Introduction
Multiple factors are involved in the occurrence of
gastric cancer, among which poor eating habits are a high risk
factor (1,2). With the development of medical
technology, both the detection and treatment rate of cancer have
increased significantly. However, when diagnosis of gastric cancer
in older patients is confirmed, it has generally developed into a
progressive stage with metastasis, resulting in a low cure rate
when chemotherapy is applied for treatment (3,4). With
developments of molecular biology, microRNA (miRNA) has gradually
become an area of intense research in medicine and life science. A
large number of studies have confirmed that miRNA is closely
associated with the occurrence and development of multiple cancers
(5,6).
The study by Gadducci et al (7) showed that overexpression of miR-21 can
significantly increase the incidence of ovarian cancer, and the
clinical use of drugs to block miR-21 expression can significantly
increase the survival time of patients. Zhang et al
(8) found that the level of miR-21
expression affects the occurrence and development of breast cancer,
and high expression of miR-182 is closely related to the
proliferation and migration of breast cancer cells. However, there
are no reports on the relationship between miR-21 and miR-182
expression and gastric cancer.
By detecting the expression of the miR-21 and
miR-182 genes in gastric cancer tissue, and investigating the
relationship between miR-21 and miR-182 expression levels and
clinicopathological features as well as prognosis of gastric cancer
patients, we aimed to determine the relationship between the
occurrence and development of gastric cancer and miR-21 and miR-182
expression, to provide a new basis for the clinical treatment of
gastric cancer.
Materials and methods
Patients
A total of 50 patients who were admitted to the
254th Hospital of PLA, and definitely diagnosed with gastric cancer
by pathological examination after surgical resection from July 2012
to July 2014 were selected. The patients were aged 48–77 years.
There were 28 males and 22 females. Other consumptive diseases were
excluded, and all patients signed the informed consent. The
included patients had complete clinical and pathological data.
Simultaneously, 50 healthy subjects without gastric cancer, who
underwent physical examinations during the same period, were
selected as the control group. Patients had 1 year of follow-up and
all treatment schemes were recorded. Peripheral blood samples were
collected from patients and stored in liquid nitrogen. This study
was approved by the Ethics Committee of 254th Hospital of PLA.
Signed written informed consents were obtained from all
participants before the study.
Instruments and reagents
MGC-803 cell line (Kunming Cell Bank of the Chinese
Academy of Sciences, Shanghai, China), methyl thiazolyl tetrazolium
(MTT) (Sigma, St. Louis, MO, USA), dimethylsulphoxide (DMSO)
(Sigma), Transwell chambers (Millipore Corp., Billerica, MA, USA),
TRIzol kit (Invitrogen, Carlsbad, CA, USA), reverse transcription
kit (Invitrogen), β-actin antibody (Sigma), fluorescence inverted
microscope (Thermo Fisher Scientific, Darmstadt, Germany), cell
culture bottles (Corning, Costar, Inc., Corning, NY, USA),
transferpettor (Eppendorf AG, Hamburg, Germany), PCR instrument
(Applied Biosystems Life Technologies, Foster City, CA, USA) and
ultraviolet imaging system (Biometra, GmbH, Goettingen,
Germany).
Detecting miR-21 and miR-182
expression in peripheral blood of gastric cancer patients by
semi-quantitative PCR
Peripheral blood samples were collected from gastric
cancer patients and healthy subjects, followed by centrifugation at
3,500 × g for 10 min. Next, the supernatant was collected, and
total RNA was extracted with a TRIzol kit. The integrity of RNA was
confirmed by agarose gel electrophoresis, which showed that the
bands corresponding to 28S, 18S, and 5S RNA were clear. The 28S
band was twice as bright as the 18S band, suggesting that RNA was
of high integrity, and could be used for follow-up experiments.
Subsequently, reverse transcription was conducted to obtain cDNA
using a reverse transcription kit. miR-21 and miR-182 expression in
peripheral blood was measured by semi-quantitative PCR, with
β-actin as the internal reference. The reaction conditions were as
follows: 95°C for 30 sec, 64°C for 25 sec, and 72°C for 30 sec, for
a total of 35 cycles. Primers were synthesized by Tiangen Biotech
(Beijing) Co., Ltd., (Beijing, China), and the sequences are shown
in Table I. Agarose gel
electrophoresis was performed after the reaction, and the products
were observed by an ultraviolet imaging system.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Genes | Sequence |
|---|
| miR-21 | F:
5-ATCCAGAGACAAGACATGTAC-3 |
|
| R:
5-TTCAGATGTTCTAAGCCTACGG-3 |
| miR-182 | F:
5-TGGCGGTTTGCGGTGGAC-3′ |
|
| R:
5-CCAGTGCAGGGTCCGAGGT-3 |
| β-actin | F:
5-GATGATTGGCATGGCTTT-3 |
|
| R:
5-CACCTTCCGTTCCAGTTT-3′ |
Establishment of miR-21 and miR-182
low expression cell lines
miR21-siRNA, miR182-siRNA, and the corresponding
control sequences were designed and synthesized by ToYoBo Co.,
Ltd., (Osaka, Japan). MGC-803 cells under healthy growth conditions
were transfected by the aforementioned synthetic si-RNA. After 48 h
of transfection, RNA was extracted and reverse transcription was
performed to obtain cDNA. PCR was adopted to detect the levels of
miR-21 and miR-182 in cells. When the transfection was successful,
the expression of miR-21 and miR-182 was significantly reduced. The
successfully transfected cells were selected and divided into three
groups: the miR21-siRNA, miR182-siRNA and siRNA-control group.
These cells were cultured in an incubator (37°C, 5% CO2)
and prepared for follow-up experiments.
MTT assay
The effects of low expression of miR-21 and miR-182
on the growth of gastric cancer cells were investigated by MTT
assay. MTT (5 mg/ml) was prepared. A total of 3×104/ml
cells were seeded in 96-well plates for continuous culture in the
incubator for 48 h, followed by the addition of MTT for further
culture. After 4 h, the culture medium was discarded, and DMSO was
added. MTT and cells were fully combined via shock treatment. The
absorbance value at 570 nm was measured with a microplate reader
(Eppendorf AG) (9).
Transwell assay
The effects of low expression of miR-21 and miR-182
on the migration of gastric cancer cells were investigated by
Transwell assay. Cells were starved for 24 h. Cell number was
adjusted to 5×105/ml, and cells were added to Transwell
chambers. After staining and fixation, the number of cells that
passed through the chamber was calculated by microscopic
examination (10).
Statistical analysis
Data are presented as mean ± standard deviation.
SPSS19.0 software (SPSS, Inc., Chicago, IL, USA) was used for data
analysis. The t-test was adopted for numerical data; intergroup
comparisons of categorical data were by chi-square test; the
correlations between miRNA expression level and clinicopathological
features were analyzed by a correlation analysis software program;
the Kaplan-Meier method was used for survival analysis, followed by
Log-rank test. P≤0.05 was considered statistically significant.
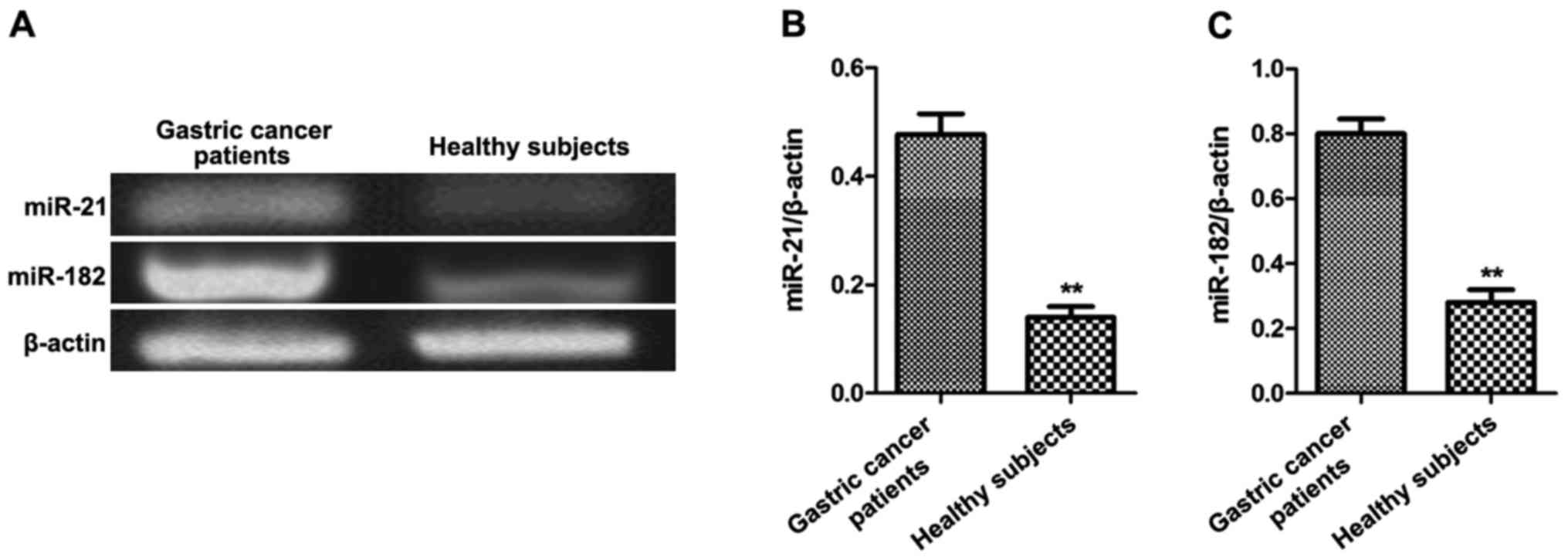
Results
Expression of miR-21 and miR-182 in
gastric cancer patients
Peripheral blood samples were collected from gastric
cancer patients and healthy subjects (n=50 each). The levels of the
miR-21 and miR-182 genes were detected by semi-quantitative PCR.
The relative levels of miR-21 and miR-182 in peripheral blood of
gastric cancer patients were significantly higher than those of
healthy subjects (p<0.01, Fig.
1).
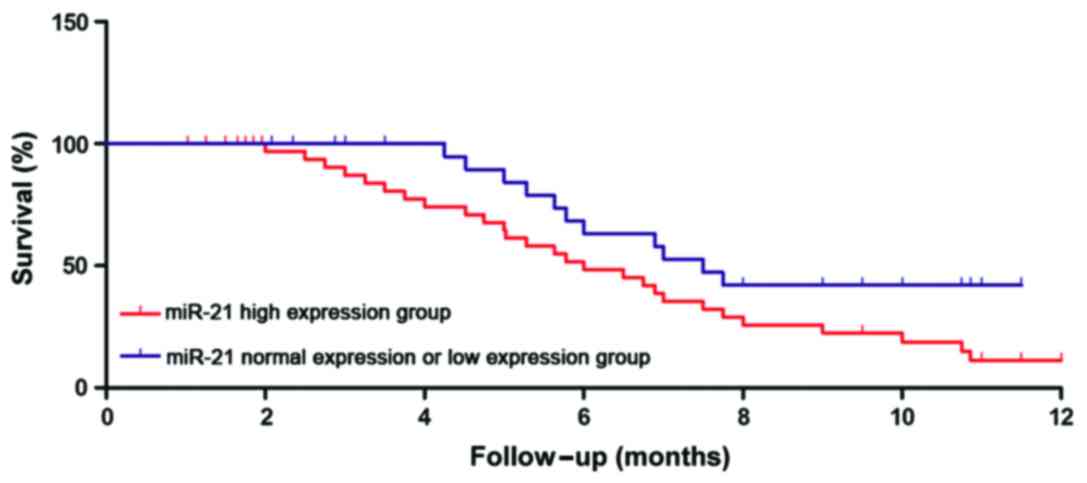
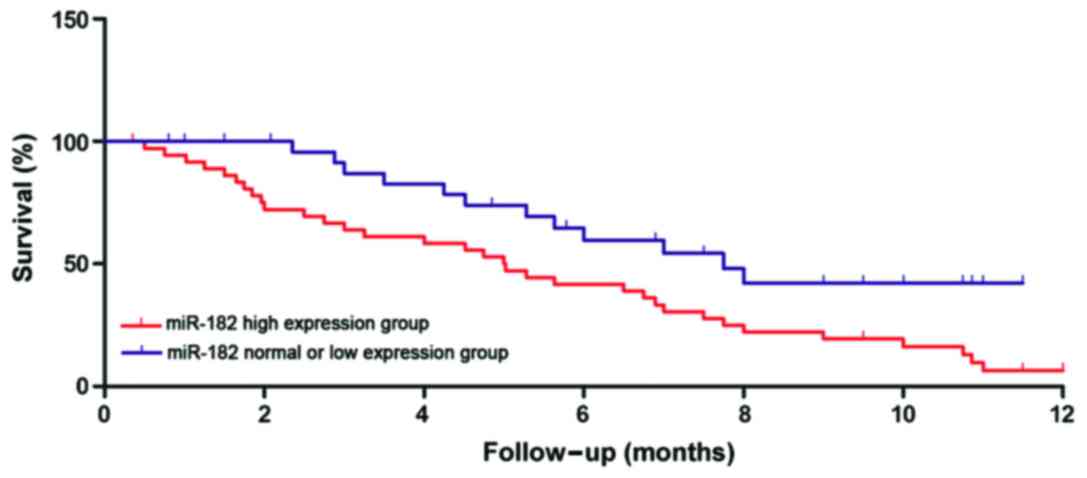
Relationship between miR-21 and
miR-182 expression and clinicopathological features as well as
prognosis of patients
The relative levels of miR-21 and miR-182 in
peripheral blood of gastric cancer patients were determined by
semi-quantitative PCR. The relative levels as well as the age,
tumor size, clinical staging, and other clinical features of
gastric cancer patients were analyzed. As shown in Table II, there was no correlations between
the relative level of miR-21 in peripheral blood of gastric cancer
patients and age, tumor size, TNM staging, lymphatic metastasis and
recurrence (p>0.05); the relative level of miR-182 had no
correlation with the age of gastric cancer patients (p=0.0682), but
it was closely related to tumor size, TNM staging, lymphatic
metastasis, and recurrence (p<0.01). According to the relative
levels of miR-21 and miR-182, the follow-up data of patients were
divided into a high expression group and normal expression or low
expression group. The survival time of the two groups was
statistically analyzed, showing that the survival times of patients
in the miR-21 and miR-182 high expression groups were significantly
shorter than those of patients in the normal expression or low
expression groups (p<0.01). Survival curves are shown in
Figs. 2 and 3.
 | Table II.Relationship between the relative
expression levels of miR-21 and miR-182 and clinical features of
patients. |
Table II.
Relationship between the relative
expression levels of miR-21 and miR-182 and clinical features of
patients.
| Category | n | miR-21 expression
level | p-value | miR-182 expression
level | p-value |
|---|
| Age (years) |
| ≤60 | 28 | 5.98±1.33 | 0.0592 | 3.53±1.08 | 0.0682 |
|
>60 | 22 | 6.26±1.28 |
| 3.85±1.12 |
|
| Tumor size |
| ≤5
cm | 36 | 5.87±1.08 | 0.0619 | 2.63±1.62 | 0.0273 |
| >5
cm | 14 | 6.16±1.22 |
| 5.17±1.29 |
|
| TNM staging |
| Stage
I–II | 21 | 6.29±1.39 | 0.0538 | 2.38±1.19 | 0.0386 |
| Stage
III–IV | 29 | 7.32±1.87 |
| 6.65±2.01 |
|
| Lymphatic
metastasis |
| Yes | 15 | 6.76±2.01 | 0.0784 | 6.73±1.78 | 0.0097 |
| No | 35 | 6.58±1.96 |
| 2.17±1.05 |
|
| Recurrence |
| Yes | 33 | 5.97±1.86 | 0.0865 | 5.32±1.25 | 0.0276 |
| No | 17 | 5.38±1.29 |
| 3.17±1.03 |
|
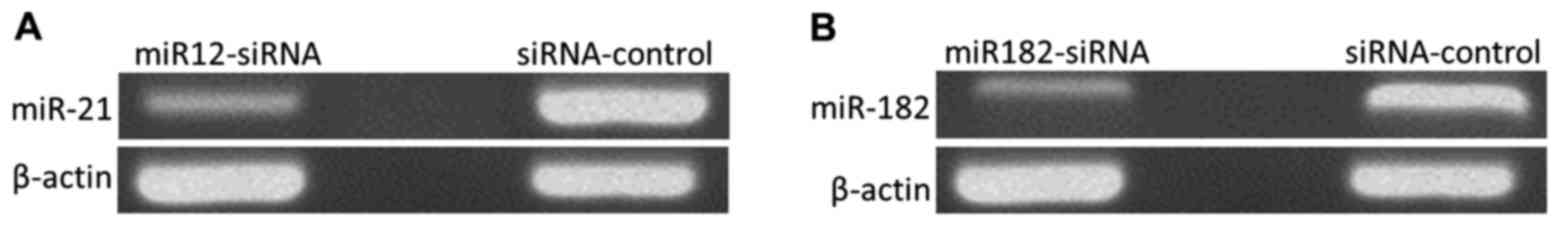
Establishment of MGC-803 cell lines
with low expression of miR-21 and miR-182
The levels of miR-21 and miR-182 in MGC-803 gastric
cancer cells were downregulated by siRNA transfection, and RNA was
extracted. After reverse transcription, RNA expression was measured
by semi-quantitative PCR. As shown in Fig. 4, compared with the siRNA-control
group, miR-21 expression in cells of the miR21-siRNA group was
decreased to 17.82±3.42% (p<0.01), and miR-182 expression in
cells of the miR182-siRNA group was decreased to 23.67±5.83%
(p<0.01), suggesting that MGC-803 cell lines with low expression
of miR-21 and miR-182 were established.
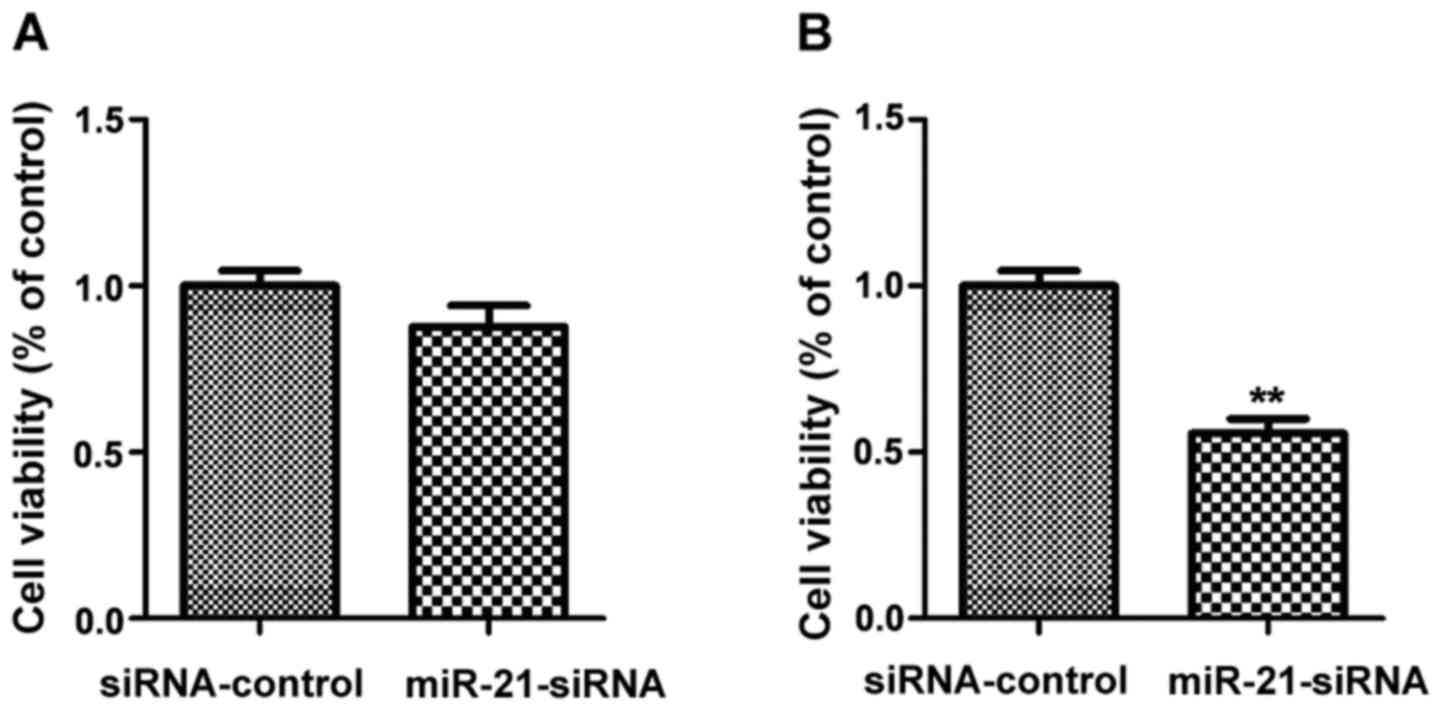
Effect of low expression of miR-21 and
miR-182 on cell proliferation
The successfully transfected MGC-803 cells with low
expression of miR-21 or miR-182 were used to investigate the
effects of miR-21 and miR-182 on cell proliferation. The quantity
of MGC-803 cells was assessed by MTT assay (Fig. 5). Compared with the siRNA-control
group, there was no obvious change in the quantity of cells in the
miR21-siRNA group (p>0.05); compared with the siRNA-control
group, the quantity of cells in the miR182-siRNA group was
significantly decreased (p<0.01).
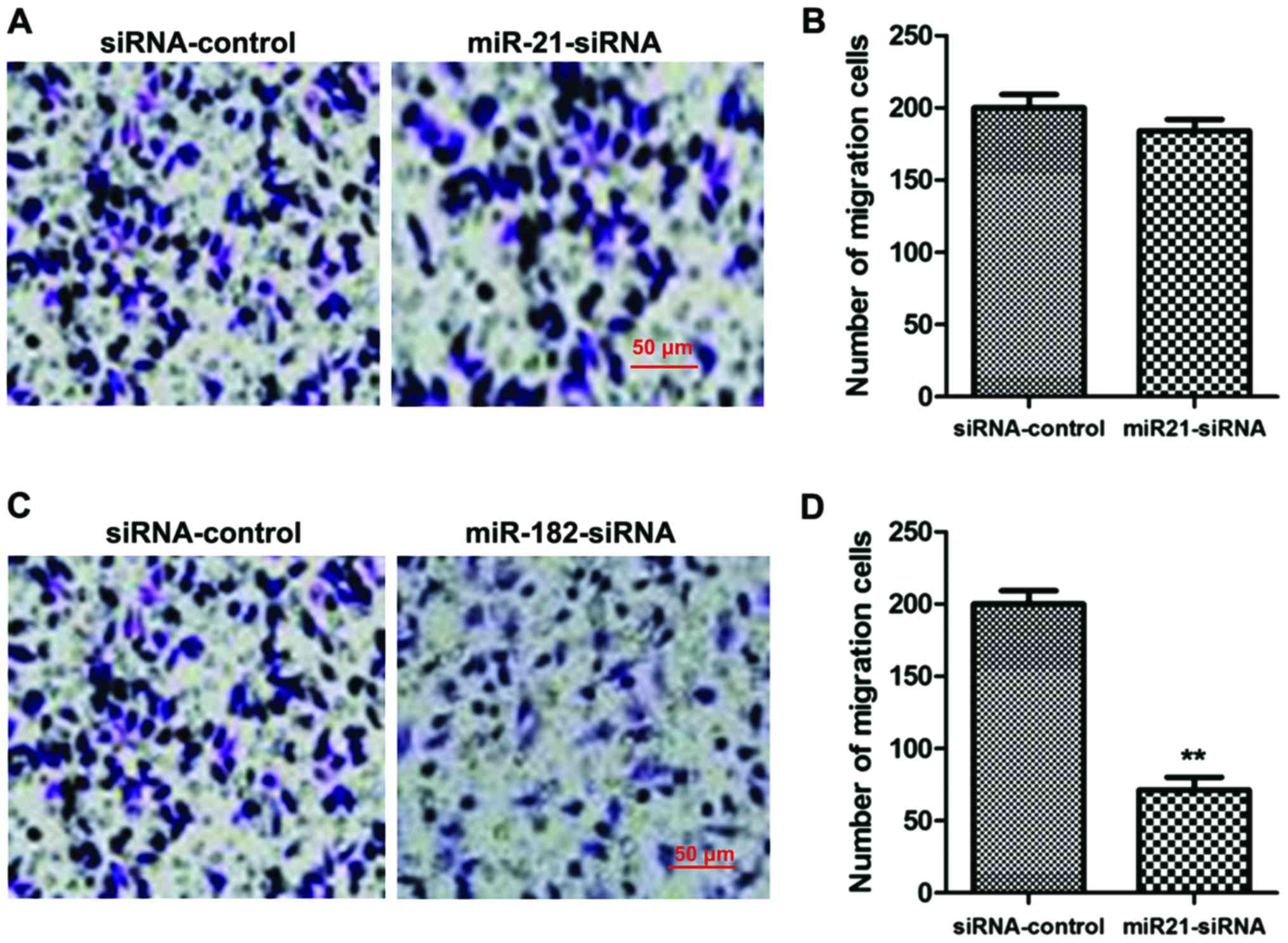
Effect of low expression of miR-21 and
miR-182 on cell migration
The successfully transfected MGC-803 cells with low
expression of miR-21 or miR-182 were used to investigate the
effects of miR-21 and miR-182 on cell migration. The migration of
gastric cancer cells was analyzed by Transwell assay (Fig. 6). Compared with the siRNA-control
group, there was no evident change in migration ability of cells in
the miR21-siRNA group (p>0.05); however, compared with the
siRNA-control group, the migration ability of cells in the
miR182-siRNA group was significantly decreased (p<0.01).
Discussion
The effects of miRNA on human pathological and
physiological processes are regulated primarily through gene
transcription and translation (11).
By affecting transcription and translation, miRNA can cause the
abnormal metabolism of tumor cells (12). The abnormal proliferation and
migration of tumor cells can be caused by high expression of miRNA,
indicating that miRNA may play a role in promoting cancer. In
contrast, some miRNA have been shown to suppress cancer (13). Studies on miRNA have shown that the
expression of miR-182 is significantly higher in multiple tumor
cells compared with normal tissues (14–16). The
study by Mirzaei et al (17)
indicated that when miR-182 is highly expressed in tumor cells, the
metastatic potential of tumors is significantly higher than tumor
cells with low expression or normal expression of miR-182. Using an
animal model of sarcoma, Gadducci et al (7) also found that the proliferation and
migration of tumor cells are affected by miR-182; when miR-182 is
knocked out, the proliferation and migration ability of tumor cells
are significantly decreased. At present, there is little research
on miR-21. Petrović (18) found that
there is a close correlation between miR-21 and renal carcinoma.
Patients with a high expression of miR-21 may have a significantly
increased risk of renal carcinoma, and miR-21 can induce the
occurrence of renal carcinoma.
In the present study, we investigated the
relationship between miR-21 and miR-182 expression levels and the
occurrence and development of gastric cancer. This was achieved by
collecting peripheral blood from gastric cancer patients, while
using peripheral blood samples from healthy subjects as controls.
The levels of miR-21 and miR-182 were closely related to the
occurrence of gastric cancer. Therefore, the occurrence of gastric
cancer may be regulated by the levels of miR-21 and miR-182. Huang
et al (19) indicated that the
levels of miR-21 and miR-182 in plasma were equivalent to those in
tumor tissues; the levels of miR-21 and miR-182 in peripheral blood
alone can be used to assess the levels of these genes in tumor
tissue samples. Therefore, collecting peripheral blood from
patients with gastric cancer as samples for analysis has the
advantages of minimal trauma, convenient to obtain, and strong
acceptability by patients, and shows great application potential
for the preliminary screening of gastric cancer patients. The
relationship between the relative levels of miR-21 and miR-182 and
clinicopathological features as well as survival time of patients
with gastric cancer indicated that miR-182 was closely related to
tumor size, TNM staging, lymphatic metastasis, and recurrence of
gastric cancer. miR-182 can regulate both the occurrence and
development of gastric cancer. Furthermore, the effects of low
expression of miR-182 on proliferation and migration ability of
gastric cancer cells were investigated by MTT and Transwell assay,
respectively. The results indicated that low expression of miR-182
significantly reduced the proliferation and migration ability of
gastric cancer cells. There was no correlation between miR-21 and
each clinicopathological feature, but high expression of miR-2
affected the survival time of patients, suggesting that miR-21 can
affect the occurrence of gastric cancer and the survival time of
patients. However, it had no direct correlation with the
differentiation and severity of gastric cancer. Currently, it is
unclear through which signaling proteins miR-21 and miR-182 affect
gastric cancer. This will be the focus of future research.
In conclusion, peripheral blood can be used to
measure the levels of miR-21 and miR-182. The relative levels of
miR-21 and miR-182 can be used as molecular markers for screening
gastric cancer, and will promote the identification of new targets
and ideas for the treatment of gastric cancer in clinical
practice.
References
|
1
|
Petrovchich I and Ford JM: Genetic
predisposition to gastric cancer. Semin Oncol. 43:554–559. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Payão SL and Rasmussen LT: Helicobacter
pylori and its reservoirs: A correlation with the gastric
infection. World J Gastrointest Pharmacol Ther. 7:126–132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cover TL: Helicobacter pylori diversity
and gastric cancer risk. MBio. 7:e01869–e15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oue T, Koshinaga T, Takimoto T, Okita H,
Tanaka Y, Nozaki M, Haruta M, Kaneko Y and Fukuzawa M; Renal Tumor
Committee of the Japanese Childrens Cancer Group, : Anaplastic
histology Wilms tumors registered to the Japan Wilms Tumor Study
Group are less aggressive than that in the National Wilms Tumor
Study 5. Pediatr Surg Int. 32:851–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barwari T, Joshi A and Mayr M: MicroRNAs
in cardiovascular disease. J Am Coll Cardiol. 68:2577–2584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garg M: Targeting microRNAs in
epithelial-to-mesenchymal transition-induced cancer stem cells:
Therapeutic approaches in cancer. Expert Opin Ther Targets.
19:285–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gadducci A, Sergiampietri C, Lanfredini N
and Guiggi I: Micro-RNAs and ovarian cancer: The state of art and
perspectives of clinical research. Gynecol Endocrinol. 30:266–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang
Q, Cheng P, Tang ZH and Huang F: Meta-analysis of microRNA-183
family expression in human cancer studies comparing cancer tissues
with noncancerous tissues. Gene. 527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hufnagel RB, Zimmerman SL, Krueger LA,
Bender PL, Ahmed ZM and Saal HM: A new frontonasal dysplasia
syndrome associated with deletion of the SIX2 gene. Am J Med Genet
A 170A. 1–491. 2016.
|
|
10
|
Berasain C, Perugorria MJ, Latasa MU,
Castillo J, Goñi S, Santamaría M, Prieto J and Avila MA: The
epidermal growth factor receptor: A link between inflammation and
liver cancer. Exp Biol Med (Maywood). 234:713–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda A, Yan IK, Foye C, Parasramka M
and Patel T: MicroRNAs as paracrine signaling mediators in cancers
and metabolic diseases. Best Pract Res Clin Endocrinol Metab.
30:577–590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nassar FJ, Nasr R and Talhouk R: MicroRNAs
as biomarkers for early breast cancer diagnosis, prognosis and
therapy prediction. Pharmacol Ther. 36:7591–7598. 2016.
|
|
13
|
Williams J, Smith F, Kumar S, Vijayan M
and Reddy PH: Are microRNAs true sensors of ageing and cellular
senescence? Ageing Res Rev. 17:435–447. 2016.
|
|
14
|
Wei Q, Lei R and Hu G: Roles of miR-182 in
sensory organ development and cancer. Thorac Cancer. 6:2–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ceribelli A, Satoh M and Chan EK:
MicroRNAs and autoimmunity. Curr Opin Immunol. 24:686–691. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chandra V, Kim JJ, Mittal B and Rai R:
MicroRNA aberrations: An emerging field for gallbladder cancer
management. World J Gastroenterol. 22:1787–1799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mirzaei H, Gholamin S, Shahidsales S,
Sahebkar A, Jaafari MR, Mirzaei HR, Hassanian SM and Avan A:
MicroRNAs as potential diagnostic and prognostic biomarkers in
melanoma. Eur J Cancer. 53:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrović N: miR-21 might be involved in
breast cancer promotion and invasion rather than in initial events
of breast cancer development. Mol Diagn Ther. 20:97–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JT, Liu SM, Ma H, Yang Y, Zhang X,
Sun H, Zhang X, Xu J and Wang J: Systematic review and
meta-analysis: Circulating miRNAs for diagnosis of hepatocellular
carcinoma. J Cell Physiol. 231:328–335. 2016. View Article : Google Scholar : PubMed/NCBI
|