation with bevacizumab and 8.3 months for
patients that received re-irradiation with fotemustin.
Due to the retrospective design of the present study
and the limited number of patients, the results should be
interpreted cautiously. A comprehensive evaluation of quality of
life and neurocognitive function in the patient cohort was not
considered; this is of particular importance, since the clinical
situation of numerous patients can improve due to resolving edema
and mass effect, and reducing steroid intake. It is also unclear
whether bevacizumab may have adverse effects on neurocognitive
function (18,41). The more general side effects of
bevacizumab are of comparably minor relevance in these heavily
pretreated patients without further treatment options.
None of the patients of the cohort from the present
study were suitable for another resection or further radiotherapy;
45.2% had already undergone radiotherapy twice. In the EU,
temozolomide and nitrosourea (lomustine) are the only drugs
approved for the treatment of glioblastoma. Besides bevacizumab, no
other therapeutic option was available for the patients. Thus, the
response rate and the PFS and OS times and rates are promising for
this heavily pre-treated cohort receiving bevacizumab as last-line
therapy subsequent to the failure of all approved options. Together
with the association of response with superior survival time and
rate, the results indicate that bevacizumab may exhibit activity in
glioblastoma as a last-line treatment. It is proposed that these
results justify the reimbursement of bevacizumab in these heavily
pretreated patients.
Considering the negative results from first-line
trials and trials at first recurrence together with the results of
the present retrospective study, a randomized trial comparing
bevacizumab with precisely defined best supportive care (42,43), or
investigator's choice of therapy, is advised.
Acknowledgements
The Dr Senckenberg Institute of Neurooncology is
supported by the Dr Senckenberg Foundation. The authors wish to
disclose the following conflicts of interest: Dr Oliver Bähr has
served as a consultant for Roche Pharma AG (Grenzach-Whylen,
Germany) and has received a travel grant from Roche Pharma AG, the
European distributor of bevacizumab (as Avastin); and Dr Joachim P.
Steinbach has served as a consultant for Roche Pharma AG.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, van den Bent M, Hopkins K, Tonn
JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D,
Henriksson R, Balana C, et al: EANO guideline for the diagnosis and
treatment of anaplastic gliomas and glioblastoma. Lancet Oncol.
15:e395–e403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keime-Guibert F, Chinot O, Taillandier L,
Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin
P, Bondiau PY, et al: Radiotherapy for glioblastoma in the elderly.
N Engl J Med. 356:1527–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kreth FW, Thon N, Simon M, Westphal M,
Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T,
Weller M, et al: Gross total but not incomplete resection of
glioblastoma prolongs survival in the era of radiochemotherapy. Ann
Oncol. 24:3117–3123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laperriere N, Zuraw L and Cairncross G;
Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology
Disease Site Group, : Radiotherapy for newly diagnosed malignant
glioma in adults: A systematic review. Radiother Oncol. 64:259–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senft C, Bink A, Franz K, Vatter H, Gasser
T and Seifert V: Intraoperative MRI guidance and extent of
resection in glioma surgery: A randomised, controlled trial. Lancet
Oncol. 12:997–1003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ; ALA-Glioma Study Group, :
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vuorinen V, Hinkka S, Färkkilä M and
Jääskeläinen J: Debulking or biopsy of malignant glioma in elderly
people-a randomised study. Acta Neurochir (Wien). 145:5–10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
De Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seystahl K, Wick W and Weller M:
Therapeutic options in recurrent glioblastoma-An update. Crit Rev
Oncol Hematol. 99:389–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montemurro N, Perrini P, Blanco MO and
Vannozzi R: Second surgery for recurrent glioblastoma: A concise
overview of the current literature. Clin Neurol Neurosurg.
142:60–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieder C, Andratschke NH and Grosu AL:
Re-irradiation for recurrent primary brain tumors. Anticancer Res.
36:4985–4995. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brandes AA, Bartolotti M, Tosoni A and
Franceschi E: Nitrosoureas in the management of malignant gliomas.
Curr Neurol Neurosci Rep. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WKA, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wick W, Weller M, van den Bent M and Stupp
R: Bevacizumab and recurrent malignant gliomas: A European
perspective. J Clin Oncol. 28:e188–e192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taal W, Oosterkamp HM, Walenkamp AM,
Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D,
De Vos FY, et al: Single-agent bevacizumab or lomustine versus a
combination of bevacizumab plus lomustine in patients with
recurrent glioblastoma (BELOB trial): A randomised controlled phase
2 trial. Lancet Oncol. 15:943–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wick W, Brandes A, Gorlia T, Bendszus M,
Sahm F, Taal W, Taphoorn M, Domont J, Idbaih A, Campone M, et al:
Lb-05 phase iii trial exploring the combination of bevacizumab and
lomustine in patients with first recurrence of a glioblastoma: The
eortc 26101 trial. Neuro Oncol. 17 Suppl 5:v12015. View Article : Google Scholar
|
|
22
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bähr O, Hattingen E, Rieger J and
Steinbach JP: Bevacizumab-induced tumor calcifications as a
surrogate marker of outcome in patients with glioblastoma. Neuro
Oncol. 13:1020–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bähr O, Harter PN, Weise LM, You SJ,
Mittelbronn M, Ronellenfitsch MW, Rieger J, Steinbach JP and
Hattingen E: Sustained focal antitumor activity of bevacizumab in
recurrent glioblastoma. Neurology. 83:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaub C, Tichy J, Schäfer N, Franz K,
Mack F, Mittelbronn M, Kebir S, Thiepold AL, Waha A, Filmann N, et
al: Prognostic factors in recurrent glioblastoma patients treated
with bevacizumab. J Neurooncol. 129:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herrlinger U, Schäfer N, Steinbach JP,
Weyerbrock A, Hau P, Goldbrunner R, Friedrich F, Rohde V, Ringel F,
Schlegel U, et al: Bevacizumab plus irinotecan versus temozolomide
in newly diagnosed O6-methylguanine-DNA methyltransferase
nonmethylated glioblastoma: The randomized GLARIUS trial. J Clin
Oncol. 34:1611–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chamberlain MC and Johnston SK: Salvage
therapy with single agent bevacizumab for recurrent glioblastoma. J
Neurooncol. 96:259–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Møller S, Grunnet K, Hansen S, Schultz H,
Holmberg M, Sorensen M, Poulsen HS and Lassen U: A phase II trial
with bevacizumab and irinotecan for patients with primary brain
tumors and progression after standard therapy. Acta Oncol.
51:797–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagane M, Nishikawa R, Narita Y, Kobayashi
H, Takano S, Shinoura N, Aoki T, Sugiyama K, Kuratsu J, Muragaki Y,
et al: Phase II study of single-agent bevacizumab in Japanese
patients with recurrent malignant glioma. Jpn J Clin Oncol.
42:887–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raizer JJ, Grimm S, Chamberlain MC,
Nicholas MK, Chandler JP, Muro K, Dubner S, Rademaker AW, Renfrow J
and Bredel M: A phase 2 trial of single-agent bevacizumab given in
an every-3-week schedule for patients with recurrent high-grade
gliomas. Cancer. 116:5297–5305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Wagner M, et al: Phase II trial of bevacizumab and
irinotecan in recurrent malignant glioma. Clin Cancer Res.
13:1253–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Sampson J, et al: Bevacizumab plus irinotecan in
recurrent glioblastoma multiforme. J Clin Oncol. 25:4722–4729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuniga RM, Torcuator R, Jain R, Anderson
J, Doyle T, Ellika S, Schultz L and Mikkelsen T: Efficacy, safety
and patterns of response and recurrence in patients with recurrent
high-grade gliomas treated with bevacizumab plus irinotecan. J
Neurooncol. 91:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wick W, Puduvalli VK, Chamberlain MC, van
den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S,
Musib L, et al: Phase III study of enzastaurin compared with
lomustine in the treatment of recurrent intracranial glioblastoma.
J Clin Oncol. 28:1168–1174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Batchelor TT, Mulholland P, Neyns B,
Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S,
Ashby LS, et al: Phase III randomized trial comparing the efficacy
of cediranib as monotherapy, and in combination with lomustine,
versus lomustine alone in patients with recurrent glioblastoma. J
Clin Oncol. 31:3212–3218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hattingen E, Jurcoane A, Daneshvar K,
Pilatus U, Mittelbronn M, Steinbach JP and Bähr O: Quantitative T2
mapping of recurrent glioblastoma under bevacizumab improves
monitoring for non-enhancing tumor progression and predicts overall
survival. Neuro Oncol. 15:1395–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sandmann T, Bourgon R, Garcia J, Li C,
Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R,
et al: Patients with proneural glioblastoma may derive overall
survival benefit from the addition of bevacizumab to first-line
radiotherapy and temozolomide: Retrospective analysis of the
AVAglio trial. J Clin Oncol. 33:2735–2744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schnell O, Thorsteinsdottir J, Fleischmann
DF, Lenski M, Abenhardt W, Giese A, Tonn JC, Belka C, Kreth FW and
Niyazi M: Re-irradiation strategies in combination with bevacizumab
for recurrent malignant glioma. J Neurooncol. 130:591–599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minniti G, Agolli L, Falco T, Scaringi C,
Lanzetta G, Caporello P, Osti MF, Esposito V and Enrici RM:
Hypofractionated stereotactic radiotherapy in combination with
bevacizumab or fotemustine for patients with progressive malignant
gliomas. J Neurooncol. 122:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fathpour P, Obad N, Espedal H, Stieber D,
Keunen O, Sakariassen PØ, Niclou SP and Bjerkvig R: Bevacizumab
treatment for human glioblastoma. Can it induce cognitive
impairment? Neuro Oncol. 16:754–756. 2014.PubMed/NCBI
|
|
42
|
Zafar SY, Currow DC, Cherny N, Strasser F,
Fowler R and Abernethy AP: Consensus-based standards for best
supportive care in clinical trials in advanced cancer. Lancet
Oncol. 13:e77–e82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zafar SY, Currow D and Abernethy AP:
Defining best supportive care. J Clin Oncol. 26:5139–5140. 2008.
View Article : Google Scholar : PubMed/NCBI
|