Introduction
Hepatocellular carcinoma (HCC) is one of the major
causes of cancer-associated mortality worldwide, accounting for
5.7% of all newly diagnosed malignancies (1–5). The
annual incidence rates of HCC are the highest in East Asia and
Sub-Saharan Africa, where >80% of all known cases develop
(1–5).
Advances in treatments for HCC during the last few decades markedly
improved the prognosis of the disease (1,2,4,5). However,
a curative therapy such as surgical resection may be applied to a
limited number (<20%) of patients with HCC (5,6).
Sorafenib is a multi-kinase inhibitor that
suppresses cancer growth and cell proliferation (7,8). Two
pivotal randomized phase III studies, namely the Sorafenib HCC
Assessment Randomized Protocol study (7) and the Asian Pacific study (8), demonstrated that patients with
unresectable HCC undergoing sorafenib therapy had significantly
longer survival time compared with the placebo group. Although
>5 years have elapsed since the introduction of sorafenib for
the treatment of unresectable HCC in daily clinical practice,
sorafenib is still regarded as first-line systemic chemotherapeutic
agent for HCC (9–11). In addition, studies on prognostic
factors in patients with HCC who underwent sorafenib therapy have
mainly focused on tumor-associated factors, liver function, serum
biomarkers and combination therapy with sorafenib (12–17).
Substantial skeletal muscle wasting, termed
sarcopenia, is an important predictor for survival in patients with
solid malignancies (18). By
contrast, sarcopenia has become a relevant clinical feature for
understanding the effects of aging on clinical outcomes (19). Sarcopenia is a commonly observed
disorder in aged populations and is associated with disability,
functional decline and frailty (19,20).
Generally, skeletal muscle mass is regulated depending on the
balance between protein synthesis and protein breakdown (21). Patients with HCC often have underlying
liver cirrhosis (LC), and skeletal muscle loss is a major
characteristic of protein energy malnutrition in patients with LC
(22,23). Age-associated sarcopenia is defined as
primary sarcopenia, whereas LC is one of the causes of secondary
sarcopenia (19,22,23).
Although commonly observed, malnutrition is frequently
underdiagnosed or overlooked, and it is poorly characterized in
patients with HCC and LC complications (21–23). In
addition, to the best of our knowledge, although sarcopenia has
been reported to be an adverse predictor in patients with HCC who
may have a potential for curative therapy such as surgical
resection, as well as in patients with several malignancies other
than HCC, there have been no studies regarding the impact of
sarcopenia on clinical outcomes in patients with HCC undergoing
sorafenib therapy (18,24–27).
Therefore, it is imperative to address these issues. The aim of the
present study was to examine the impact of sarcopenia prior to
sorafenib therapy on the clinical outcomes in patients with HCC
receiving sorafenib.
Materials and methods
Patients and indications of sorafenib
treatment
Between June 2009 and August 2015, 234 patients with
HCC treated with sorafenib (median age=72 years, range; 40–91
years, 182 males and 52 females) were admitted to the Division of
Hepatobiliary and Pancreatic Disease, Department of Internal
Medicine, Hyogo College of Medicine (Hyogo, Japan) and the
Department of Gastroenterology and Hepatology, Osaka Red Cross
Hospital (Osaka, Japan). Of these, two patients with insufficient
clinical data were excluded from the analysis. Thus, 232 patients
with HCC treated with sorafenib were analyzed in the present study.
The majority of analyzed patients received previous therapies for
HCC. Sorafenib therapy was recommended for patients with
unresectable HCC and the following features, as determined by
dynamic computed tomography (CT): i) The presence of distant
metastases; ii) refractory response to previous transcatheter
arterial therapies for HCC [transcatheter arterial
chemoembolization (TACE) or transcatheter arterial infusion (TAI)
chemotherapy]; iii) unsuitability for TACE or TAI due to anatomical
reasons; iv) vascular invasion such as tumor thrombus in the portal
vein (28–30). Patients with poor performance status
[PS; Eastern Cooperative Oncology Group (ECOG) classification ≥3]
were not recommended for sorafenib therapy (28,29).
Definition of sarcopenia and the study
protocol
Assessment of sarcopenia was performed using CT
scans obtained prior to sorafenib therapy. The tissue Hounsfield
unit (HU) limit for skeletal muscles on the CT image was −29 HU to
+150 HU, as previously reported (27). The third lumbar vertebra (L3) was used
as a standard landmark. Skeletal muscles at the L3 level included
the erector spinae, transverse abdominis, psoas, quadratus
lumborum, internal and external oblique abdominal muscle and the
rectus abdominis muscle; these muscles were identified on the CT
images. Cross-sectional areas (cm2) of the muscles were
measured by manual tracing on the CT images, and their sum was
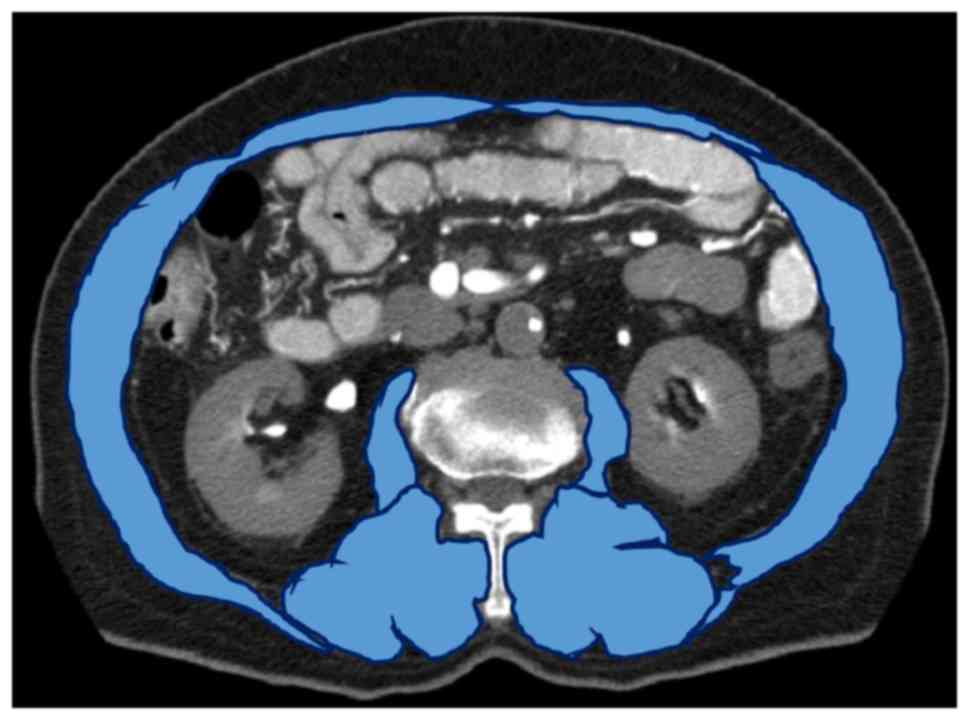
calculated (27). A representative
case is presented in Fig. 1. The
cross-sectional areas were normalized for patient height [skeletal
muscle index (SMI), cm2/m2]. Male patients
with SMI ≤36.2 cm2/m2 and female patients
with SMI ≤29.6 cm2/m2 were defined as having
sarcopenia, based on the findings of a previous study (31).
The present study retrospectively compared baseline
characteristics, overall survival (OS), progression-free survival
(PFS), best treatment response of sorafenib and serious adverse
events [SAEs; grade ≥3 as defined by the Common Terminology
Criteria for Adverse Events (CTCAE); version 3 (32)] in the sarcopenia and the
non-sarcopenia groups, and investigated factors associated with OS
and PFS using univariate and multivariate analysis. The current
study was performed in accordance with the Declaration of Helsinki
and with approval from the Ethics Committees of each hospital
(Hyogo College of Medicine and Osaka Red Cross Hospital). The
requirement to obtain written informed consent for inclusion in the
present study from patients was waived.
HCC diagnosis and sorafenib
therapy
HCC was diagnosed according to the previously
described methods (28,29). Briefly, dynamic CT of the liver was
performed prior to initiating sorafenib therapy. For patients with
atypical imaging findings, ultrasound-guided tumor biopsy was
conducted for histological assessment. HCC was finally diagnosed
based on radiological or histological findings in accordance with
the guidelines of the European Association for the Study of the
Liver (33).
For patients with no evident risk factors, the
recommended initial dose of 800 mg/day of sorafenib (400 mg twice a
day) was administered (7,8). The reduced initial dose was administered
to a number of patients (n=166) based on clinical features,
including body weight, age, ECOG-PS and liver function. During
sorafenib treatment, each attending physician adjusted the daily
dose of sorafenib according to the degree of adverse events. In
patients who received an initial reduced dose of sorafenib and
exhibited good tolerability, dose escalation from 400–600 mg/day or
from 400–800 mg/day was permitted. In patients with adverse events
of grade ≥3, sorafenib treatment was discontinued until the
clinical symptoms resolved to grade 1 or 2. In principle, the
treatment efficacy of sorafenib was assessed every 4–8 weeks
following the initiation of therapy, according to the modified
Response Evaluation Criteria in Solid Tumors (mRECIST) and/or the
levels of tumor markers (28,29,34,35).
Patients continued sorafenib until the development of the following
conditions: Unacceptable sorafenib-associated toxicity, disease
progression or the patient's wish to discontinue treatment.
Following discontinuation of sorafenib therapy for any reason,
physicians evaluated the clinical conditions (tumor status or the
general status) of each patient and investigated the suitability of
other therapies (TACE, TAI or systemic chemotherapy other than
sorafenib) for achieving the best clinical outcome (28,29).
Evaluation of treatment efficacy
The best treatment efficacy achieved during
sorafenib therapy was determined according to the mRECIST criteria
and/or tumor marker levels as previously indicated (28,29,34). The
treatment efficacy was classified into the following four
categories: Complete response (CR); partial response (PR); stable
disease (SD); progressive disease (PD). A patient with CR was
characterized by the absence of enhancement in the arterial phase
within all targeted nodules. A patient with PR was characterized by
a ≥30% reduction in tumor size, which was determined by calculating
the sum of the diameters of the targeted nodules. The size of the
nodules was estimated via unidirectional measurement. A patient
with PD was characterized by a ≥20% increase in the tumor size via
calculating the sum of the maximal dimensions of the targeted
nodules. A patient with SD was characterized by the absence of CR,
PR or PD (29,34). The objective response rate (ORR) was
defined as the percentage of patients with the best tumor response
rates considering CR and PR. The disease control rate (DCR) was
defined as the percentage of patients with the best tumor response
rates considering CR, PR and SD.
Safety evaluation of sorafenib
therapy
Sorafenib associated adverse events, including rash,
diarrhea, hand-foot skin reaction, hypertension, liver damage,
fatigue, gastrointestinal hemorrhage and lung injury, were
evaluated using CTCAE version 3.0 (32).
Statistical analysis
The categorical variables of the sarcopenia and
non-sarcopenia groups were analyzed by Fisher's exact test, while
the numerical variables were analyzed with the unpaired Student's
t-test or with the Mann-Whitney U test as applicable. OS and PFS
curves were generated using the Kaplan-Meier method and compared
using the log-rank test. Factors with values of P<0.05 in
univariate analysis were included in the multivariate analysis with
the Cox proportional hazards model. In order to analyze the
significance of predictors in multivariate analysis, numerous
variables were divided by the median values for all cases (n=232)
and treated as dichotomous covariates. OS was defined as the period
from the initiation of sorafenib therapy until mortality (due to
any cause) or the last follow-up visit. PFS was defined as the
period from the initiation of sorafenib therapy until the date of
the detection of progression-free disease or mortality (due to any
cause) (28,29). Data are expressed as median values
(range). P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using the JMP 11 software (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
The baseline characteristics of the patients (n=232)
are presented in Table I. There were
181 male and 51 female patients with a median age of 72 years
(range, 40–91). Sarcopenia was observed in 151 (65.1%) patients.
There were 165 patients with Child-Pugh class A and 67 patients
with Child-Pugh class B cirrhosis (36). In 66 (28.4%) patients, the standard
dose of sorafenib (800 mg/day) was administered at the beginning of
therapy. Previously, the most common therapies for were
transcatheter arterial therapies, including TACE or TAI, followed
by percutaneous ablative therapies and surgical resection.
 | Table I.Baseline characteristics (n=232). |
Table I.
Baseline characteristics (n=232).
| Variables | Number of patients
or median value, n (range) |
|---|
| Age, years | 72 (40–91) |
| Sarcopenia |
|
|
Yes | 151 |
| No | 81 |
| Gender |
|
|
Male | 181 |
|
Female | 51 |
| Causes of liver
disease |
|
| B | 33 |
| C | 144 |
|
Non-B/non-C | 49 |
| B and
C | 4 |
|
Unknown | 2 |
| Initial dose of
sorafenib, mg/day |
|
|
800 | 66 |
|
600 | 1 |
|
400 | 162 |
|
200 | 3 |
| Child-Pugh |
|
| A | 165 |
| B | 67 |
| ECOG performance
status |
|
| 0 | 197 |
| 1 | 30 |
| 2 | 5 |
| HCC stage |
|
| I | 1 |
| II | 18 |
|
III | 79 |
|
IVA | 46 |
|
IVB | 88 |
| Previous therapies
for HCC |
|
|
Transcatheter arterial
therapies |
|
|
Yes | 211 |
|
No | 21 |
| Percutaneous
ablative therapies |
|
|
Yes | 133 |
| No | 99 |
| Surgical
resection |
|
|
Yes | 73 |
| No | 159 |
| Tumor burden
≥50% |
|
|
Yes | 23 |
| No | 209 |
| Total bilirubin,
mg/dl | 0.8 (0.2–5.1) |
| Serum albumin,
g/dl | 3.4 (1.7–4.8) |
| Prothrombin time,
% | 80 (48–116) |
| Platelets,
×104/mm3 | 11.7
(3.4–56.7) |
| AST, IU/l | 50 (15–791) |
| ALT, IU/l | 34 (6–380) |
| ALP, IU/l | 401
(124–4,535) |
| GGT,
IU/la | 72 (14–2,172) |
| AFP,
ng/mlb | 139.2
(1.7–688,400) |
| DCP,
mAU/mlc | 748
(10–421,210) |
Comparison of baseline characteristics
between patients with and without sarcopenia
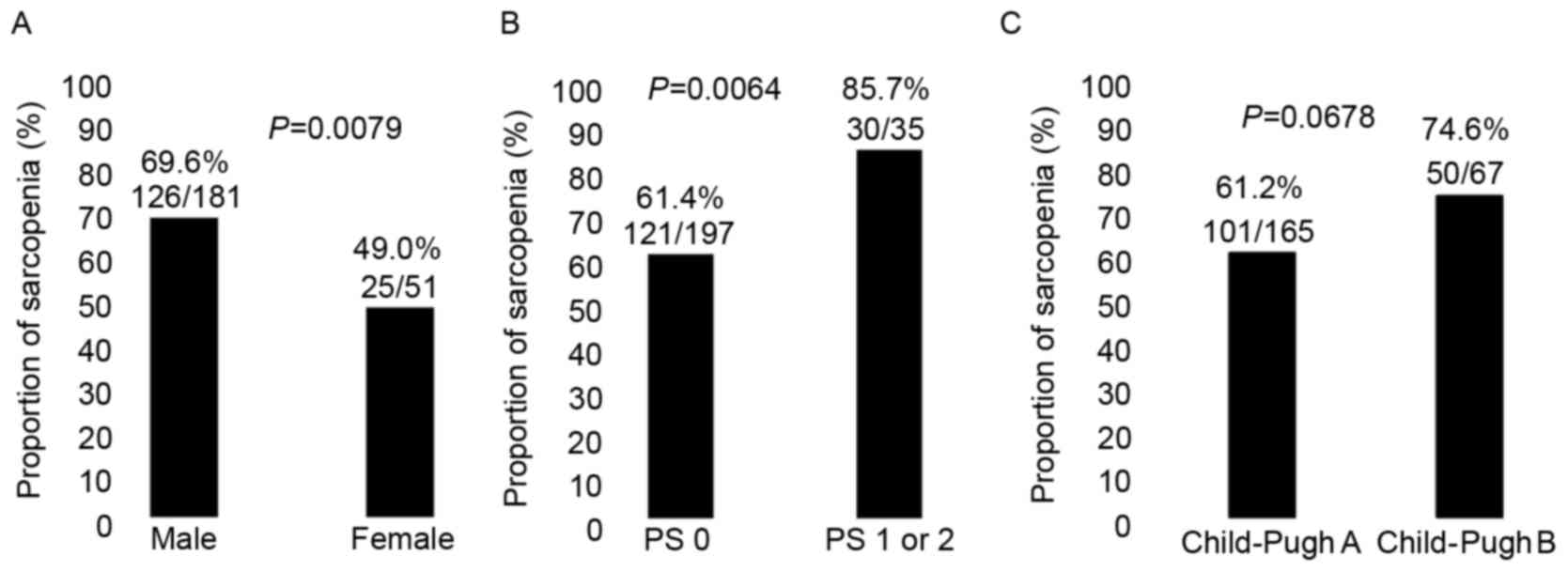
Compared with those in the non-sarcopenia group, the
proportion of sarcopenia in male patients as compared with that in
female patients was significantly higher (P=0.0079; Fig. 2A) and the proportion of sarcopenia in
patients with poorer ECOG-PS as compared with that in patients with
PS-0 was significantly higher (P=0.0137; Fig. 2B), whereas the proportion of
sarcopenia in patients with Child-Pugh class A cirrhosis as
compared with Child-Pugh class B cirrhosis tended to be lower
(P=0.0678; Fig. 2C) and patients
treated with an initial sorafenib dose of 800 mg/day (P=0.096) in
the sarcopenia group tended to be significantly lower compared with
those in the non-sarcopenia group (Table
II). Laboratory analysis revealed that the differences between
the sarcopenia and non-sarcopenia groups were significant with
regard to the levels of serum albumin (P=0.0022), aspartate
aminotransferase (AST; P=0.0062) and des-γ-carboxy prothrombin
(DCP; P=0.0007; Table II).
 | Table II.Comparisons between patients with and
without sarcopenia. |
Table II.
Comparisons between patients with and
without sarcopenia.
| Variables | Sarcopenia, n
(range) | Non-sarcopenia, n
(range) | P-value |
|---|
| Total | 151 | 81 |
|
| Age, years | 72 (46–91) | 71 (40–85) | 0.1456 |
| Gender |
|
| 0.0079 |
|
Male | 126 | 55 |
|
|
Male | 25 | 26 |
|
| Causes of liver
disease |
|
| 0.6426 |
| B | 22 | 11 |
|
| C | 93 | 52 |
|
|
Non-B/non-C | 31 | 17 |
|
| B and
C | 4 | 0 |
|
|
Unknown | 1 | 1 |
|
| Child-Pugh,
A/B |
|
| 0.0678 |
| A | 101 | 64 |
|
| B | 50 | 17 |
|
| ECOG performance
status |
|
| 0.0137 |
| 0 | 121 | 76 |
|
| 1 | 26 | 4 |
|
| 2 | 4 | 1 |
|
| Initial dose of
sorafenib, mg/day |
|
| 0.0960 |
|
800 | 37 | 29 |
|
|
600 | 0 | 1 |
|
|
400 | 112 | 50 |
|
|
200 | 2 | 1 |
|
| HCC stage |
|
| 0.3353 |
| I | 1 | 0 |
|
| II | 10 | 8 |
|
|
III | 48 | 31 |
|
|
IVA | 35 | 11 |
|
|
IVB | 57 | 31 |
|
| Tumor burden
≥50% |
|
| 0.4900 |
|
Yes | 17 | 6 |
|
| No | 134 | 75 |
|
| Total bilirubin,
mg/dl | 0.8 (0.3–2.5) | 0.8 (0.2–5.1) | 0.4279 |
| Serum albumin,
g/dl | 3.4 (1.7–4.8) | 3.5 (2.0–4.8) | 0.0022 |
| Prothrombin time,
% | 79 (48–116) | 81 (60–111) | 0.4466 |
| Platelets,
×104/mm3 | 11.6
(3.4–47.9) | 11.8
(3.6–56.7) | 0.8461 |
| AST, IU/l | 55 (15–791) | 43 (17–679) | 0.0062 |
| ALT, IU/l | 36 (6–380) | 30 (9–290) | 0.8302 |
| ALP, IU/l | 429 (161–4535) | 391
(124–3,265) | 0.6496 |
| GGT,
IU/la | 79.5 (15–941) | 68 (14–2,172) | 0.2377 |
| AFP,
ng/mlb | 138.3
(1.8–688,400) | 162.9
(1.7–98,435) | 0.7965 |
| DCP,
mAU/mlc | 1,305
(10–421,210) | 292.5
(10–53,857) | 0.0007 |
| Serious adverse
events, grade ≥3 | 41.1% (62/151) | 33.3% (27/81) | 0.2610 |
| Best treatment
response |
|
| 0.0185 |
| CR | 1 | 3 |
|
| PR | 5 | 8 |
|
| SD | 40 | 27 |
|
| PD | 61 | 30 |
|
| NE | 44 | 13 |
|
| Objective response
rate | 4.0% (6/151) | 13.6% (11/81) | 0.0146 |
| Disease control
rate | 30.5% (46/151) | 46.9% (38/81) | 0.0151 |
Comparison of OS and PFS rates between
patients with and without sarcopenia
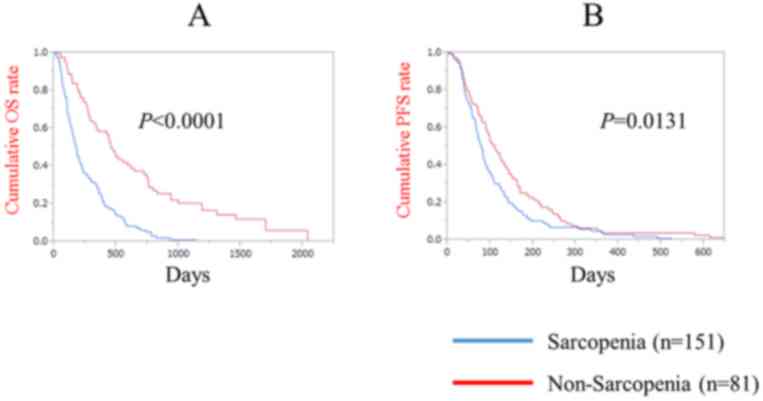
The median follow-up periods subsequent to sorafenib
treatment were 170 days (range, 12–1,145) in the sarcopenia group
and 419 days (range, 50–2,036) in the non-sarcopenia group. The
median OS was 174 days in the sarcopenia group and 454 days in the
non-sarcopenia group (P<0.0001; Fig.
3A). The median PFS was 77 days in the sarcopenia group and 106
days in the non-sarcopenia group (P=0.0131; Fig. 3B).
Comparison of treatment duration and
SAEs of grade ≥3 between patients with and without sarcopenia
The median treatment duration was 66 days in the
sarcopenia group, and 103 days in the non-sarcopenia group
(P=0.001). The prevalence of sorafenib-associated SAEs of grade ≥3,
as assessed by CTCAE version 3.0, was 41.1% (62/151) in the
sarcopenia group and 33.3% (27/81) in the non-sarcopenia group
(P=0.261).
Best tumor treatment response in the
sarcopenia and non-sarcopenia groups
In the analysis of the best tumor response in the
sarcopenia group, CR was achieved in 1 patient, PR in 5, SD in 40
and PD in 61, while 44 were not evaluated (NE); the ORR and DCR
were calculated to be 4.0% (6/151) and 30.5% (46/151),
respectively. In the analysis of the best tumor response in the
non-sarcopenia group, CR was achieved in 3 patients, PR in 8, SD in
27, PD in 30 and 13 were NE; the ORR and DCR were calculated to be
13.6% (11/81) and 46.9% (38/81), respectively. The best treatment
efficacy significantly differed between the sarcopenia and
non-sarcopenia groups (ORR, P=0.0146; DCR, P=0.0151; Table II).
Causes of mortality
In the sarcopenia group, 136 (90.1%) patients
expired during the follow-up period: 111 due to HCC progression; 6
of liver failure; 19 of other causes. In the non-sarcopenia group,
63 (77.8%) patients perished during the follow-up period: 60 due to
HCC progression; 1 of liver failure; 2 of other causes.
Univariate and multivariate analysis
of factors contributing to OS
The univariate analysis identified that the
following factors significantly contributed to OS for all cases
(n=232): Sex (P=0.0079); initial dose of sorafenib (P=0.0394);
sarcopenia (P<0.0001); ECOG-PS (P=0.0041); extrahepatic
metastases (P=0.0024); portal vein invasion (P=0.0029); tumor
burden ≥50% (P=0.0001); presence of ascites (P<0.0001); AST ≥50
IU/l (P=0.0081); alkaline phosphatase ≥401 IU/l (P=0.0301); serum
albumin ≥3.4 g/dl (P=0.0010); α-fetoprotein ≥139.2 ng/ml
(P=0.0286); DCP ≥748 mAU/ml (P=0.0037; Table III). The hazard ratios (HRs) and 95%
confidence intervals (CIs) determined by multivariate analysis for
the 13 variables (selected based on P<0.05 values in univariate
analysis) are detailed in Table
III. Using multivariate analysis, sarcopenia (P<0.0001),
extrahepatic metastases (P<0.0001), tumor burden ≥50% (P=0.0004)
and the presence of ascites (P=0.0002) were identified to be
significant predictors of OS.
 | Table III.Univariate and multivariate analysis
of factors contributing to overall survival. |
Table III.
Univariate and multivariate analysis
of factors contributing to overall survival.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Patients, n | Univariate
analysis | Hazard ratio (95%
CI) |
P-valuea |
|---|
| Gender |
| 0.0079 | 0.736
(0.477–1.111) | 0.1464 |
|
Male | 181 |
|
|
|
|
Male | 51 |
|
|
|
| Age, years |
| 0.5742 |
|
|
|
≥72 | 119 |
|
|
|
|
<72 | 113 |
|
|
|
| Initial dose of
sorafenib |
| 0.0394 | 1.072
(0.750–1.547) | 0.7068 |
| 800
mg/day | 66 |
|
|
|
| Reduced
dose of sorafenib | 166 |
|
|
|
| Sarcopenia |
| <0.0001 | 0.365
(0.255–0.516) | <0.0001 |
|
Yes | 151 |
|
|
|
| No | 81 |
|
|
|
| ECOG-PS 0 |
| 0.0041 | 1.098
(0.717–1.636) | 0.6581 |
|
Yes | 197 |
|
|
|
| No | 35 |
|
|
|
| Extrahepatic
metastases |
| 0.0024 | 0.523
(0.383–0.715) | <0.0001 |
|
Yes | 88 |
|
|
|
| No | 144 |
|
|
|
| Portal vein
invasion |
| 0.0229 | 0.734
(0.521–1.051) | 0.0900 |
|
Yes | 52 |
|
|
|
| No | 180 |
|
|
|
| Tumor burden
≥50% |
| 0.0001 | 0.357
(0.218–0.614) | 0.0004 |
|
Yes | 23 |
|
|
|
| No | 209 |
|
|
|
| Ascites |
| <0.0001 | 0.427
(0.283–0.715) | 0.0002 |
|
Yes | 195 |
|
|
|
| No | 37 |
|
|
|
| AST, IU/l |
| 0.0081 | 0.774
(0.564–1.061) | 0.1116 |
|
≥50 | 121 |
|
|
|
|
<50 | 111 |
|
|
|
| ALT, IU/l |
| 0.0833 |
|
|
|
≥34 | 117 |
|
|
|
|
<34 | 115 |
|
|
|
| ALP, IU/l |
| 0.0301 | 0.993
(0.724–1.356) | 0.9643 |
|
≥401 | 116 |
|
|
|
|
<401 | 116 |
|
|
|
| GGT, IU/l |
| 0.0823 |
|
|
|
≥72 | 115 |
|
|
|
|
<72 | 114 |
|
|
|
| Prothrombin time,
% |
| 0.1215 |
|
|
|
≥80 | 117 |
|
|
|
|
<80 | 115 |
|
|
|
| Serum albumin
level, g/dl |
| 0.0010 | 1.160
(0.827–1.622) | 0.3879 |
|
≥3.4 | 127 |
|
|
|
|
<3.4 | 105 |
|
|
|
| Total bilirubin,
mg/dl |
| 0.1166 |
|
|
|
≥0.8 | 129 |
|
|
|
|
<0.8 | 103 |
|
|
|
| Platelet count,
×104/mm3 |
| 0.5146 |
|
|
|
≥11.7 | 116 |
|
|
|
|
<11.7 | 116 |
|
|
|
| Serum AFP,
ng/ml |
| 0.0286 | 0.743
(0.542–1.015) | 0.0619 |
|
≥139.2 | 116 |
|
|
|
|
<139.2 | 115 |
|
|
|
| DCP, mAU/ml |
| 0.0037 | 0.858
(0.622–1.182) | 0.3492 |
|
≥748 | 115 |
|
|
|
|
<748 | 114 |
|
|
|
Univariate and multivariate analysis
of factors contributing to PFS
Univariate analysis identified sarcopenia
(P=0.0131), ECOG-PS (P=0.0021), extrahepatic metastases (P=0.0019),
portal vein invasion (P=0.0203), tumor burden ≥50% (P=0.0244),
presence of ascites (P=0.0429) and DCP ≥748 mAU/ml (P=0.0266) to be
significantly associated with PFS for all cases (n=232; Table IV). The HRs and 95% CIs determined by
multivariate analysis for these seven factors (selected based on
P<0.05 values in univariate analysis) are presented in Table IV. Multivariate analysis identified
ECOG-PS (P=0.0452) and extrahepatic metastasis (P=0.0014) to be
significant prognostic factors associated with PFS.
 | Table IV.Univariate and multivariate analysis
of factors contributing to progression-free survival. |
Table IV.
Univariate and multivariate analysis
of factors contributing to progression-free survival.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Patients, n | Univariate
analysis | Hazard ratio (95%
CI) |
P-valuea |
|---|
| Gender |
| 0.3319 |
|
|
|
Male | 181 |
|
|
|
|
Male | 51 |
|
|
|
| Age, years |
| 0.7418 |
|
|
|
≥72 | 119 |
|
|
|
|
<72 | 113 |
|
|
|
| Initial dose of
sorafenib |
| 0.1065 |
|
|
| 800
mg/day | 66 |
|
|
|
| Reduced
dose of sorafenib | 166 |
|
|
|
| Sarcopenia |
| 0.0131 | 0.831
(0.612–1.123) | 0.2300 |
|
Yes | 151 |
|
|
|
| No | 81 |
|
|
|
| ECOG-PS 0 |
| 0.0021 | 1.509
(1.009–2.192) | 0.0452 |
|
Yes | 197 |
|
|
|
| No | 35 |
|
|
|
| Extrahepatic
metastases |
| 0.0019 | 0.627
(0.475–0.833) | 0.0014 |
|
Yes | 88 |
|
|
|
| No | 144 |
|
|
|
| Portal vein
invasion |
| 0.0203 | 0.715
(0.516–1.007) | 0.0547 |
|
Yes | 52 |
|
|
|
| No | 180 |
|
|
|
| Tumor burden
≥50% |
| 0.0244 | 0.686
(0.441–1.118) | 0.1255 |
|
Yes | 23 |
|
|
|
| No | 209 |
|
|
|
| Ascites |
| 0.0429 | 0.695
(0.485–1.025) | 0.0656 |
|
Yes | 195 |
|
|
|
| No | 37 |
|
|
|
| AST, IU/l |
| 0.1455 |
|
|
|
≥50 | 121 |
|
|
|
|
<50 | 111 |
|
|
|
| ALT, IU/l |
| 0.6526 |
|
|
|
≥34 | 117 |
|
|
|
|
<34 | 115 |
|
|
|
| ALP, IU/l |
| 0.0977 |
|
|
|
≥401 | 116 |
|
|
|
|
<401 | 116 |
|
|
|
| GGT, IU/l |
| 0.3614 |
|
|
|
≥72 | 115 |
|
|
|
|
<72 | 114 |
|
|
|
| Prothrombin time,
% |
| 0.3787 |
|
|
|
≥80 | 117 |
|
|
|
|
<80 | 115 |
|
|
|
| Serum albumin
level, g/dl |
| 0.1266 |
|
|
|
≥3.4 | 127 |
|
|
|
|
<3.4 | 105 |
|
|
|
| Total bilirubin,
mg/dl |
| 0.6683 |
|
|
|
≥0.8 | 129 |
|
|
|
|
<0.8 | 103 |
|
|
|
| Platelet count,
×104/mm3 |
| 0.1255 |
|
|
|
≥11.7 | 116 |
|
|
|
|
<11.7 | 116 |
|
|
|
| Serum AFP,
ng/ml |
| 0.2879 |
|
|
|
≥139.2 | 116 |
|
|
|
|
<139.2 | 115 |
|
|
|
| DCP, mAU/ml |
| 0.0266 | 0.852
(0.643–1.129) | 0.2641 |
|
≥748 | 115 |
|
|
|
|
<748 | 114 |
|
|
|
Discussion
Recently, sarcopenia has attracted a high level of
attention in the fields of several types of malignancies due to its
impact on clinical outcomes (18,24,25).
However, to the best of our knowledge, reliable data regarding the
impact of sarcopenia on the clinical outcomes of patients with HCC
receiving sorafenib therapy have yet to be obtained. Therefore, the
present study was conducted; to the best of our knowledge, it is
the first study to evaluate the associations between sarcopenia and
clinical outcomes in patients with unresectable HCC receiving
sorafenib therapy. The major advantage of the current study was the
large patient cohort.
Multivariate analysis identified sarcopenia to be an
independent predictor of OS (HR=0.365; P<0.0001) and
demonstrated its association with treatment efficacy. These results
indicated that sarcopenia may be a significant predictor of
prognosis in patients with HCC who underwent sorafenib therapy, and
potentially in patients with other types of malignancies.
Individualized nutritional assessment and interventional strategies
may be recommended for patients with HCC and sarcopenia treated
with sorafenib (18,24,25). By
contrast, it should be noted that sarcopenia was identified in 151
(65.1%) patients in the present analysis. A potential explanation
for the high prevalence is that the median age of the patients was
72 years. In Japan, the number of elderly patients with HCC has
been increasing (5). These trends may
be critical, as the incidence of sarcopenia in patients with HCC is
predicted to increase in the future. Another possible reason is
that, in the majority of cases, patients with HCC frequently
underwent other treatments prior to sorafenib therapy. Popular HCC
therapies may cause the deterioration of liver function,
potentially leading to a decreased quality of life and the
occurrence of sarcopenia in patients with HCC (37).
Consistent with previous studies, the presence of
sarcopenia was associated with poor PS and poor liver function in
the present study (38,39). Furthermore, the ORR and DCR for the
sarcopenia group were significantly lower than for the
non-sarcopenia group. This may be attributed to the fact that the
duration of treatment with sorafenib in the sarcopenia group was
significantly shorter than that in the non-sarcopenia group
(P=0.001). Mir et al (40)
reported that the presence of sarcopenia is associated with early
dose-limiting toxicities and the pharmacokinetics of sorafenib in
patients with HCC. These results are possibly associated with the
results in the present study.
The recent increase in the prevalence of obesity has
surfaced a novel clinical condition termed sarcopenic obesity,
which is the combination of obesity and sarcopenia (41). As patients with cirrhosis develop
sarcopenia even if they have obesity, a considerable number of
cirrhotic patients are established to have sarcopenic obesity
(41). Sarcopenic obesity has also
been associated with poorer clinical outcomes in numerous types of
malignancies (42). However, in the
present study, the differences between the sarcopenia group with
obesity (BMI ≥25 kg/m2; n=21) and the sarcopenia group
without obesity (n=130) were insignificant in terms of OS
(P=0.8767) and PFS (P=0.2064; data not presented) (43,44). The
reasons for this observation are unclear, and additional studies
concerning the impact of sarcopenic obesity on the survival of
patients with HCC treated with sorafenib are required.
Multivariate analysis identified the presence of
extrahepatic metastasis as an independent predictor of OS
(HR=0.523; P<0.0001) and PFS (HR=0.627; P=0.0014). These results
are consistent with the results from previous studies (7–9).
Tumor-associated factors, including extrahepatic metastasis,
maximum tumor size and vascular invasion, may potentially have the
strongest prognostic impact on sorafenib therapy instead of liver
function. In patients with HCC with significantly poor liver
function, sorafenib therapy must be contraindicated (7,8).
There are several limitations of the present study.
First, it is a retrospective observational study. Second, the
initial dose of sorafenib differed between the patients, creating
bias. Third, various anticancer therapies were employed following
the discontinuation of sorafenib, and these therapies may have
potentially caused bias in the clinical outcomes of the patients.
Fourth, certain data were missing in the analysis. However, owing
to the small number of patients with missing data, this may not
have affected the interpretation of the data. Finally, the present
study population only included Japanese patients with relatively
low body weights compared with patients in Western countries.
Therefore, the present study results may not be directly applied to
various ethnic populations. However, the results of the current
study demonstrated that sarcopenia is associated with the clinical
outcomes of patients with HCC undergoing sorafenib therapy. In
conclusion, sarcopenia may be a significant predictor of prognosis
in patients with HCC receiving sorafenib therapy. In such patients,
appropriate interventions, such as nutritional therapies or
exercise, may be required for improving the clinical outcomes.
Acknowledgements
The authors would like to thank Mrs. Haruko Takada
(Osaka Red Cross Hospital), Mrs. Nozomi Kanazawa (Hyogo College of
Medicine), Mrs. Yoko Matsushita (Hyogo College of Medicine) and
Miss. Sayaka Fujii (Hyogo College of Medicine) for data
collection.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
LC
|
liver cirrhosis
|
|
CT
|
dynamic computed tomography
|
|
TACE
|
transcatheter arterial
chemoembolization
|
|
TAI
|
transcatheter arterial infusion
chemotherapy
|
|
PS
|
performance status
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HU
|
Hounsfield unit
|
|
L3
|
third lumbar vertebra
|
|
SMI
|
skeletal muscle index
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
SAE
|
serious adverse event
|
|
mRECIST
|
modified Response Evaluation Criteria
in Solid Tumors
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR
|
objective response rate
|
|
DCR
|
disease control rate
|
|
CTCAE
|
Common Terminology Criteria for
Adverse Events
|
|
AST
|
aspartate aminotransferase
|
|
DCP
|
des-on Termxy prothrombin
|
|
NE
|
not evaluated
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singal AG and El-Serag HB: Hepatocellular
carcinoma from epidemiology to prevention: Translating knowledge
into practice. Clin Gastroenterol Hepatol. 13:2140–2151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osaki Y and Nishikawa H: Treatment for
hepatocellular carcinoma in Japan over the last three decades: Our
experience and literature review. Hepatol Res. 45:59–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Marco V, De Vita F, Koskinas J, Semela
D, Toniutto P and Verslype C: Sorafenib: From literature to
clinical practice. Ann Oncol. 24 Suppl 2:ii30–ii37. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sangiovanni A and Colombo M: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 36 Suppl 1:S124–S129. 2016. View Article : Google Scholar
|
|
11
|
Arizumi T, Ueshima K, Minami T, Kono M,
Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, et al:
Effectiveness of sorafenib in patients with transcatheter arterial
chemoembolization (TACE) refractory and intermediate-stage
hepatocellular carcinoma. Liver Cancer. 4:253–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J, Raoul JL, Sherman M, Mazzaferro
V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M,
Sangiovanni A, et al: Efficacy and safety of sorafenib in patients
with advanced hepatocellular carcinoma: Subanalyses of a phase III
trial. J Hepatol. 57:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Peña CE, Lathia CD, Shan M,
Meinhardt G and Bruix J: SHARP Investigators Study Group. Plasma
biomarkers as predictors of outcome in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 18:2290–2300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ogasawara S, Chiba T, Ooka Y, Suzuki E,
Kanogawa N, Saito T, Motoyama T, Tawada A, Kanai F and Yokosuka O:
Liver function assessment according to the Albumin-Bilirubin (ALBI)
grade in sorafenib-treated patients with advanced hepatocellular
carcinoma. Invest New Drugs. 33:1257–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY,
Kang YK, Shin YM, Kim KM, Lim YS and Lee HC: Sorafenib alone versus
sorafenib combined with transarterial chemoembolization for
advanced-stage hepatocellular carcinoma: Results of propensity
score analyses. Radiology. 269:603–611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kudo M, Matsui O, Izumi N, Kadoya M,
Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka
A, et al: Transarterial chemoembolization failure/refractoriness:
JSH-LCSGJ criteria 2014 update. Oncology. 87 Suppl 1:S22–S31. 2014.
View Article : Google Scholar
|
|
17
|
Printz C: Clinical trials of note.
Sorafenib as adjuvant treatment in the prevention of disease
recurrence in patients with hepatocellular carcinoma (HCC) (STORM).
Cancer. 115:46462009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumors of
the respiratory and gastrointestinal tracts: A population based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenberg IH: Sarcopenia: Origins and
clinical relevance. J Nutr. 127:990S–991S. 1997.PubMed/NCBI
|
|
20
|
Wang C and Bai L: Sarcopenia in the
elderly: Basic and clinical issues. Geriatr Gerontol Int.
12:388–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dasarathy S: Consilience in sarcopenia of
cirrhosis. J Cachexia Sarcopenia Muscle. 3:225–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Periyalwar P and Dasarathy S: Malnutrition
in cirrhosis: Contribution and consequences of sarcopenia on
metabolic and clinical responses. Clin Liver Dis. 16:95–131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishikawa H, Yoh K, Enomoto H, Iwata Y,
Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T,
et al: Factors associated with protein-energy malnutrition in
chronic liver disease: Analysis using indirect calorimetry.
Medicine (Baltimore). 95:e24422016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukushima H, Nakanishi Y, Kataoka M,
Tobisu K and Koga F: Prognostic significance of sarcopenia in
patients with metastatic renal cell carcinoma. J Urol. 195:26–32.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levolger S, van Vugt JL, de Bruin RW and
Ijzermans JN: Systematic review of sarcopenia in patients operated
on for gastrointestinal and hepatopancreatobiliary malignancies. Br
J Surg. 102:1448–1458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi A, Kaido T, Hamaguchi Y, Okumura
S, Taura K, Hatano E, Okajima H and Uemoto S: Impact of
postoperative changes in sarcopenic factors on outcomes after
hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat
Sci. 23:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harimoto N, Shirabe K, Yamashita YI,
Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A and
Yamanaka T: Sarcopenia as a predictor of prognosis in patients
following hepatectomy for hepatocellular carcinoma. Br J Surg.
100:1523–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeda H, Nishikawa H, Osaki Y, Tsuchiya
K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y and Izumi N;
Japanese Red Cross Liver Study Group, : Clinical features
associated with radiological response to sorafenib in unresectable
hepatocellular carcinoma: A large multicenter study in Japan. Liver
Int. 35:1581–1589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishikawa H, Takeda H, Tsuchiya K, Joko K,
Ogawa C, Taniguchi H, Orito E, Uchida Y, Osaki Y and Izumi N;
Japanese Red Cross Liver Study Group, : Sorafenib therapy for BCLC
stage B/C hepatocellular carcinoma; clinical outcome and safety in
aged patients: A multicenter study in Japan. J. Cancer. 5:499–509.
2014. View Article : Google Scholar
|
|
30
|
Cheng AL, Amarapurkar D, Chao Y, Chen PJ,
Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, et al:
Re-evaluating transarterial chemoembolization for the treatment of
hepatocellular carcinoma: Consensus recommendations and review by
an International Expert Panel. Liver Int. 34:174–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujiwara N, Nakagawa H, Kudo Y, Tateishi
R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami
T, et al: Sarcopenia, intramuscular fat deposition, and visceral
adiposity independently predict the outcomes of hepatocellular
carcinoma. J Hepatol. 63:131–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer,
. EASL-EORTC Clinical Practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salvaggio G, Furlan A, Agnello F, Cabibbo
G, Marin D, Giannitrapani L, Genco C, Midiri M, Lagalla R and
Brancatelli G: Hepatocellular carcinoma enhancement on
contrast-enhanced CT and MR imaging: Response assessment after
treatment with sorafenib: Preliminary results. Radiol Med.
119:215–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albers I, Hartmann H, Bircher J and
Creutzfeldt W: Superiority of the Child-Pugh classification to
quantitative liver function tests for assessing prognosis of liver
cirrhosis. Scand J Gastroenterol. 24:269–276. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saito M, Seo Y, Yano Y, Miki A, Yoshida M
and Azuma T: Short-term reductions in non-protein respiratory
quotient and prealbumin can be associated with the long-term
deterioration of liver function after transcatheter arterial
chemoembolization in patients with hepatocellular carcinoma. J
Gastroenterol. 47:704–714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanai T, Shiraki M, Ohnishi S, Miyazaki T,
Ideta T, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H and
Shimizu M: Rapid skeletal muscle wasting predicts worse survival in
patients with liver cirrhosis. Hepatol Res. 46:743–751. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chindapasirt J: Sarcopenia in Cancer
Patients. Asian Pac J Cancer Prev. 16:8075–8077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mir O, Coriat R, Blanchet B, Durand JP,
Boudou-Rouquette P, Michels J, Ropert S, Vidal M, Pol S, Chaussade
S and Goldwasser F: Sarcopenia predicts early dose-limiting
toxicities and pharmacokinetics of sorafenib in patients with
hepatocellular carcinoma. PLoS One. 7:e375632012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shiraki M, Nishiguchi S, Saito M, Fukuzawa
Y, Mizuta T, Kaibori M, Hanai T, Nishimura K, Shimizu M, Tsurumi H
and Moriwaki H: Nutritional status and quality of life in current
patients with liver cirrhosis as assessed in 2007–2011. Hepatol
Res. 43:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prado CM, Wells JC, Smith SR, Stephan BC
and Siervo M: Sarcopenic obesity: A Critical appraisal of the
current evidence. Clin Nutr. 31:583–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McCurry J: Japan battles with obesity.
Lancet. 369:451–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Examination Committee of Criteria for
‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity,
. New criteria for ‘obesity disease’ in Japan. Circ J. 66:987–992.
2002. View Article : Google Scholar : PubMed/NCBI
|