Introduction
It is well known that the steroid hormone estrogen
performs a role in the pathogenesis of breast cancer, and the
majority of breast cancer types involve estrogen signaling pathways
in cancer initiation, progression and metastasis (1). The majority of estrogen-associated
biological actions are traditionally attributed to activate classic
estrogen receptors (ERs), ERα and ERβ (2). Thus, endocrine therapies that interfere
with ER functions are currently applied in patients with
ER-positive breast cancer. Although targeted inhibition of ERα is a
successful approach, numerous patients fail to respond (de
novo resistance) or relapse despite an initial response
(acquired resistance) to anti-estrogen therapy (3). The selective ER modulator tamoxifen,
which antagonizes estrogen-induced genomic-nuclear ERα activity, is
widely used in the treatment of ERα-positive breast cancer. It has
significantly reduced the mortality risk among patients, however,
not all patients achieve beneficial effects as disease recurrence
occurs in ~25–30% of patients (4).
The identification and characterization of the G
protein-coupled receptor 30 (GPR30) represents an additional
mechanism of estrogen effects, which mediate a wide range of
estrogenic responses, leading to changes in gene expression and
relevant biological responses (5). It
has been revealed that GPR30 expression is associated with clinical
and pathological biomarkers of poor outcome in breast cancer
(6). In addition, GPR30 is
overexpressed in invasive breast cancer and positively correlated
with the development of distant metastases, furthermore, mouse
mammary tumor virus-polyoma middle T (MMTV-PyMT) mice deficient in
GPR30 expression exhibit a substantially decreased incidence of
metastasis compared with GPR30 wild-type (6–8). These
observations suggest that GPR30 is implicated in breast cancer
metastasis and may provide a novel potential target for treatment.
Although tamoxifen exhibits antagonistic properties with respect to
ERα, it performs as a GPR30 agonist (9), which demonstrates the complex
physiological and therapeutic actions it possesses.
Tumor metastasis consists of a series of relevant
biological processes including cell adhesion, migration, and
invasion. Degradation extracellular matrix (ECM) of tumor cells is
a hallmark of tumor metastasis and an essential step for invading
distant organs (10). The expression
and activation of matrix metalloproteinases (MMPs) facilitate tumor
cells to invade the ECM underlying their basement membrane and
stroma (11). Among the MMPs, MMP-2
and MMP-9, two zinc-dependent endopeptidases, perform essential
roles in ECM degradation. Overexpression of MMP-2 and MMP-9 were
associated with high potential of metastasis and they are poor
prognostic factors in patients with breast cancer (12). A positive association between GPR30
and MMP-9 expression was identified in malignant ovarian
endometriotic cysts and epithelial ovarian cancer (13,14).
Furthermore, E2 and selective GPR30 agonist G-1 increased the
expression and proteolytic activity of MMP-9 in ovarian cancer
cells, which could be inhibited by small interfering RNA targeting
GPR30 or G protein inhibitor pertussin toxin (15), indicating a possible linkage between
GPR30 and MMP-9. Nevertheless, the contribution of GPR30 in the
regulation of MMP-9 in breast cancer cells remains unclear.
The incidence of breast cancer is much higher in
Western countries compared with in Asian countries. Certain
epidemiological studies contribute one factor of these differences
to dietary flavonoids (16–18). Furthermore, as their polyphenolic ring
is similar to the steroid nucleus of 17β-estradiol (E2), certain
flavonoids have been considered to exert an anti-estrogenic effect
and are candidates for chemopreventive agents to reduce the risk of
breast cancer (19). Baicalein
(5,6,7-trihydroxyflavone) is the primary flavonoid derived from
Scutellaria baicalensis Georgi, whose structure is composed
of a three-ring flavone backbone with phenolic hydroxyl at the 5′,
6′, and 7′ position. Several studies have revealed that baicalein
possesses antitumor activity in breast cancer and exhibits
anti-estrogenic activity (20–22). In
our previous study, it was demonstrated that baicalein is able to
suppress E2 enhanced migration, adhesion and invasion of breast
cancer cells and interferes with E2 or G-1 induced GPR30 signaling
activation (23). However, whether
this compound could inhibit E2 promoted MMP-9 upregulation and
activation, as well as take effects via GPR30 remains unclear.
In the present study, the role of GPR30 in the
regulation of the invasion process was evaluated, and the effects
of baicalein on E2-induced cell invasion, MMP-9 activity and
expression were investigated in ERα/GPR30-positive MCF-7 human
breast cancer cells. In addition, the effects of baicalein with the
active form of the selective ER modulator tamoxifen
4-hydroxytamoxifen (OHT) and the GPR30 antagonist G15.
Materials and methods
Reagents and antibodies
Baicalein (purity >98%) was provided by Professor
Qinglong Guo and Zhiyu Li (China Pharmaceutical University,
Nanjing, China). It was dissolved in dimethyl sulfoxide (DMSO) as a
stock solution at 0.1 M and stored at −20°C. E2 (cat. no. E2758)
and OHT (cat. no. H7904) were dissolved in DMSO as a stock solution
at 10−1 M and stored at 4°C (both from Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). G-1 (cat. no. 10008933) and G15
(cat. no. 14,673) were purchased from Cayman Chemical Company (both
from Ann Arbor, MI, USA) and dissolved in DMSO as a stock solution
at 10−1 M and stored at −20°C. Matrigel (cat. no.
356237) was obtained from BD Biosciences (Franklin Lakes, NJ, USA).
Primary antibodies directed against MMP-9 (W680) (cat. no. BS1241;
polyclonal rabbit anti-human; dilution 1:1,000), and GAPDH (1A6)
(cat. no. MB001; monoclonal mouse anti-human; dilution, 1:4,000)
were purchased from Bioworld Technology, Inc. (St. Louis Park, MN,
USA). The anti-mouse (cat. no. sc-2005) or anti-rabbit (cat. no.
sc-2030) ImmunoglobulinG horseradish peroxidase-conjugated
secondary antibodies (dilution, 1:3,000) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture and treatment
The human breast cancer MCF-7 cell line from the
Kunming Cell Bank of the Chinese Academy of Sciences (Kunming,
China) was maintained in DMEM (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) with 10% fetal bovine serum (FBS; Gibco,
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (both from Sigma-Aldrich;
Merck KGaA). The cells were incubated at 37°C in a humidified
atmosphere with 5% CO2. Prior to the indicated
treatments, cells were pre-cultured for 24 h at 37°C in phenol red
(PR)-free DMEM (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) without serum to remove endogenous estrogen. Subsequently,
cells were treated accordingly in PR-free DMEM (Hyclone; GE
Healthcare Life Sciences). Control cells were incubated in DMSO
(0.01% v/v). The concentrations of each drug were used as follows:
E2 (1 nM), OHT (1 µM), G1 (1 µM), G15 (1 µM) and baicalein (10
µM).
Invasion assay
Invasive ability of cells was measured using an
assay in a Transwell chamber (EMD Millipore, Billerica, MA, USA)
containing membranes with an 8-µm pore size, coated with Matrigel
as previously described (24).
Following treatment, cells were trypsinized and suspended at a
final concentration of 5×105 cells/ml in serum-free
PR-free DMEM. The cell suspension was then added into each 10-mm
upper chamber and PR-free DMEM with 10% FBS was added into the
bottom chamber as a chemo-attractant. Subsequent to a 24 h
incubation at 37°C in a humidified atmosphere with 5%
CO2, the upper surfaces of the membranes were swabbed to
remove non-invasive cells and the cells attached onto the lower
surface were fixed in 100% methanol, stained with hematoxylin and
eosin (Beyotime Institute of Biotechnology, Haimen, China), and
counted under an inverted light microscope equipped (Eclipse 50i)
with a color camera (DS-Fi2) (both Nikon Corporation, Tokyo, Japan)
at magnification, ×200. A total of 5 randomly selected fields were
analyzed from each group. Values represent the percentage of
invasive cells relative to the controls (as 100%).
Western blot analysis
Cells were collected and lysed in lysis buffer
(Beyotime Institute of Biotechnology). The lysates were separated
using centrifugation at 4°C for 15 min at 13,000 × g. Protein
concentration was determined using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.) with a Varioskan multimode
microplate spectrophotometer (Thermo Fisher Scientific, Inc.).
Total protein (30 µg/lane) was separated using 12% SDS-PAGE and
transferred to a PVDF membrane (EMD Millipore). The membrane was
blocked using 5% non-fat milk at 37°C for 1 h, and subsequently
incubated with aforementioned appropriate antibodies at 37°C for 1
h. Blots were visualized using an enhanced chemiluminescence kit
(EMD Millipore). Digital images of blots were captured using a
ChemiDoc XRS+ system and analyzed using Image Lab™
software (version 5.2) (both Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Gelatin zymography assay
The activity of MMP-2 and MMP-9 were analyzed using
a gelatin zymography assay as previous described (25). Cells (2×105 cells/ml) were
seeded (2 ml) into a six-well plate. When 80% confluence was
achieved, the cells were treated as indicated. Following treatment,
the conditioned medium was collected and centrifuged at 112 × g for
5 min at 4°C to remove the dead cell debris. The conditioned medium
with sample buffer (0.5 M Tris-HCl pH 6.8, buffer with 30%
glycerin, 0.05% Bromophenol blue and 6% SDS, v/v at 2:1) was
subjected to 10% SDS-PAGE containing 0.1% gelatin. Following
electrophoresis, the gels were washed twice with rinsing buffer (50
mM Tris-HCl pH 7.6, with 5 mM, CaCl2, 1 µM
ZnCl2 and 2.5% Triton X-100) to remove the SDS and
incubated for 36 h at 37°C in incubating buffer (50 mM Tris-HCl
buffer with 5 mM CaCl2, 1 µM ZnCl2). The gels
were subsequently stained with 0.1% Coomassie Brilliant Blue R250
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) for 1 h at room temperature, followed with destaining using
10% acetic acid and 10% methanol. The images of the gels were
captured using the ChemiDoc XRS+ system and analyzed
with Image Lab™ software (version 5.2).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted following treatment using an
RNA Extraction kit (cat. no. 9767; Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. cDNA was
synthesized using a strand complementary DNA synthesis kit (cat.
no. 6210A; Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. mRNA expression was measured using SYBR
Green PCR Core reagents (cat. no. RR820A, TaKaRa). The reaction
were conducted in 96-well plate and the reaction volume for per
well was 20 µl, including 2 µl (100 ng) cDNA, 10 µl SYBR Premix Ex
Taq II, 0.8 µl (10 µM) of both forward and reverse primers and 6.4
µl ddH2O. The reaction was performed with a CFX Connect™
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.). The
following primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China) and used in the present study: MMP-9 forward,
5′-GTGGGGATTTACATGGCACT-3′ and reverse, 5′-AAAGCCTATTTCTGCCAGGAC-3′
(26); β-actin forward,
5′-AGTTGCGTTACACCCTTTC-3′ and reverse, 5′-CCTTCACCGTTCCAGTTT-3′
(27). The thermocycling conditions
maintained were as follows: 30 sec at 95°C; followed by 40 cycles
at 95°C for 5 sec; and 60°C for 30 sec. The melting curve was
analyzed at 65–95°C to detect a single gene-specific peak and
verify the absence of primer dimer peaks. The MMP-9 mRNA level was
quantified relative to β-actin mRNA expression using the
2−ΔΔCq method (28).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean following ≥3 independent experiments. Statistically
significant differences were calculated using one-way analysis of
variance followed by the Bonferroni's post hoc test for
multiple-group comparisons by using SPSS Statistics v.17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Unlike OHT, baicalein is able to
prevent E2-induced invasion and MMP-9 expression and activity in
MCF-7 human breast cancer cells
To assess the effects of anti-estrogenic agents
tamoxifen and baicalein on E2-induced invasion, estrogen-sensitive
MCF-7 breast cancer cells were treated with OHT or baicalein in the
presence of E2 for 24 h. It was observed that OHT failed to
suppress E2-induced invasion, while baicalein was able to
significantly suppress this effect compared with the E2-treated
control group (Fig. 1A). MMPs perform
essential roles in processes associated with tumor invasion and
metastasis, when cells become invasive they often produce
proteolytic enzymes, which can degrade the majority of the
extracellular matrix (29). Next, the
enzyme activity of MMP-2 and MMP-9 was addressed following the
above treatment. It was demonstrated that E2 or E2 plus OHT
promoted MMP-9 activity, while baicalein exerted a significant
suppression of E2-enhanced MMP-9 activity compared with the
E2-treated group (Fig. 1B). However,
no significant differences were identified among the activity of
MMP-2 across all treatment groups (Fig.
1B). Additionally, western blot analysis and RT-qPCR analyses
were performed to further confirm the changes observed in MMP-9
activity. The protein and mRNA expression levels were upregulated
following E2 or E2 plus OHT treatment compared with the untreated
control group. However, the two expression levels were
significantly downregulated in the presence of baicalein compared
with the E2-treated group (Fig. 1C and
D).
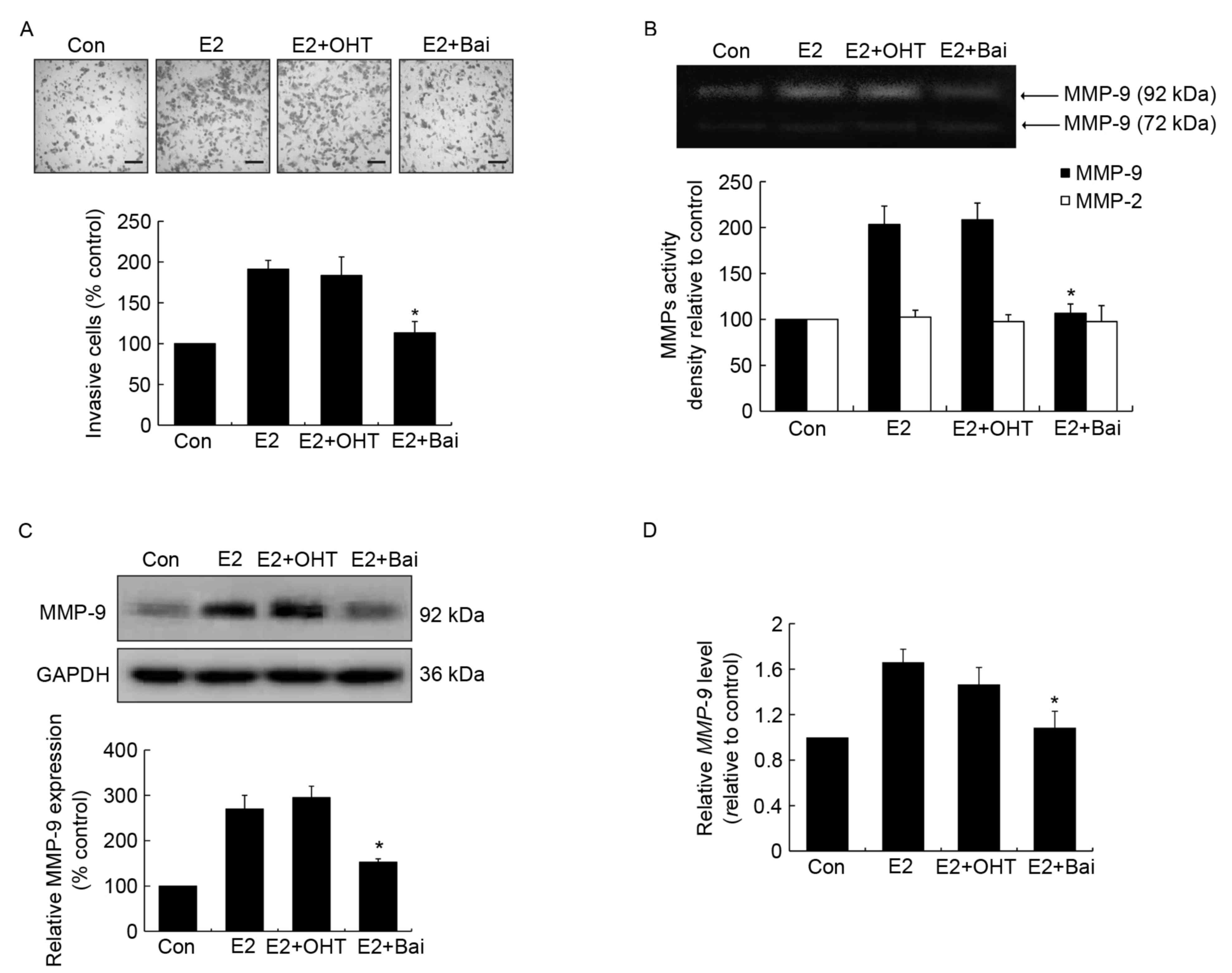 | Figure 1.Effects of OHT and baicalein on
E2-induced cell invasion, and the activity and expression of MMP-9
in MCF-7 cells. Cells were treated with DMSO (0.01%), E2 (1 nM), E2
(1 nM) plus OHT (1 µM) or E2 (1 nM) plus baicalein (Bai, 10 µM) for
24 h. OHT and baicalein was added 30 min prior to E2 stimulation.
(A) Invasive cells were stained using hematoxylin and eosin
(magnification, ×200, scale bar=500 µm). (B) The activity of
MMP-2/9 was measured using a gelatin zymography assay. A
representative zymographic gel is illustrated, where the clear
bands represent collagenases MMP-2 and MMP-9. Results of
densitometrical analysis are illustrated. (C) The expression of
MMP-9 was determined using western blotting. Densitometric analysis
represents the ratios of MMP-9 relative to GAPDH. (D) MMP-9
mRNA expression was detected using reverse
transcription-quantitative polymerase chain reaction analysis and
fold-changes were normalized to β-actin mRNA level. Data are
presented as the mean ± standard error of the mean, and normalized
as a percentage of the control. *P<0.05 vs. the E2-treated
group. OHT, 4-hydroxytamoxifen; E2, 17β-estradiol; MMP, matrix
metalloproteinase; cont, control; Bai, baicalein. |
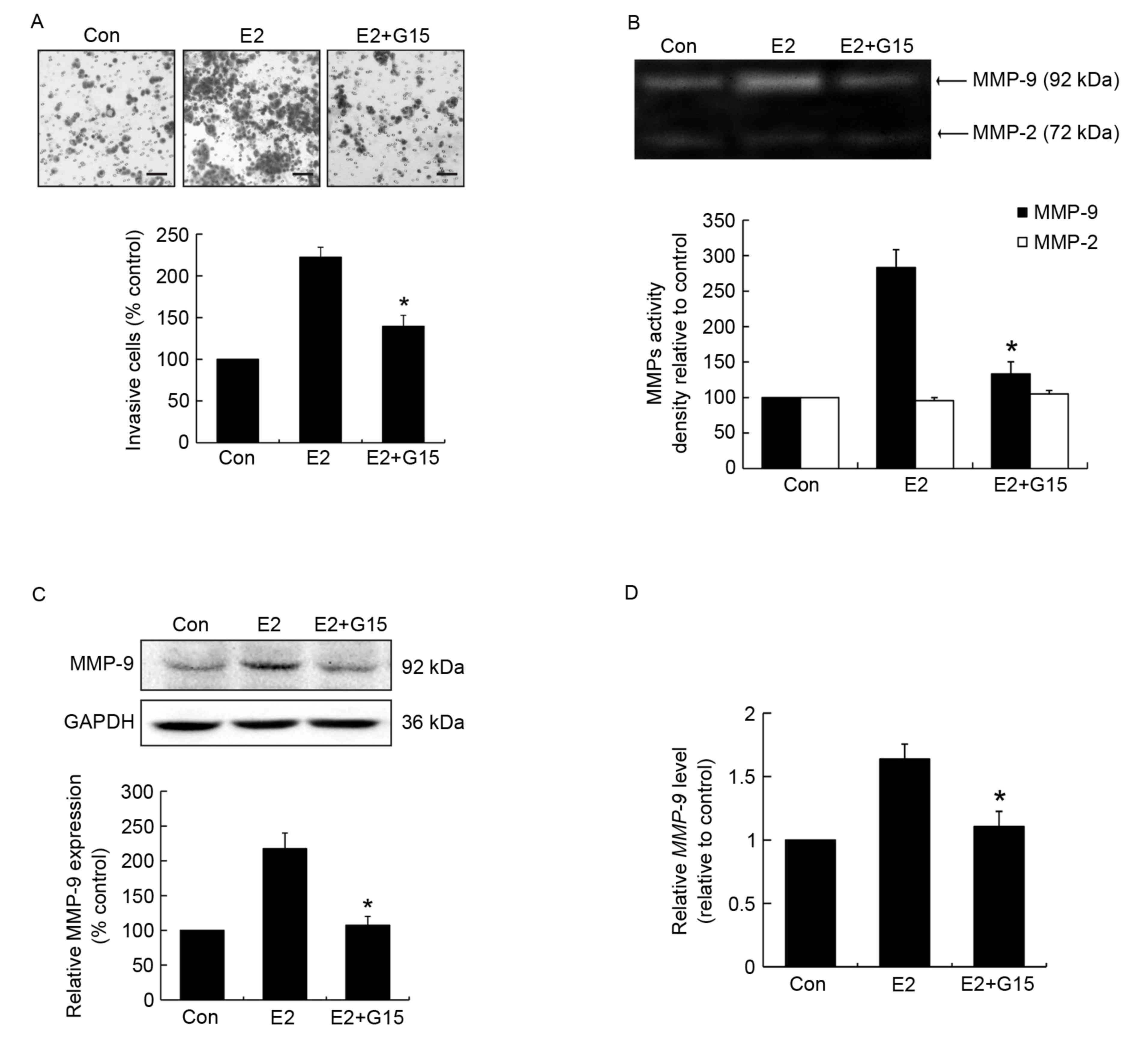
GPR30 mediates cell invasion and MMP-9
activity and expression induced by E2
Since tamoxifen acts as an ERα antagonist but
agonist for the GPR30, it was hypothesized that the mechanism by
which estrogen induced invasiveness occurs through GPR30
activation. To validate the potential role of GPR30 in cell
invasion, and MMP-9 activity and expression, MCF-7 cells were
pretreated with the GPR30 antagonist G15 prior to stimulation with
E2. The results demonstrated that G15 effectively suppressed
E2-induced cell invasion compared with the E2-treated group
(Fig. 2A). G15 significantly reduced
MMP-9 activity compared with the E2-treated group (Fig. 2B). Furthermore, MMP-9 protein
expression in G15 pretreated groups was significantly lower
compared with that in the E2-induced group (Fig. 2C). Similar changes in MMP-9 mRNA
expression were observed (Fig. 2D).
This suggests that the effects of E2 on cell invasion and MMP-9 are
mediated through the activation of GPR30.
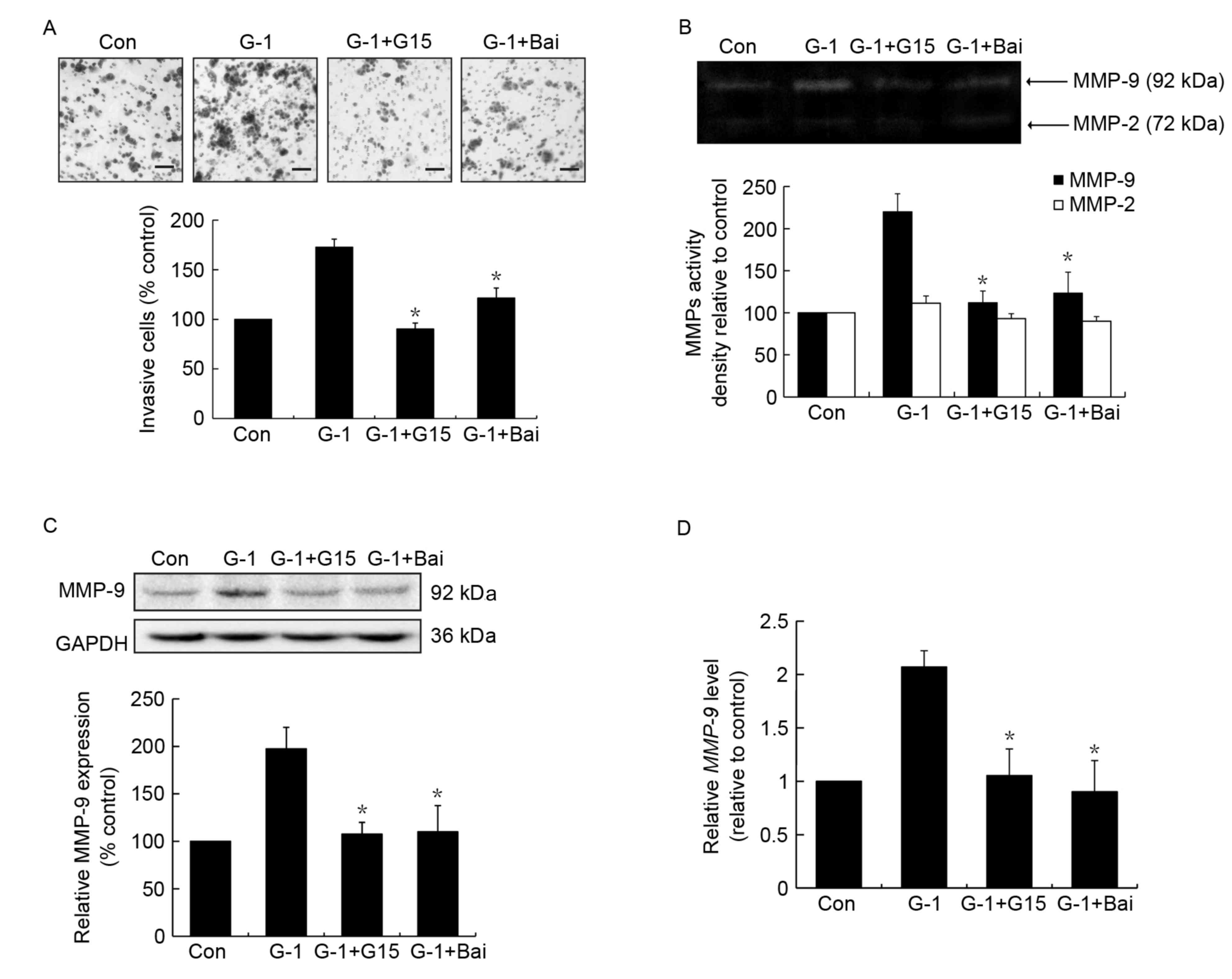
Similar to the effects of G15,
baicalein inhibits G-1 induced cell invasion and MMP-9 activity and
expression in MCF-7 cells
To confirm whether baicalein exerted its effects by
interfering with GPR30 activation, the action of baicalein on GPR30
agonist G-1 induced invasion and MMP-9 activity and expression was
investigated, whereby G15 was used as a positive control. As
expected, treatment with G-1 resulted in an increase in cell
invasion, which was significantly suppressed by G15 or baicalein
pretreatment (Fig. 3A). In addition,
it was revealed that treatment with baicalein led to significant
suppression of G-1-induced MMP-9 activation (Fig. 3B), similar to the effects of G15. In
addition, the upregulation of MMP-9 protein and mRNA expression
induced by G-1 was significantly inhibited following baicalein
treatment (Fig. 3C and D).
Discussion
While anti-estrogenic agents have been used
successfully, breast cancer remains the leading cause of
cancer-associated mortality among females worldwide (30). Additionally, aromatase inhibitors,
which depress E2 synthesis, are more efficacious and produce
significantly lower recurrence rates when compared with tamoxifen
(31), indicating that solely
targeting ERα to inhibit the action of estrogen may be suboptimal.
GPR30 is independent of ERα status in breast cancer cells and
tissue samples, and its action differs from the classical nuclear
ERs, ERα and ERβ (32). Upregulation
and activation of GPR30 promotes the progression of breast cancer,
and GPR30 is considered a biological target for innovative
therapeutic strategies (33).
Research has demonstrated that overexpression of GPR30 in primary
tumors is positively correlated with the metastatic phenotype of
breast cancer (6,7), and GPR30 knockout MMTV-PyMT mice possess
less aggressive tumors and fewer metastases (8). In the present study, an ERα- and
GPR30-positive MCF-7 breast cancer cell line was used as a model
system. It was demonstrated that the inhibition of GPR30 activation
by its specific antagonist G15 significantly suppressed E2- or
G-1-induced invasion, which is the initial stage of metastasis.
Furthermore, GPR30 inhibition significantly reduced the E2- or
G-1-induced increase in MMP-9 expression and proteolytic activity,
suggesting that GPR30 regulates metastasis by enhancing cell
invasive ability.
Tamoxifen was initially designed as an
anti-estrogenic agent, but has also been demonstrated to partially
induce estrogenic activity (34). It
has been revealed to enhance the migration and invasion abilities
of ERα-negative SK-BR-3 breast cancer cells (35), and promotes the proliferation and
invasion of endometrial cancer cells, similar to the effects of E2
(36). Furthermore, previous studies
have revealed that tamoxifen can increase invasiveness and the
expression of MMPs in MCF-7 cells in vitro (37,38). In
the current study, it was demonstrated that although OHT could
inhibit the function of ERα, it failed to prevent E2-induced cell
invasion, and MMP-9 activation and upregulation. However, the
E2-stimulated activation and upregulation of MMP-9 were
significantly inhibited by treatment of cells with the GPR30
antagonist G15. In addition, tamoxifen could perform as a GPR30
agonist. The results of the present study indicate that E2 promoted
MMP-9 upregulation and expression through GPR30, instead of ERα.
Furthermore, these results suggest that GPR30 performs an important
role in maintaining the responsiveness of breast cancer cells to E2
in the pharmacological blockade of ERα. Therefore, antagonistic
action on ERα and GPR30 may be a rational strategy for the
treatment of ERα-positive breast cancer.
Emerging evidence suggests that administering
endocrine agents, including tamoxifen has effects beyond their
initially described mechanism of action. For example, tamoxifen has
been demonstrated to promote the induction of epidermal growth
factor receptor, human epidermal growth factor receptor-2 and
insulin-like growth factor I (IGF-I) receptor, which subsequently
activates various cellular kinase cascades, and elicits tamoxifen
resistance (39). In addition,
previous studies demonstrated that GPR30 is involved in the
development of tamoxifen resistance and appearance of metastasis
(40,41). GPR30 expression is significantly
increased in tamoxifen resistant tumor tissue compared with primary
tumors from the same patients (40).
Furthermore, Mo et al (41)
revealed that the expression of GPR30 was significantly increased
in metastatic tumor compared with their corresponding primary tumor
samples from 53 GPR30-positive patients with tamoxifen recurrence
and that the GPR30 antagonist was able to reverse tamoxifen-induced
resistance. Thus, it is suggested that inhibiting GPR30 signaling
activation may provide an alternative therapeutic strategy for
treating tamoxifen-resistant patients with breast cancer.
A previous study demonstrated that baicalein
exhibits effective inhibitory activities against E2/IGF-1-induced
cellular proliferation and colony formation in human breast
carcinoma cells (42). In addition,
the binding ability of baicalein to the ER was confirmed through a
competitive ligand-binding assay (43,44).
Notably, baicalein antagonized the estradiol-induced estrogen
responsive elements response in a dose dependent manner (21). Unlike genistein, an isomer of
baicalein, it does not exhibit a biphasic effect on ERα, thus there
is no exhibition of estrogenic activity to transactivate ERα at low
concentrations (21). These
investigations propose that baicalein exerts anti-estrogenic
activity and inhibitory effects on ERα transcriptional function.
Recently, the authors of the current study demonstrated that
baicalein suppresses E2-promoted migration and invasion in MCF-7
(GPR30/ERα-positive) and SK-BR-3 (GPR30-positive/ERα-negative)
breast cancer cells (23).
Furthermore, it was revealed that baicalein significantly inhibited
E2- or G1-induced GPR30 signal activation and GPR30 target genes,
cysteine-rich 61, and connective tissue growth factor upregulation
(23).
In the present study, it was demonstrated that
baicalein significantly suppressed E2- or G-1 induced cell
invasion, and MMP-9 upregulation and activation, exhibiting a
similar effect to G15, but a reverse effect compared with OHT. This
indicates that baicalein exerts a different activity to tamoxifen,
and may possess dual inhibitory effects on ERα and GPR30 signaling.
However, this hypothesis requires further investigation. To further
investigate the underlying mechanism of baicalein, a detailed
explanation of the signaling pathway by which GPR30 regulates MMP-9
expression and activation is required, as well as the molecular
mechanisms by which baicalein influences GPR30 signaling. In
addition, in vivo studies are warranted to confirm the
effects observed in the current study.
In conclusion, baicalein, but not OHT, significantly
attenuated E2-induced invasion, and MMP-9 upregulation and
activation in MCF-7 breast cancer cells, which may be due to their
different actions on GPR30. As cell invasion and MMP-9 activation
positively correlates with cancer metastasis, it could be suggested
that the inhibition of GPR30 activation may be a promising approach
to reduce metastasis, and improve the efficacy of anti-estrogens.
Targeting ERα and GPR30 receptors may achieve additional
therapeutic benefits for the treatment of patients with breast
cancer.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81302804 and
81560598), The Natural Science Foundation of Guizhou Province of
China [grant no. QKHJ (2014) 2007], Postdoctoral Science Foundation
of China (grant no. 2015M582749XB), Science Foundation of Guiyang
Science and Technology Bureau [grant no. (20141001)06], Science and
technology innovation advanced individual of Guizhou educational
department [grant no. QJHKY (2015)492], Startup Foundation for
Doctors of Guiyang Medical University [grant no. (2013)09], The
Foundation for Training Programs of Innovation and Entrepreneurship
for Undergraduates of Guiyang Medical University (grant no.
201410660038), The Innovated Team of the Education Department of
Guizhou Province (grant no. 2014-31), The Innovation team of
Guizhou Province [grant no. (2015)4025], The Program for New
Century Excellent Talents in University (grant no. NCET-13-0747),
The High level Innovation Talents (grant no. 2015-4029).
References
|
1
|
Cordera F and Jordan VC: Steroid receptors
and their role in the biology and control of breast cancer growth.
Semin Oncol. 33:631–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang B, Warner M and Gustafsson JÅ:
Estrogen receptors in breast carcinogenesis and endocrine therapy.
Mol Cell Endocrinol. 418:240–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnston SR: New strategies in estrogen
receptor-positive breast cancer. Clin Cancer Res. 16:1979–1987.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jager NG, Linn SC, Schellens JH and
Beijnen JH: Tailored tamoxifen treatment for breast cancer
patients: A perspective. Clin Breast Cancer. 15:241–244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prossnitz ER and Arterburn JB:
International union of basic and clinical pharmacology. XCVII. G
protein-coupled estrogen receptor and its pharmacologic modulators.
Pharmacol Rev. 67:505–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Filardo EJ, Graeber CT, Quinn JA, Resnick
MB, Giri D, DeLellis RA, Steinhoff MM and Sabo E: Distribution of
GPR30, a seven membrane-spanning estrogen receptor, in primary
breast cancer and its association with clinicopathologic
determinants of tumor progression. Clin Cancer Res. 12:6359–6366.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Q, Li JG, Zheng XY, Jin F and Dong HT:
Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast
carcinomas. Chin Med J (Engl). 122:2763–2769. 2009.PubMed/NCBI
|
|
8
|
Marjon NA, Hu C, Hathaway HJ and Prossnitz
ER: G protein-coupled estrogen receptor regulates mammary
tumorigenesis and metastasis. Mol Cancer Res. 12:1644–1654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Revankar CM, Cimino DF, Sklar LA,
Arterburn JB and Prossnitz ER: A transmembrane intracellular
estrogen receptor mediates rapid cell signaling. Science.
307:1625–1630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS,
Di GH, Liu G, Li FM, Ou ZL, et al: Prognostic value of matrix
metalloproteinases (MMP-2 and MMP-9) in patients with lymph
node-negative breast carcinoma. Breast Cancer Res Treat. 88:75–85.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu HD, Yan Y, Cao XF, Tan PZ, Wen HX, Lv
CM, Li XM and Liu GY: The expression of a novel estrogen receptor,
GPR30, in epithelial ovarian carcinoma and its correlation with
MMP-9. Sheng Li Xue Bao. 62:524–528. 2010.(In Chinese). PubMed/NCBI
|
|
14
|
Long L, Cao Y and Tang LD: Transmembrane
estrogen receptor GPR30 is more frequently expressed in malignant
than benign ovarian endometriotic cysts and correlates with MMP-9
expression. Int J Gynecol Cancer. 22:539–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan Y, Liu H, Wen H, Jiang X, Cao X, Zhang
G and Liu G: The novel estrogen receptor GPER regulates the
migration and invasion of ovarian cancer cells. Mol Cell Biochem.
378:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi RE, Pericleous M, Mandair D, Whyand
T and Caplin ME: The role of dietary factors in prevention and
progression of breast cancer. Anticancer Res. 34:6861–6875.
2014.PubMed/NCBI
|
|
17
|
Hui C, Qi X, Qianyong Z, Xiaoli P, Jundong
Z and Mantian M: Flavonoids, flavonoid subclasses and breast cancer
risk: A meta-analysis of epidemiologic studies. PLoS One.
8:e543182013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho YA, Kim J, Park KS, Lim SY, Shin A,
Sung MK and Ro J: Effect of dietary soy intake on breast cancer
risk according to menopause and hormone receptor status. Eur J Clin
Nutr. 64:924–932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takemura H, Sakakibara H, Yamazaki S and
Shimoi K: Breast cancer and flavonoids - a role in prevention. Curr
Pharm Des. 19:6125–6132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon YJ, Wang X and Morris ME: Dietary
flavonoids: Effects on xenobiotic and carcinogen metabolism.
Toxicol In Vitro. 20:187–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Po LS, Chen ZY, Tsang DS and Leung LK:
Baicalein and genistein display differential actions on estrogen
receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer
Lett. 187:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang HT, Chou CT, Kuo DH, Shieh P, Jan CR
and Liang WZ: The mechanism of Ca(2+) movement in the involvement
of baicalein-induced cytotoxicity in ZR-75-1 human breast cancer
cells. J Nat Prod. 78:1624–1634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang
X and Chen Y: Baicalein suppresses 17-β-estradiol-induced
migration, adhesion and invasion of breast cancer cells via the G
protein-coupled receptor 30 signaling pathway. Oncol Rep.
33:2077–2085. 2015.PubMed/NCBI
|
|
24
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen P, Lu N, Ling Y, Chen Y, Hui H, Lu Z,
Song X, Li Z, You Q and Guo Q: Inhibitory effects of wogonin on the
invasion of human breast carcinoma cells by downregulating the
expression and activity of matrix metalloproteinase-9. Toxicology.
282:122–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pello OM, De Pizzol M, Mirolo M, Soucek L,
Zammataro L, Amabile A, Doni A, Nebuloni M, Swigart LB, Evan GI, et
al: Role of c-MYC in alternative activation of human macrophages
and tumor-associated macrophage biology. Blood. 119:411–421. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferreira E and Cronjé MJ: Selection of
suitable reference genes for quantitative real-time PCR in
apoptosis-induced MCF-7 breast cancer cells. Mol Biotechnol.
50:121–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dowsett M, Cuzick J, Ingle J, Coates A,
Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, et al:
Meta-analysis of breast cancer outcomes in adjuvant trials of
aromatase inhibitors versus tamoxifen. J Clin Oncol. 28:509–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maggiolini M and Picard D: The unfolding
stories of GPR30, a new membrane-bound estrogen receptor. J
Endocrinol. 204:105–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Hu L, Zhang G, Zhang L and Chen C:
G protein-coupled receptor 30 in tumor development. Endocrine.
38:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catalano S, Giordano C, Panza S, Chemi F,
Bonofiglio D, Lanzino M, Rizza P, Romeo F, Fuqua SA, Maggiolini M,
et al: Tamoxifen through GPER upregulates aromatase expression: A
novel mechanism sustaining tamoxifen-resistant breast cancer cell
growth. Breast Cancer Res Treat. 146:273–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruan SQ, Wang ZH, Wang SW, Fu ZX, Xu KL,
Li DB and Zhang SZ: Heregulin-β1-induced GPR30 upregulation
promotes the migration and invasion potential of SkBr3 breast
cancer cells via ErbB2/ErbB3-MAPK/ERK pathway. Biochem Biophys Res
Commun. 420:385–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du GQ, Zhou L, Chen XY, Wan XP and He YY:
The G protein-coupled receptor GPR30 mediates the proliferative and
invasive effects induced by hydroxytamoxifen in endometrial cancer
cells. Biochem Biophys Res Commun. 420:343–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thompson EW, Reich R, Shima TB, Albini A,
Graf J, Martin GR, Dickson RB and Lippman ME: Differential
regulation of growth and invasiveness of MCF-7 breast cancer cells
by antiestrogens. Cancer Res. 48:6764–6768. 1988.PubMed/NCBI
|
|
38
|
Nilsson UW, Garvin S and Dabrosin C: MMP-2
and MMP-9 activity is regulated by estradiol and tamoxifen in
cultured human breast cancer cells. Breast Cancer Res Treat.
102:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fedele P, Calvani N, Marino A, Orlando L,
Schiavone P, Quaranta A and Cinieri S: Targeted agents to reverse
resistance to endocrine therapy in metastatic breast cancer: Where
are we now and where are we going? Crit Rev Oncol Hematol.
84:243–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ignatov A, Ignatov T, Weissenborn C,
Eggemann H, Bischoff J, Semczuk A, Roessner A, Costa SD and
Kalinski T: G-protein-coupled estrogen receptor GPR30 and tamoxifen
resistance in breast cancer. Breast Cancer Res Treat. 128:457–466.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mo Z, Liu M, Yang F, Luo H, Li Z, Tu G and
Yang G: GPR30 as an initiator of tamoxifen resistance in
hormone-dependent breast cancer. Breast Cancer Res. 15:R1142013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin CW, Yang LY, Shen SC and Chen YC:
IGF-I plus E2 induces proliferation via activation of ROS-dependent
ERKs and JNKs in human breast carcinoma cells. J Cell Physiol.
212:666–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Branham WS, Dial SL, Moland CL, Hass BS,
Blair RM, Fang H, Shi L, Tong W, Perkins RG and Sheehan DM:
Phytoestrogens and mycoestrogens bind to the rat uterine estrogen
receptor. J Nutr. 132:658–664. 2002.PubMed/NCBI
|
|
44
|
Shenouda NS, Zhou C, Browning JD, Ansell
PJ, Sakla MS, Lubahn DB and Macdonald RS: Phytoestrogens in common
herbs regulate prostate cancer cell growth in vitro. Nutr Cancer.
49:200–208. 2004. View Article : Google Scholar : PubMed/NCBI
|