Introduction
Thyroid carcinoma is a common endocrine malignancy.
It accounts for 1.3% of systemic malignancies and 5.1% of head and
neck cancer. Thyroid cancer has a variety of pathological types,
and the most common type is papillary thyroid carcinoma (PTC),
which accounts for 59.5–89.0% of thyroid carcinoma (1). At present, it is commonly believed that
the occurrence and development of cancer are closely related to
changes at the genetic level. Various genetic techniques have been
applied for the diagnosis and treatment of tumors. Several genes
have been identified that are related to the occurrence,
development, and prognosis of thyroid cancer (2). In recent years, genetic testing of
thyroid benign and malignant tumors has been extensively performed,
and the results suggest there is a high frequency of BRAF mutations
in PCT. By contrast, BRAF mutations have not been detected in any
other benign or malignant thyroid tumors (1–3). This
indicates that BRAF mutations are important genetic events in PTC,
and are closely related to its occurrence and development. BRAF,
also known as murine sarcoma viral oncogene homolog B1, is a member
of the RAF gene family (4). At
present, over 30 types of BRAF mutations have been identified, of
which approximately 90% are detected in exon 15, and 10% in exon 11
(4). An important BRAF mutation in
PTC is thymine substituted with adenine at nucleotide 1799 of the
11th and 15th exons, which results in a missense mutation of codon
600 (V600E) (4,5). This leads to valine being substituted by
glutamate. It has been reported that the frequency of the BRAF
V600E mutation varies greatly (28–83%) (5–8).
Thyroid B ultrasound is a form of ultrasound
examination. The principle of imaging is based on the differences
of reflection, absorption, and attenuation of ultrasonic waves in
thyroid tissue and surrounding neck tissues. Thyroid B ultrasound
can clearly show the anatomical morphology of the thyroid, its
size, internal tissue echo, the presence or absence of nodules, and
tumors. In addition, it can detect deep thyroid nodules which are
undetectable by common physical examination. Therefore, it is a
preferred auxiliary examination for patients with thyroid diseases
(9–13), and a preferred method for the
evaluation of anatomic abnormalities of the thyroid and for the
guidance of fine needle aspiration biopsy. High frequency and high
resolution ultrasound probes, as well as color Doppler ultrasound
further improve the accuracy of ultrasound diagnosis. Some features
of ultrasound such as low echo, microcalcification, and increased
blood flow in nodules detected by Doppler ultrasound are important
for the diagnosis of malignant nodules and thyroid cancer (6). However, the association between the BRAF
V600E mutation and thyroid ultrasound features in patients with PTC
remains unclear.
The aim of the present study was to investigate the
incidence of BRAF mutations in patients with PTC, by analyzing BRAF
mutations in fresh thyroid carcinoma tissue and paracarcinoma
tissue, and to compare the differences in PTC patients with and
without BRAF V600E mutations, thus providing a basis for the
clinical diagnosis and treatment of thyroid cancer.
Materials and methods
General information
Fresh thyroid carcinoma tissue and paracarcinoma
tissue (over 5 cm away from the edge of carcinoma tissue) were
collected from 34 patients undergoing surgery for PTC from December
2009 to June 2010 in People's Hospital of Zhengzhou University
(Henan, China). Thyroid carcinoma was confirmed by
histopathological analysis. The 34 patients included 28 females and
6 males, aged 16–75 years. The thyroid ultrasound features of
patients were obtained using GE Logiq 9 and GE Logiq 700 (both from
GE Healthcare, Piscataway, NJ, USA) color Doppler ultrasound
scanners (frequency: 10–14 MHz). For each patient, tumor size
(recorded at its largest diameter), border (clear or unclear), and
the presence or absence of calcifications were analyzed. This study
was approved by the Ethics Committee of Henan Provincial People's
Hospital. Signed written informed consents were obtained from all
participants before the study.
Reagents
Tissue lysis solution, protease K solution (20
mg/ml), phenol:chloroform:isoamyl alcohol (25:24:1), isoamyl
alcohol, chloroform, 3 M sodium acetate solution, 75% ethanol, and
double-distilled water were purchased from Guangzhou Chemical
Reagent Factory (Guangzhou, China). Taq enzymes and related
reagents were purchased from Tiangen Biotech Co., Ltd. (Beijing,
China). Protein molecular weight marker and PCR marker were
purchased from Takara Bio, Inc. (Otsu, Japan). Agarose powder was
purchased from the Shanghai Institute of Medicine of Chinese
Academy of Medical Sciences (Shanghai, China). TBE buffer, shrimp
alkaline phosphatase, and exonuclease were purchased from Zhong
Shan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Instruments and equipment
Instruments used in the present study were:
Horizontal agarose electrophoresis equipment (Shanghai Jingyi
Organic Glass Products and Instruments Factory, Shanghai, China),
centrifuge, 37°C incubator (Thermo Fisher Scientific, Waltham, MA,
USA), −20°C refrigerator (Qingdao Haier Pharmaceutical Co., Ltd.,
Qingdao, China), DNA thermal cycler 480 (Perkin-Elmer, Norwalk, CT,
USA), ABI 3730 DNA Sequencer (Perkin-Elmer Applied Biosystems,
Foster City, CA, USA); PTC-200 high throughput PCR machine (MJ
Research, Inc., South San Francisco, CA, USA); and ultraviolet
spectrophotometer (Mettler-Toledo, Schwerzenbach, Switzerland).
Extraction of genomic DNA
Thyroid tissue was placed in a 2 ml centrifuge tube.
Tissue lysis buffer (800 µl) and 20 µl of pre-cooled proteinase K
solution were added. Centrifuge tubes were placed on a shaker for
gentle agitation (100–150 times/min) at 50°C overnight. A total of
820 µl of phenol:chloroform:isoamyl alcohol (25:24:1) was added and
then gently mixed for 10 min until the solution turned milky-white.
The samples were centrifuged at 188.942 × g for 15 min at 4°C. The
supernatant was aspirated and transferred to another centrifuge
tube. An equal volume of chloroform was then added to the
supernatant, mixed gently for 10 min, and centrifuged at 11,500 × g
for 15 min at 4°C. The supernatant was transferred to another 2 ml
centrifuge tube. An equal volume of pre-cooled (−20°C) isopropanol
and 10% of the supernatant volume of pre-cooled (0°C) sodium
acetate were added, and gently mixed by inversion 10 times until
the flocculation precipitate was observed. The samples were then
centrifuged at 11,500 × g for 5 min at 4°C. The supernatant was
discarded, 1 ml of 75% ethanol was added, and the samples were
centrifuged at 11,500 × g and 4°C for 5 min. Ethanol was removed,
and the precipitate was washed once with 75% ethanol. Ethanol was
discarded and samples were centrifuged at 11,500 × g for 5 min at
4°C. The remaining ethanol was aspirated, and the precipitate was
left to air dry for 20–30 min until it became semi-translucent.
Next, 30–50 µl of double-distilled water was added to dissolve the
sample. Samples were placed at 37°C for 30 min for better
dissolution, then cooled and stored at −20°C; or placed at 4°C
overnight until DNA was completely dissolved and then stored at
−20°C.
Identification of genomic DNA and
determination of concentration
After DNA was completely dissolved at room
temperature, absorbance (OD value) at 260 and 280 nm was measured.
Distilled water was used as a blank control.
Identification of DNA purity and
quality
The OD 260/280 nm ratio was calculated. The purity
of DNA was considered high if the ratio was close to 1.8, while a
ratio of >2.0 indicated RNA contamination, and ratio of <1.6
indicated organic solvent contamination.
Polymerase chain reaction
Primers used for amplification of the BRAF
gene and BRAF mutant genes were designed using Primer
Premier 7.0 (Primer, Quebec, Canada), and produced by the Shanghai
Bioengineering Co., Ltd. (Shanghai, China). The upstream and
downstream primers, respectively, were:
5′-CATCCTAACACATTTCAAGCCCC-3′, and 5′-TCACACCTGCCTTAAATTGCATAC-3′.
The amplified fragment was 795 bp in length. The volume of the PCR
reaction system was 20 µl. The PCR cycles were performed on a
PTC-200 high-throughput PCR machine. The thermal profile was as
follows: pre-denaturation at 95°C for 5 min (denaturation at 95°C
for 30 sec, annealing at 59°C for 30 sec, extension at 72°C for 55
sec) × 35 cycles, extension at 72°C for 10 min.
DNA sequencing
The PCR products were sent to Shanghai Biotechnology
Co., Ltd. (Shanghai, China) for sequencing to detect the BRAF V600E
mutation. The sequencing results were compared using BLAST. The
results included the base pairs TT, TA, and AA, which were likely
present at nucleotide 1799.
Statistical analysis
Data were analyzed using SPSS 17.0 (IBM Corp.,
Armonk, NY, USA) software. Statistical analyses were performed
using the t-test and the exact probability test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Results of DNA sequencing
Part of exon 15 of BRAF was amplified using specific
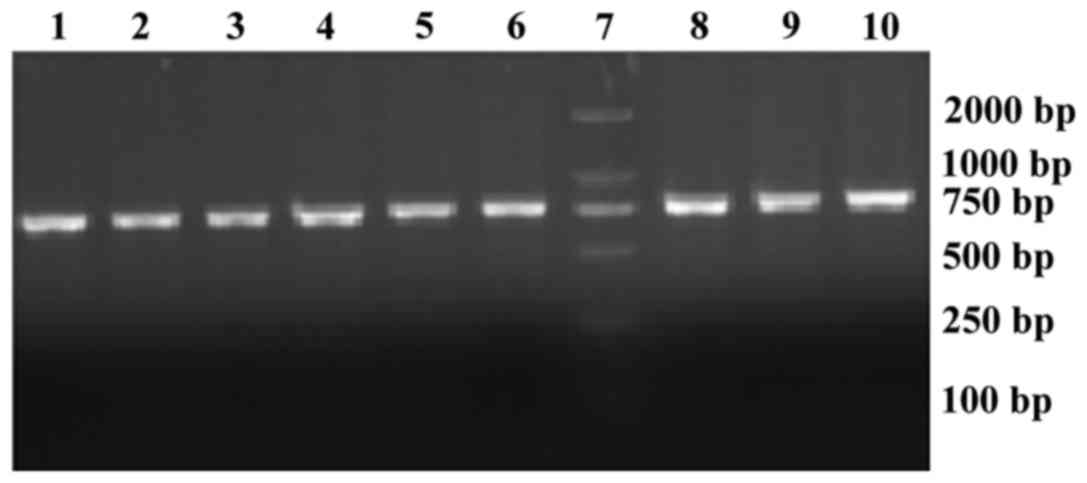
primers, and the length of the product was 795 bp. The results of
electrophoresis of the PCR reaction products are shown in Fig. 1. The 7th lane shows the marker
(500–2,000 bp), while the remaining lanes represent the
electrophoresis bands of the PCR reaction products from nine
samples. All the products were between 750 and 1,000 bp. The BRAF
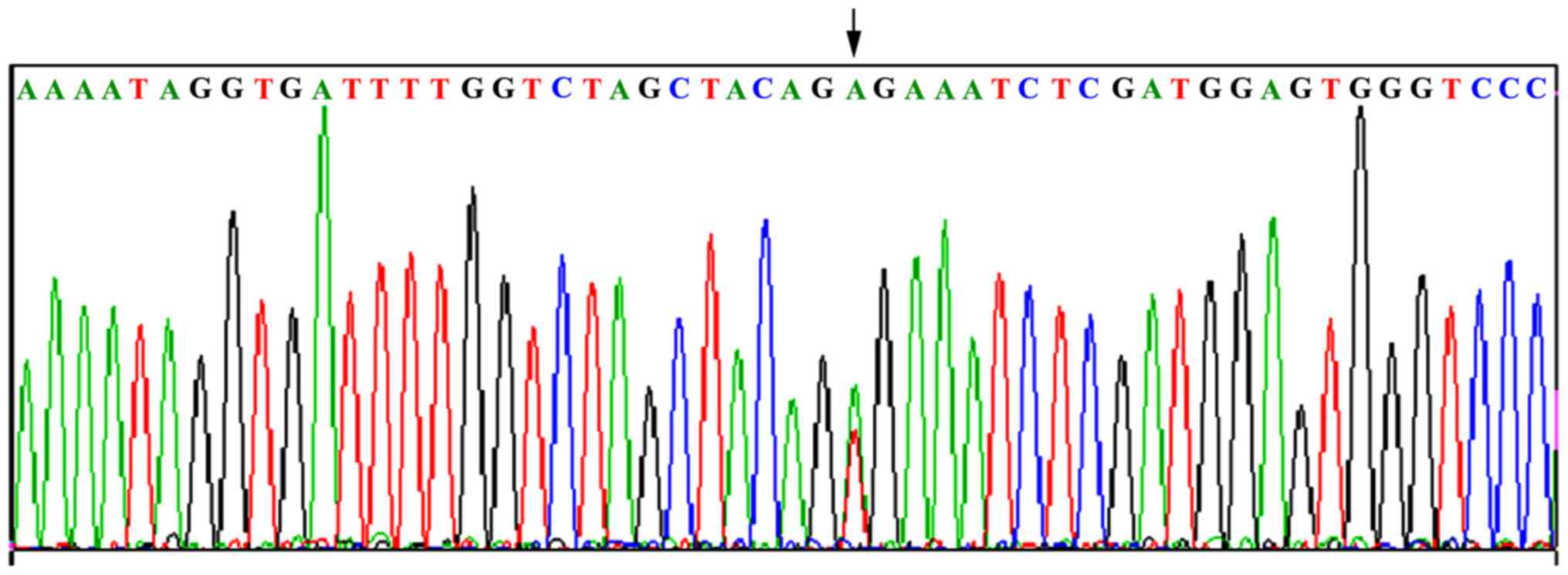
V600E mutation was detected in 18 out of 34 cases (52.9%), with a T
to A transition at nucleotide 1799 in exon 15 (Fig. 2, two signal peaks superimposed),
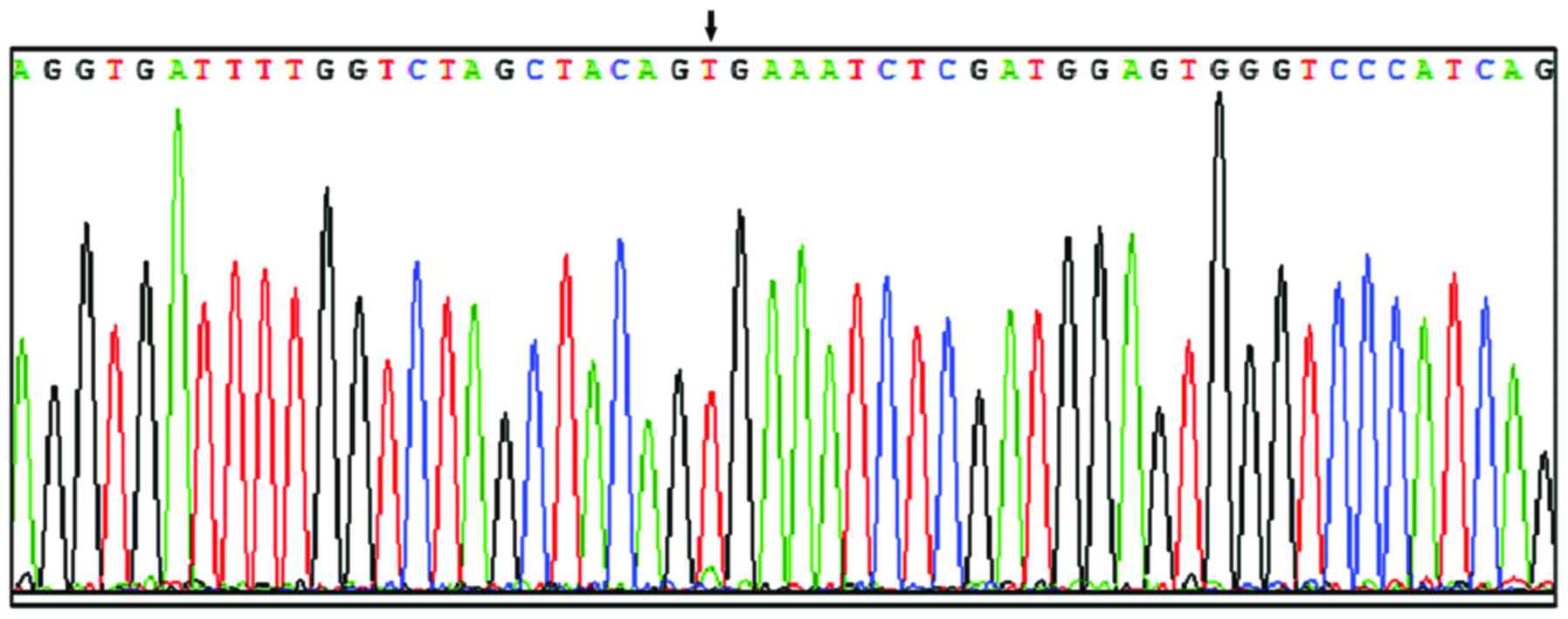
resulting in valine 600 being substituted by tryptophan. No BRAF
V600E mutations were detected in 16 out of 34 cases (47.1%)
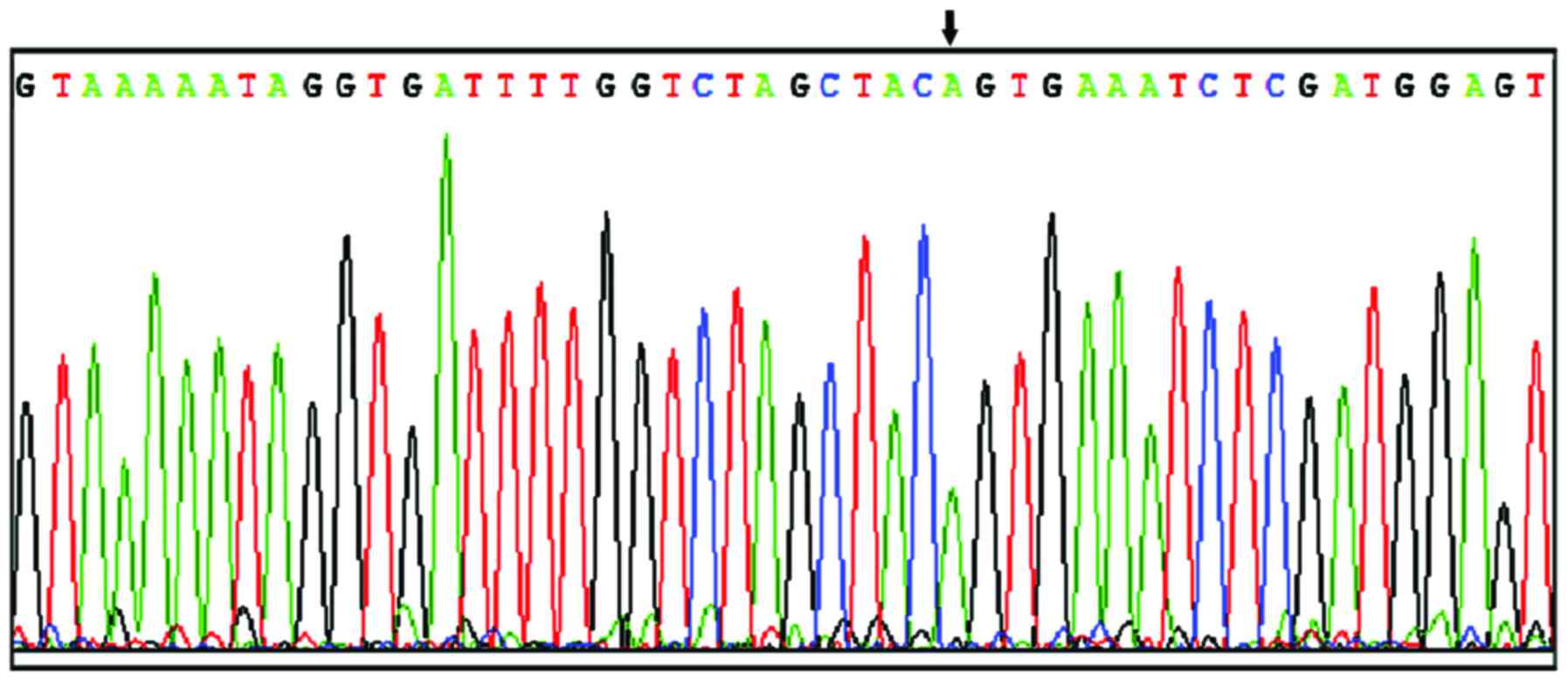
(Fig. 3) and all 34 paracarcinoma
tissue samples (Fig. 4). All 18 cases
with mutations were heterozygous mutations with base TA type. The
genotypic and allelic frequencies in each group are presented in
Table I.
 | Table I.Frequency of genotype and alleles. |
Table I.
Frequency of genotype and alleles.
|
| Genotype (%) | Allele (%) |
|---|
|
|
|
|
|---|
| Tissue | T/T | T/A | A/A | T | A |
|---|
| Carcinoma | 16 (47.1) | 18 (52.9) | 0 (0) | 50
(73.5) | 18 (26.5) |
| Paracarcinoma | 34 (100) | 0 (0) | 0 (0) | 68 (100) | 0 (0) |
Comparison of thyroid ultrasound
features between patients with and without BRAF V600E
mutations
The features and differences of thyroid ultrasound
between patients with and without BRAF V600E mutations are shown in
Table II. There were no significant
differences (P>0.05) in tumor size, tumor border, or
calcification between the BRAF V600E mutation group and the
non-mutation group according to the ultrasound examination.
 | Table II.Comparison of thyroid ultrasound
features between patients with and without BRAF V600E
mutations. |
Table II.
Comparison of thyroid ultrasound
features between patients with and without BRAF V600E
mutations.
|
|
|
| Tumor border | Calcification |
|---|
|
|
|
|
|
|
|---|
| BRAF V600E | No. of cases | Tumor size | Clear | Less clear | Yes | No |
|---|
| Mutation | 18 | 19.33±8.32 | 7 | 11 | 14 | 4 |
| No mutation | 16 | 17.32±7.32 | 4 | 12 | 13 | 3 |
| t-test | – | 0.63 |
|
|
|
|
| P-value | – | >0.05 | 0.487a | 1.000a |
Discussion
BRAF, also known as murine sarcoma viral
carcinogenic homology B1, was identified by Ikawa et al 28
years ago as an oncogene (4). Its
biological role is to induce the proliferation of avian primary
cells and the transformation of NIH3T3 cells. BRAF is a member of
the RAF family of proteins. It is a specific serine/threonine
kinase, and the most critical activator of MEK/ERK (8–11).
It was reported that the BRAF V600E protein induced
the transformation of thyroid cells in vitro (6). Moreover, thyroid cancer was induced in
transgenic mice with thyroid tissue that specifically expressed the
BRAF V600E protein, suggesting that BRAF protein activation is
closely related to the occurrence of PTC. The role and potential
mechanism of BRAF mutation in the occurrence and development of PTC
has become an area of great interest (7). It is currently believed that the V600E
mutation is located in the activation region of BRAF (CR3). A
negative charge induced by mutation mimics the phosphorylation of
threonine 598 and serine 601, resulting in aberrant activation of
BRAF, which enhances its ability to activate downstream kinases by
up to 12.5 times compared with the wild-type protein. Activated
BRAF then transfers signals to downstream molecules, through the
RAS/RAF/MEK/ERK/MAPK pathway, promoting the proliferation of
thyroid cells and tumor formation (8). Sithanandam et al (9) and Mercer and Pritchard (10) found that BRAF was the strongest
activator of the MAPK pathway. When a BRAF mutant was transfected
into NIH3T3 (mouse embryonic fibroblast cell line) cells,
transformation efficiency was enhanced by 70- to 138-fold compared
with wild-type (10,11), which further supported the observation
that BRAF mutation induces carcinogenesis through the
RAS/RAF/MEK/ERK/MAPK pathway. The continuous activation of the
RAS/RAF/MEK/MAPK signaling pathway also promoted the change from
well-differentiated papillary carcinoma cells to poorly
differentiated and undifferentiated cancer cells (11–13).
Recent studies indicated that BRAF likely affects the occurrence
and development of thyroid cancer through the expression of other
oncogenes (11).
There are different points of view regarding BRAF
mutations. For patients with thyroid cancer induced by nuclear
radiation, RET/PTC rearrangements, especially RET/PTC3, were
significantly increased (13).
However, BRAF is likely not a radiosensitive gene. Nikiforova et
al (12) demonstrated that the
BRAF mutation rate of patients with PCT induced by the Chernobyl
nuclear accident in Ukraine was significantly lower than in
sporadic PTC patients. Another study suggested that the relatively
low BRAF mutation rate was associated with the young age of
subjects selected in that study (13). BRAF mutation leading to tumorigenesis
likely requires a longer, more latent process compared with RET/PTC
rearrangement. It has also been suggested that BRAF mutation was
likely associated with high iodine intake (14) and high thyroid stimulating hormone
level (15). BRAF not only induces
tumorigenesis, but plays an important role in tumor development and
evolution. In addition, it is closely related to the
clinicopathological characteristics of tumors, which is another
area of great interest. Lee et al concluded that the BRAF
mutation rate was 49% according to a statistical analysis of 1168
PTC patients from 12 units (16).
Nikiforova et al indicated that the BRAF V600E gene mutation
was found in 11.1% of cases of poorly differentiated thyroid cancer
and anaplastic thyroid cancer (ATC), while the nipple structure was
found in cancer tissue, suggesting that part of the ATC likely
evolved from well-differentiated thyroid cancer (17). BRAF V600E gene mutation and tumor
prognosis is another popular topic of study. Kim et al
reported that BRAF V600E mutation in the primary lesion could
predict the lymph node metastasis of PTC, and its predictive value
was higher than that of other clinicopathological factors such as
age, clinical stage, and tumor size (18). However, the study by Costa et
al suggested that it could not clearly predict the poor
prognosis of PTC unless BRAF mutation and other genetic changes
existed simultaneously (19). The
data obtained by Xing et al from 219 multicenter studies
indicated that there was a significant correlation between BRAF
mutations in PTC and the following events: the invasion of cancer
tissue into surrounding tissue at the time of the first operation,
cervical lymph node metastasis, and thyroid cancer stage III–IV
(20). Elisei et al showed
that BRAF V600E was the only independent factor for predicting poor
prognosis by postoperative 15-year follow-up (21). Multivariate analysis revealed that
only BRAF V600E mutation and cervical lymph node metastasis were
independent prognostic factors for the postoperative recurrence and
metastasis of PTC. The BRAF T1799A gene mutation likely plays an
important role in the growth, invasion, and dedifferentiation
processes of differentiated thyroid cancer. However, factors
including the number of cases, PTC subtype, clinical stage, and
experimental method likely affected the detection results of the
BRAF T1799A mutation.
Thus far, the BRAF V600E mutation rates reported
from different studies are varied (6–8), ranging
from 28 to 83%, which is primarily related to epidemiological
factors such as different geographical and ethnic backgrounds. In
addition, some studies suggested it was related to the subtype
distribution of PTC. However, BRAF V600E has not been identified in
other thyroid benign or malignant tumors except for PTC, which
contributes to the diagnosis and differential diagnosis of thyroid
carcinoma. In the present study, the BRAF V600E mutation was
detected in 52.9% of cases of PTC, which was consistent with
previous studies. Detecting BRAF V600E, as a genetic test for PTC,
can be combined with ultrasound-guided fine-needle aspiration (FNA)
cytology. Studies suggested that detection of the BRAF Tl799A
mutation in cells obtained by FNA contributed to the diagnosis and
differential diagnosis of PTC (22–25). With
the rapid development of ultrasound technology, the advent of high
resolution color Doppler ultrasound instruments, and improvement of
the diagnostic level of thyroid tumors, ultrasound has become an
important method for diagnosing thyroid tumors that is increasingly
valuable to clinicians. To improve the differential diagnosis of
thyroid benign and malignant tumors, numerous relevant studies have
been carried out, and a series of diagnostic standards have been
proposed. Ultrasound examination and the BRAF V600E test are two
different methods for the diagnosis of thyroid cancer, and
determining the association between them was one of the objectives
of this study. Hwang et al suggested that in PTC patients
with BRAF V600E mutation, thyroid ultrasound features had a
tendency to have an anteroposterior diameter greater than the
diameter (26). The incidence of
calcification was also lower compared with patients without BRAF
V600E mutation, but the difference was not statistically
significant. In addition, there were no significant differences in
nodule size, border, or echo. In the present study, we focused on
tumor size, with clear or less clear border, and with or without
calcification, and compared the data with the ultrasound features
in patients with or without BRAF V600E mutations. There were no
significant differences in ultrasound features between the two
groups of patients. Therefore, ultrasound is unable to predict the
presence or absence of BRAF V600E mutations. Ultrasound and BRAF
V600E test are two different methods for the diagnosis and
differential diagnosis of PTC, and should be analyzed in
combination. The present study had limitations. The sample size was
small, and the standards of thyroid ultrasound examination were not
sufficiently detailed because of limitations of technological
conditions. These factors restricted the study to some degree and
require further investigation.
In conclusion, the BRAF V600E mutation rate is
relatively high in PTC. There is no significant association between
BRAF V600E mutation and thyroid ultrasound features. Thyroid
ultrasound is therefore unable to predict the presence of BRAF
V600E mutations in patients with PTC.
References
|
1
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukushima T, Suzuki S, Mashiko M, Ohtake
T, Endo Y, Takebayashi Y, Sekikawa K, Hagiwara K and Takenoshita S:
BRAF mutations in papillary carcinomas of the thyroid. Oncogene.
22:6455–6457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu X, Quiros RM, Gattuso P, Ain KB and
Prinz RA: High prevalence of BRAF gene mutation in papillary
thyroid carcinomas and thyroid tumor cell lines. Cancer Res.
63:4561–4567. 2003.PubMed/NCBI
|
|
4
|
Ikawa S, Fukui M, Ueyama Y, Tamaoki N,
Yamamoto T and Toyoshima K: B-raf, a new member of the raf family,
is activated by DNA rearrangement. Mol Cell Biol. 8:2651–2654.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen Y, Xing M, Mambo E, Guo Z, Wu G,
Trink B, Beller U, Westra WH, Ladenson PW and Sidransky D: BRAF
mutation in papillary thyroid carcinoma. J Natl Cancer Inst.
95:625–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fugazzola L, Mannavola D, Cirello V,
Vannucchi G, Muzza M, Vicentini L and Beck-Peccoz P: BRAF mutations
in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf).
61:239–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KH, Kang DW, Kim SH, Seong IO and Kang
DY: Mutations of the BRAF gene in papillary thyroid carcinoma in a
Korean population. Yonsei Med J. 45:818–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sithanandam G, Druck T, Cannizzaro LA,
Leuzzi G, Huebner K and Rapp UR: B-raf and a B-raf pseudogene are
located on 7q in man. Oncogene. 7:795–799. 1992.PubMed/NCBI
|
|
10
|
Mercer KE and Pritchard CA: Raf proteins
and cancer: B-Raf is identified as a mutational target. Biochim
Biophys Acta. 1653:25–40. 2003.PubMed/NCBI
|
|
11
|
Soares P, Trovisco V, Rocha AS, Lima J,
Castro P, Preto A, Máximo V, Botelho T, Seruca R and
Sobrinho-Simões M: BRAF mutations and RET/PTC rearrangements are
alternative events in the etiopathogenesis of PTC. Oncogene.
22:4578–4580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikiforova MN, Ciampi R, Salvatore G,
Santoro M, Gandhi M, Knauf JA, Thomas GA, Jeremiah S, Bogdanova TI,
Tronko MD, et al: Low prevalence of BRAF mutations in
radiation-induced thyroid tumors in contrast to sporadic papillary
carcinomas. Cancer Lett. 209:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lima J, Trovisco V, Soares P, Máximo V,
Magalhães J, Salvatore G, Santoro M, Bogdanova T, Tronko M,
Abrosimov A, et al: Reply to: Low prevalence of BRAF mutations in
radiation-induced thyroid tumors in contrast to sporadic papillary
carcinomas. Cancer Lett. 230:149–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P,
Zhang Y, Shan Z, Teng W and Xing M: Association of high iodine
intake with the T1799A BRAF mutation in papillary thyroid cancer. J
Clin Endocrinol Metab. 94:1612–1617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe R, Hayashi Y, Sassa M, Kikumori
T, Imai T, Kiuchi T and Murata Y: Possible involvement of BRAF
V600E in altered gene expression in papillary thyroid cancer.
Endocr J. 56:407–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Lee ES and Kim YS:
Clinicopathologic significance of BRAF V600E mutation in papillary
carcinomas of the thyroid: A meta-analysis. Cancer. 110:38–46.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nikiforova MN, Kimura ET, Gandhi M,
Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G,
Fusco A, et al: BRAF mutations in thyroid tumors are restricted to
papillary carcinomas and anaplastic or poorly differentiated
carcinomas arising from papillary carcinomas. J Clin Endocrinol
Metab. 88:5399–5404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim J, Giuliano AE, Turner RR, Gaffney RE,
Umetani N, Kitago M, Elashoff D and Hoon DS: Lymphatic mapping
establishes the role of BRAF gene mutation in papillary thyroid
carcinoma. Ann Surg. 244:799–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa AM, Herrero A, Fresno MF, Heymann J,
Alvarez JA, Cameselle-Teijeiro J and García-Rostán G: BRAF mutation
associated with other genetic events identifies a subset of
aggressive papillary thyroid carcinoma. Clin Endocrinol (Oxf).
68:618–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing M, Westra WH, Tufano RP, Cohen Y,
Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et
al: BRAF mutation predicts a poorer clinical prognosis for
papillary thyroid cancer. J Clin Endocrinol Metab. 90:6373–6379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elisei R, Ugolini C, Viola D, Lupi C,
Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A and Basolo F:
BRAF(V600E) mutation and outcome of patients with papillary thyroid
carcinoma: A 15-year median follow-up study. J Clin Endocrinol
Metab. 93:3943–3949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim DL, Song KH and Kim SK: High
prevalence of carcinoma in ultrasonography-guided fine needle
aspiration cytology of thyroid nodules. Endocr J. 55:135–142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xing M, Tufano RP, Tufaro AP, Basaria S,
Ewertz M, Rosenbaum E, Byrne PJ, Wang J, Sidransky D and Ladenson
PW: Detection of BRAF mutation on fine needle aspiration biopsy
specimens: A new diagnostic tool for papillary thyroid cancer. J
Clin Endocrinol Metab. 89:2867–2872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung KW, Yang SK, Lee GK, Kim EY, Kwon S,
Lee SH, Park DJ, Lee HS, Cho BY, Lee ES, et al: Detection of BRAF
V600E mutation on fine needle aspiration specimens of thyroid
nodule refines cyto-pathology diagnosis, especially in BRAF600E
mutation-prevalent area. Clin Endocrinol (Oxf). 65:660–666. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumagai A, Namba H, Akanov Z, Saenko VA,
Meirmanov S, Ohtsuru A, Yano H, Maeda S, Anami M, Hayashi T, et al:
Clinical implications of pre-operative rapid BRAF analysis for
papillary thyroid cancer. Endocr J. 54:399–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang J, Shin JH, Han BK, Ko EY, Kang SS,
Kim JW and Chung JH: Papillary thyroid carcinoma with BRAF V600E
mutation: Sonographic prediction. AJR Am J Roentgenol.
194:W425–W430. 2010. View Article : Google Scholar : PubMed/NCBI
|