Introduction
Colorectal cancer (CRC) is the third most common
cancer and the fourth leading cause of cancer-related death
worldwide (1,2). In 2012, 1,360,600 new cases were
diagnosed and 693,900 deaths were attributed to CRC (2). The carcinogenetic mechanism of CRC has
been extensively investigated and intracellular signaling pathways
related to cell survival, cell fate, and genome maintenance have
been implicated (3).
Membrane traffic is a fundamental intracellular
transport system in eukaryotic cells, and recent studies have
revealed that molecules involved in membrane traffic play an
important role in the initiation and progression of several types
of tumor (4–6). Soluble N-ethylmaleimide-sensitive
factor attachment protein receptors (SNAREs) located on
intracellular vesicles and their target membranes are the central
coordinators of membrane traffic (7),
and syntaxin family proteins are the main components of SNARE
complexes. Taxilin was identified as a novel binding partner of
syntaxins (8). The taxilin family
consists of at least three members: α-, β-, and γ-taxilins.
α-taxilin binds to free syntaxins that are not part of a SNARE
complex (9), suggesting that
α-taxilin may act as a regulator of vesicular transport by
affecting the assembly of SNARE complexes. In the physiological
setting α-taxilin was proposed to be involved in
Ca2+-dependent exocytosis in neuroendocrine cells
(8), and it was recently reported
that α-taxilin is normally expressed in gastrointestinal epithelial
cells (10). α-taxilin is also
expressed in stromal cells, such as mouse fibroblast NIH3T3
(10) and human fibrosarcoma HT1080
(11) cell lines. Overexpression of
α-taxilin mRNA has been reported in human glioblastoma
compared with normal tissues of the central nervous system
(12). α-taxilin expression has also
been found to be associated with proliferative activity and
dedifferentiation of hepatocellular carcinoma (HCC) (13) and local invasiveness and poor
prognosis of renal cell carcinoma (RCC) (14).
In the present study, we analyzed α-taxilin
expression in human colorectal tumors and explored the associations
between α-taxilin overexpression and prognosis of CRC. This is the
first study to investigate the clinical significance of α-taxilin
in human CRC.
Materials and methods
Tumor samples, pathological diagnosis,
and staging
CRCs that were endoscopically or surgically resected
at the Dokkyo Medical University Hospital (DMUH) and the
International University of Health and Welfare, Shioya Hospital
(IUHWSH) were analyzed. Clinicopathologic classification was based
on the World Health Organization classification of colorectal
tumors and stage grouping was according to tumor, node, metastases
(TNM) staging of the American Joint Committee on Cancer (AJCC)
Colon and Rectum Cancer Staging (15,16).
Colorectal intramucosal adenocarcinomas (IMAs; pTis
defined by AJCC) with adenoma components were selected from all
CRCs resected at IUHWSH from 2013 to 2015 and at DMUH from 2012 to
2014. A total of 20 IMAs, pathologically diagnosed as
well-differentiated and/or moderately differentiated
adenocarcinoma, were analyzed. For pathological analysis,
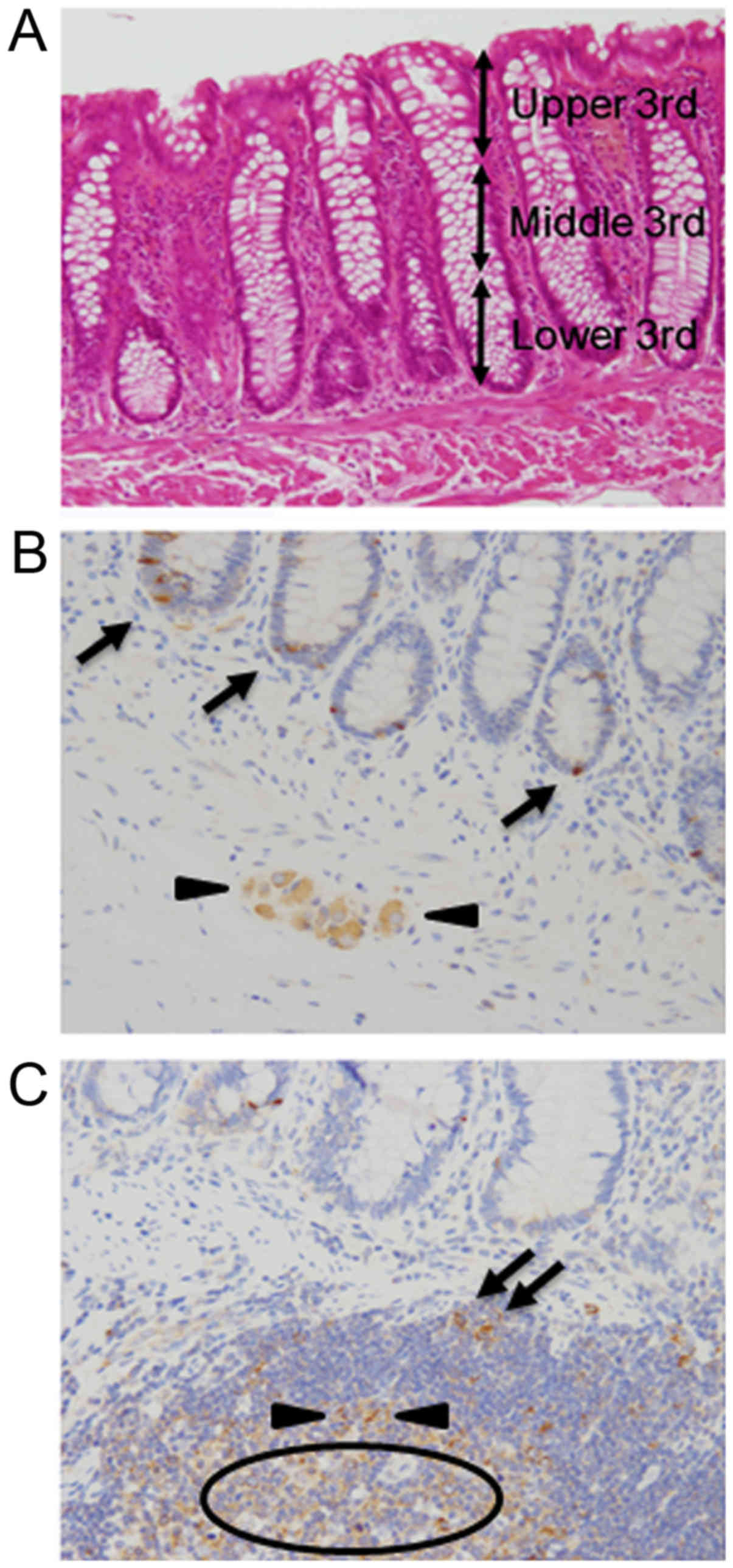
intramucosal glands were divided into three anatomical components:
Upper third (UT), middle third (MT), and lower third (LT) (Fig. 1A). Next, among all CRCs surgically
resected at DMUH and IUHWSH from 2009 to 2011, histologically
proven well-differentiated and/or moderately differentiated
adenocarcinomas in the left-sided colon with anatomic stage II
and/or III were selected. All CRCs included in this study were
diagnosed clinically and pathologically as primary tumors and
confirmed to be advanced cancers that invaded the muscularis
propria or more (pT2 to 4). We aimed to study the pure effect of
α-taxilin expression on prognosis by normalizing other possible
prognostic factors, such as tumor histology, differentiation grade,
location, and initial anatomic stage (17). Patients with complete medical records
were included in the survival analyses, but individuals with
invasive cancers originating from other sites were excluded. As a
result, a total of 57 cases were subjected to prognostic analyses.
This study protocol was approved by the ethical review boards of
the participating hospitals (IUHWSH, FK-94; DMUH, 26067).
Immunohistochemistry
Tumor specimens were fixed in 10% neutral-buffered
formalin for 48 h, embedded in paraffin, and then cut into 4-µm
sections. Antigen retrieval was performed in 10 mM citrate buffer
(pH 6.0) using microwave irradiation (400 W) at 95°C for 40 min.
After quenching endogenous peroxidase activity, sections were
incubated with primary antibody detecting α-taxilin (1:1,000) or
Ki-67 (1:50, clone: Mib-1; Dako, Glostrup, Denmark) for 60 min at
room temperature. Characterization of the anti-α-taxilin antibody
was described in previous studies (10,18).
Intensity of α-taxilin staining was classified into four
categories: Level 3, comparable to that of proliferating cells in
the lower crypt, follicular dendritic cells, and immunoblasts:
Level 2, comparable to that of ganglion cells and follicular
B-lymphocytes: Level 1, between level 0 and 2: Level 0, no staining
(Fig. 1B and C). α-taxilin expression
of levels 2 and 3 was regarded as overexpression. Ki-67 indices
were calculated as the percentage of Ki-67-positive cells among 500
to 1,000 cells in the areas with the strongest nuclear
labeling.
Statistics
Comparisons between two sets of data were performed
by non-paired/paired two-tailed Student's t-test. Correlation
between α-taxilin expression levels and Ki-67 indices were analyzed
by Spearman's rank correlation analysis. Specific parameters
between two patient cohorts were compared using the χ2
test with/without Yates' correction or by Fisher's exact test. Age
was compared using the Mann-Whitney U test, and survival curves
were analyzed using the Kaplan-Meier method and log-rank tests.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using IBM SPSS
Statistics 23 (IBM SPSS, Armonk, NY, USA).
Results
α-taxilin expression in IMA and
adenoma
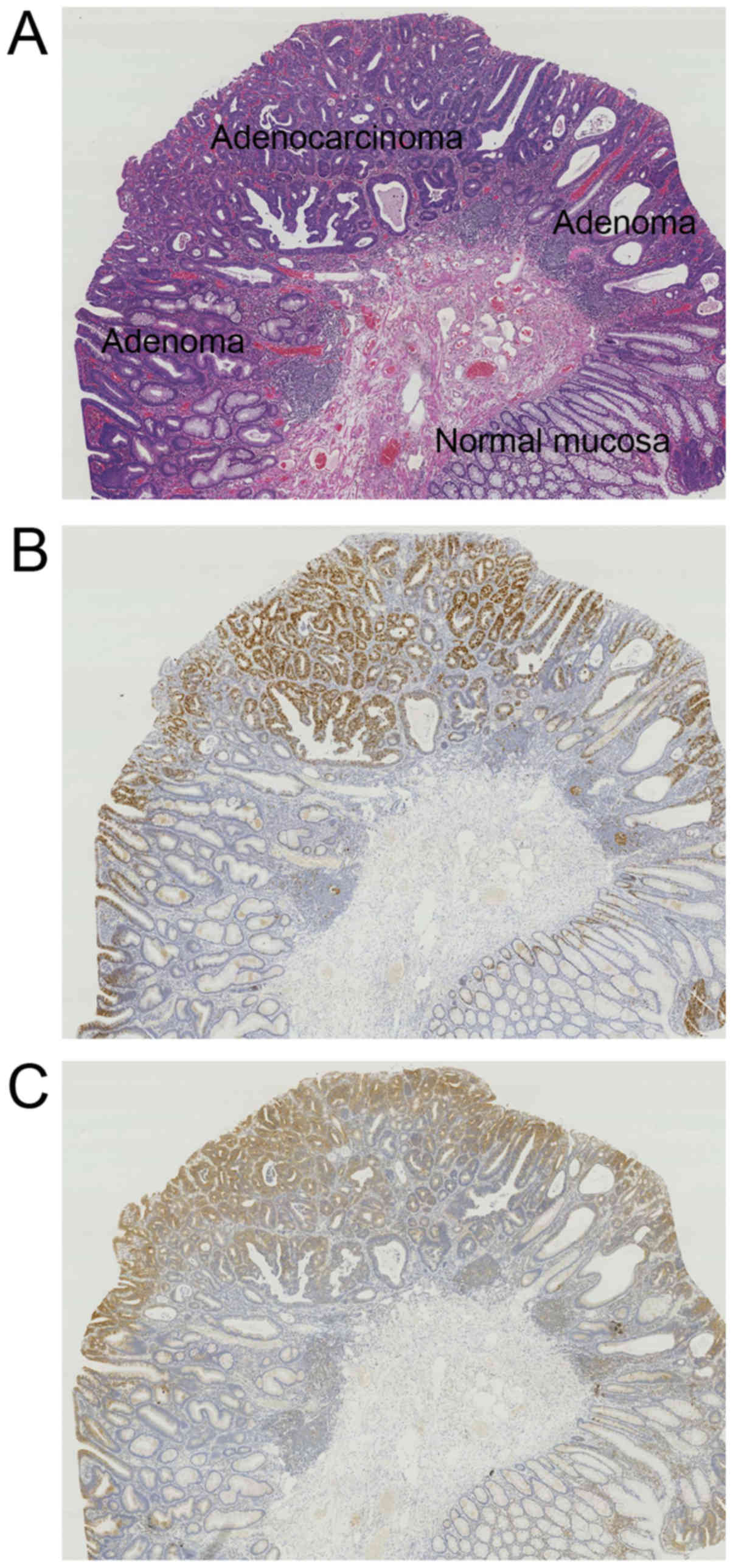
A total of 20 cases of IMA with adenoma were
analyzed (Fig. 2A). The proliferative
zone was in the LT in the normal colonic crypt, whereas it shifted
to UT in adenoma and spread from the UT downward in IMA (Fig. 2B). α-taxilin expression paralleled
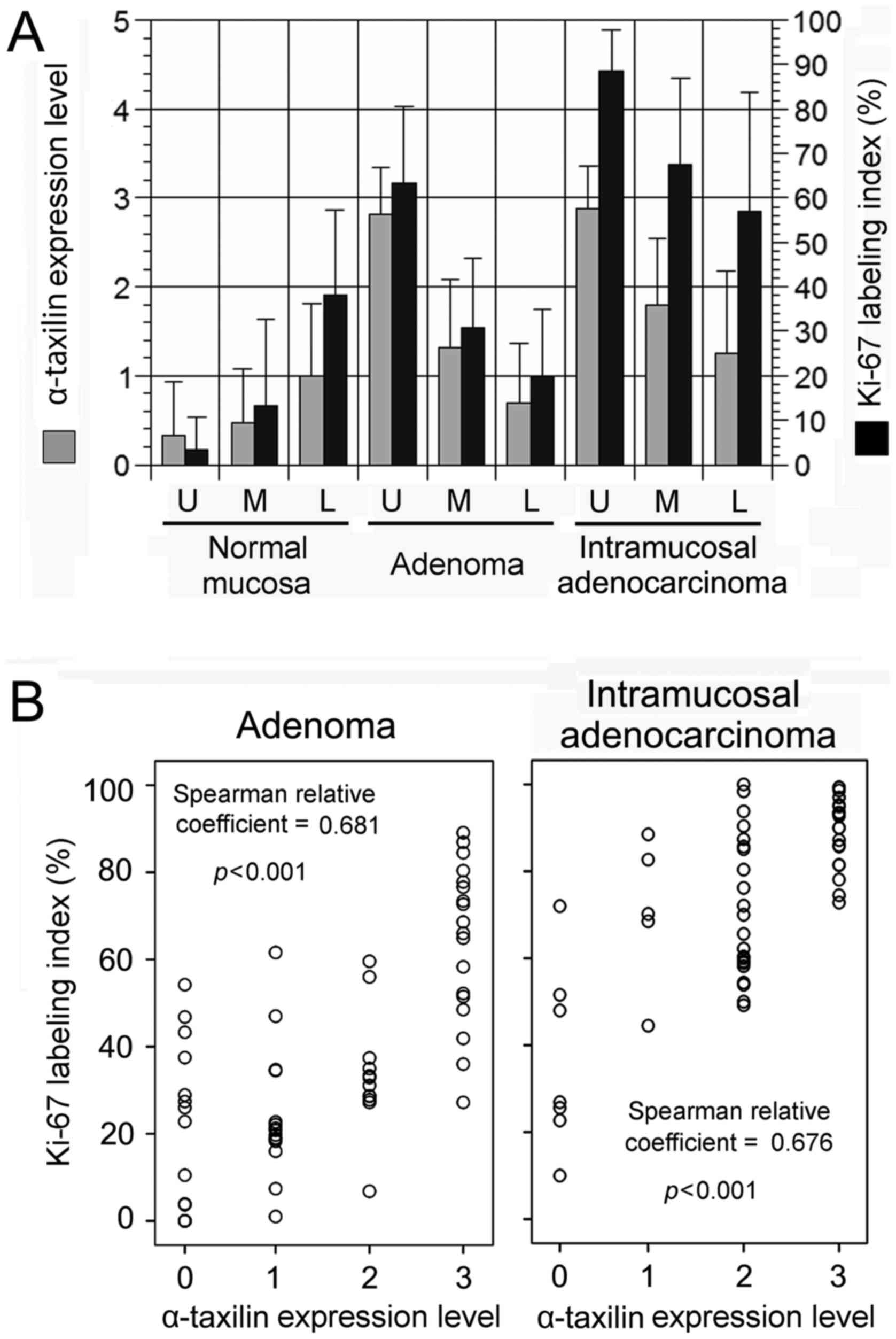
Ki-67 expression (Fig. 2B and C). In
the normal mucosa, expression of both α-taxilin and Ki-67 was
strongest in LT followed by MT, and weakest in UT of the crypt
(Fig. 3A). In contrast, for glands in
adenoma and IMA, expression of both α-taxilin and Ki-67 was
strongest in UT followed by MT, and weakest in LT (Fig. 3A). α-taxilin levels were similarly
high in UT of glands of adenoma and IMA (2.813±0.527 and
2.875±0.484, respectively), although Ki-67 indices were
significantly higher in IMA than in adenoma (88.46±9.343 and
63.35±17.2, respectively; P<0.01) (Fig. 3A).
Coordinate points consisting of α-taxilin level in
the x-axis and Ki-67 index in the y-axis in respective anatomical
components (LT, MT and UT) of intramucosal neoplastic glands were
plotted in a scattergram (Fig. 3B).
α-taxilin level and Ki-67 index showed a significant positive
correlation in both adenoma and IMA.
α-taxilin expression in CRC and
prognosis
We next investigated α-taxilin expression in
histologically proven well-differentiated and/or moderately
differentiated adenocarcinoma in the left-sided colon with anatomic
stage II and/or III and examined its association with local
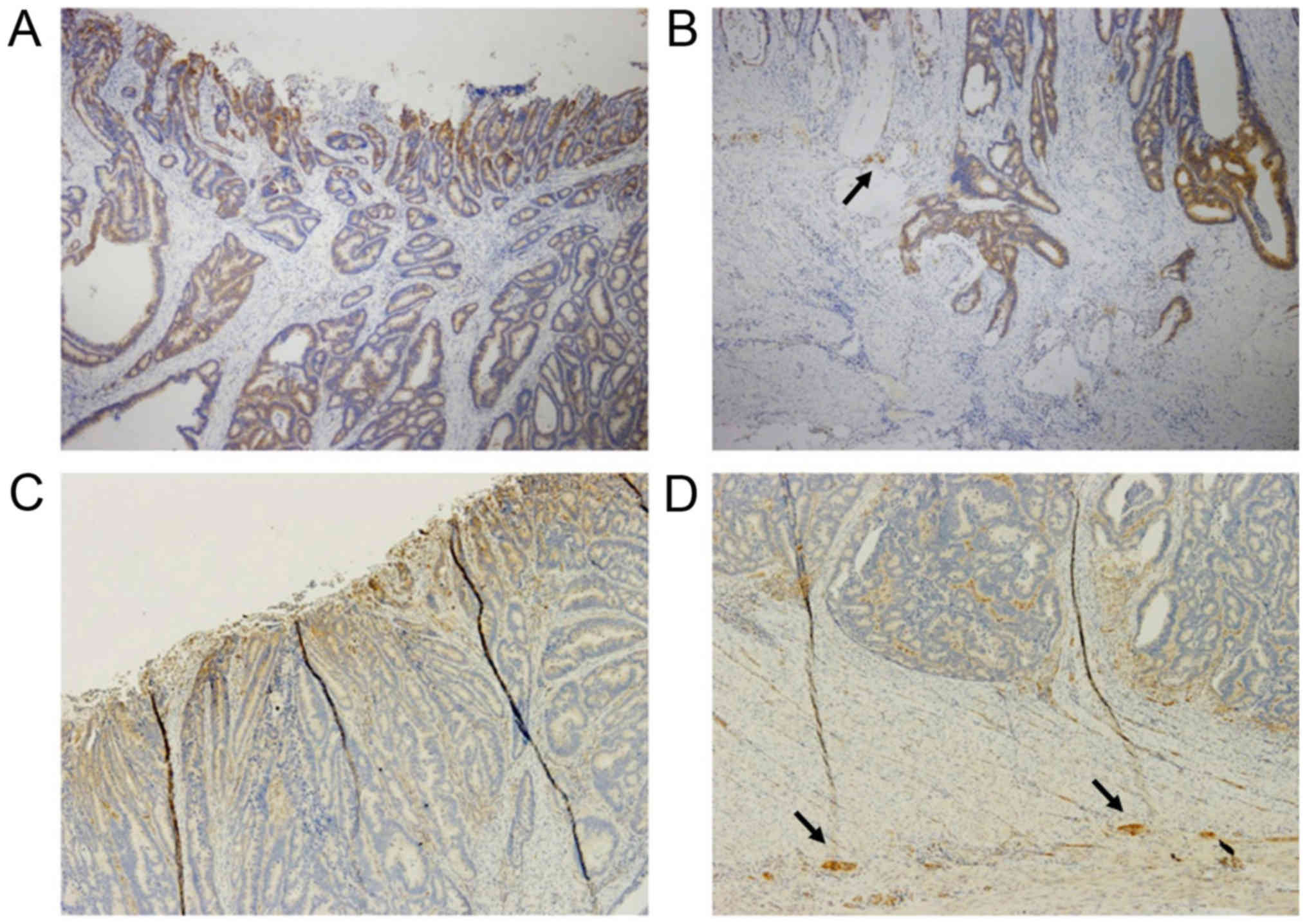
invasiveness and prognosis. α-taxilin was strongly expressed at the
cancer surface of nearly all CRCs: Level 1 in 6 cases, level 2 in
15 cases, level 3 in 35 cases, not determined in one case (Fig. 4A and C). In contrast, α-taxilin
expression levels at the cancer deep advancing edge in the
colorectal wall were more variable: Level 0 in 4 cases, level 1 in
12 cases, level 2 in 25 cases, and level 3 in 16 cases (Fig. 4B and D). Sixteen of 57 cases (28.1%)
showed low expression of α-taxilin. On average, there was a
significant difference in α-taxilin expression levels between the
surface area and the advancing edge (2.52±0.09 and 1.91±0.12,
respectively; P<0.01) in the present CRC cohorts.
Malignant tumor is characterized by destructive
downward growth and metastasis. We therefore investigated
associations of α-taxilin expression in cancer cells at the
advancing edge with local invasiveness and prognosis. α-taxilin
overexpression (levels 2 and 3) was not associated with any of the
clinical parameters tested, including depth of tumor (pT), venous
invasion, lymphatic permeation, nodal metastasis, and clinical
stage (Table I). In the survival
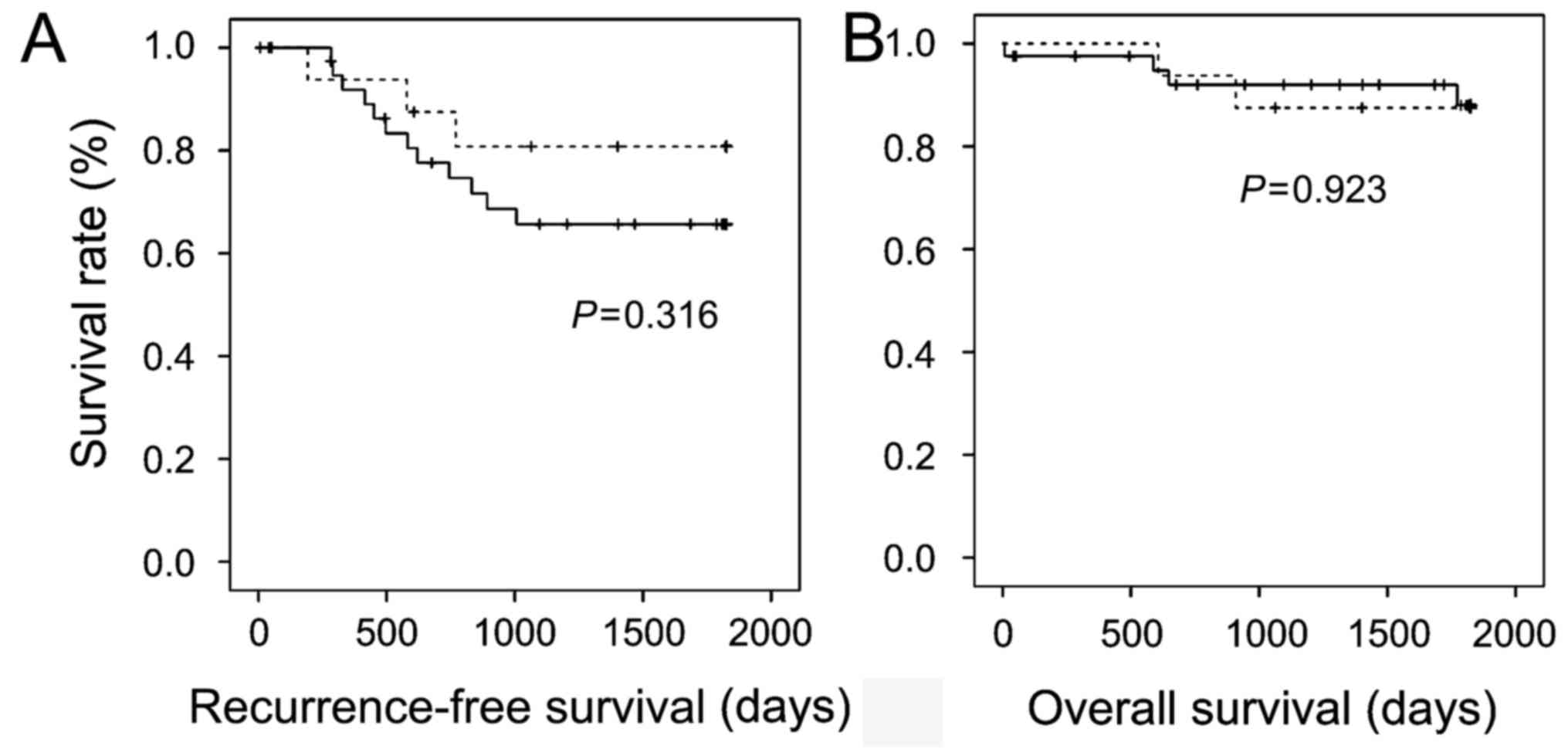
analyses, CRCs with α-taxilin overexpression showed a trend for
worse 5-year recurrence-free survival than those without α-taxilin
overexpression, but the difference did not reach statistical
significance (Fig. 5A). Advanced CRCs
with and without α-taxilin overexpression demonstrated similar
5-year overall survival (Fig. 5B).
These results suggest that the α-taxilin expression level may not
affect prognosis of CRCs.
 | Table I.Association between α-taxilin
overexpression in cancer cells at the deep advancing edge and
clinicopathologic characteristics of advanced CRC. |
Table I.
Association between α-taxilin
overexpression in cancer cells at the deep advancing edge and
clinicopathologic characteristics of advanced CRC.
|
| α-Taxilin
overexpression |
|
|---|
|
|
|
|
|---|
| Characteristics | Yes (n=41) | No (n=16) | P-value |
|---|
| Age |
|
|
|
| Range
(mean) | 42–83 (67) | 58–81 (69.5) | 0.505 |
| Gender |
|
|
|
| Male | 23 | 8 | 0.771 |
|
Female | 18 | 8 |
|
| pT4 |
|
|
|
| Yes | 8 | 4 | 0.450 |
| No | 33 | 12 |
|
| Venous invasion |
|
|
|
| Yes | 28 | 11 | 1.000 |
| No | 13 | 5 |
|
| Lymphatic
permeation |
|
|
|
| Yes | 32 | 13 | 1.000 |
| No | 9 | 3 |
|
| Nodal metastasis |
|
|
|
| Yes | 19 | 8 | 1.000 |
| No | 22 | 8 |
|
| Clinical stage |
|
|
|
| II | 22 | 8 | 1.000 |
| III | 19 | 8 |
|
Discussion
It has been reported that α-taxilin is related to
cell proliferation of neuroepithelial cells and malignancies of
epithelial origin, such as HCC and RCC (8,13,14). Recently, Horii et al reported
α-taxilin expression status in the normal gastrointestinal tract in
mice (10). They demonstrated that
α-taxilin was expressed in the majority of the gastrointestinal
tract and prominently present in the cytoplasm of epithelial cells
expressing Ki-67 in C57BL/6 mice. Although some epithelial cells
expressed α-taxilin without Ki-67 expression, the majority of
α-taxilin-positive/Ki-67-negative epithelial cells were observed in
the vicinity of α-taxilin/Ki-67 double-positive cells, and the
authors reasoned that α-taxilin remained in these cells following
division and subsequent loss of Ki-67 expression. They also
reported that treatment with dibenzazepine, a γ-secretase inhibitor
that inhibits cell proliferation in the stomach, resulted in a
decrease in the number of Ki-67-positive cells and
α-taxilin-positive cells in the lower part of the gastric glands,
suggesting that expression of α-taxilin was dependent on cell
proliferation. In the present study, we investigated α-taxilin
expression status in human colorectal tumors for the first time.
Assuming that α-taxilin might be a marker of CRC, we first studied
its expression focusing on the pathological sequence of colorectal
carcinogenesis. In normal mucosa, the proliferation zone of the
colonic crypt is located in the lower third of the crypt, where
stem cells are present. However, the proliferation zone represented
by high Ki-67 indices shifted to the upper third of the crypt in
adenoma and was found to extend downward from the UT in IMA. These
observations corresponded well with one of the two models
explaining colon carcinogenesis: The top-down model. This model
proposes that transformation is initiated in a fully differentiated
cell in which adenomatous polyposis coli is highly expressed and
β-catenin is downregulated (19). The
fully differentiated villus cell proliferates and replaces the
normal mucosa from the top down. In contrast, the bottom-up model
proposes that transformation is initiated in a stem cell at the
base of the crypt, which proliferates and replaces the normal
mucosa with transformed cells from the bottom up. In the present
study, α-taxilin levels were significantly associated with
proliferation activity in both adenoma and IMA. We observed that
α-taxilin was similarly upregulated in the upper third of the
neoplastic glands in both adenoma and IMA. These data suggested
that α-taxilin expression status might be similar between adenoma
and adenocarcinoma, and that high α-taxilin levels could not be a
marker of malignancy in the large intestine.
We next investigated α-taxilin expression in
histologically proven well-differentiated and/or moderately
differentiated adenocarcinoma in the left-sided colon with anatomic
stage II and/or III in order to investigate its prognostic
significance in CRC. Since α-taxilin expression levels were
similarly high in the surface area of adenoma and adenocarcinoma,
we reasoned that α-taxilin expression on the surface could not be a
marker of malignancy or prognosis in CRCs. However, the malignant
potential of cancer would be determined by proliferation activity,
destructive downward growth, and metastasis, suggesting that
malignant potential may be determined by the invasiveness and
proliferation activity of cancer cells at the deep advancing edge.
We therefore investigated associations of α-taxilin expression at
the advancing edge with local invasiveness (pT and vessel invasion)
and prognosis; however, α-taxilin expression levels were not
associated with local invasiveness and also did not affect 5-year
recurrence-free survival or overall survival of patients with
advanced CRCs.
Previously, Ohtomo et al reported that
α-taxilin upregulation was significantly associated with
proliferative activity, less-differentiated histological grade,
positivity of vascular invasion, and/or intrahepatic metastasis of
HCC (13). In addition, Mashidori
et al reported that α-taxilin upregulation was significantly
associated with depth of tumor (pT), vessel invasion, and
unfavorable overall and disease-free survival of patients with RCC
(14). In the present study,
α-taxilin expression levels were associated with proliferation
activity but its overexpression did not significantly affect
prognosis of patients with CRC. There are some possible
explanations for this discrepancy. First, it is well known that a
majority of CRCs develop via the adenoma-carcinoma sequence.
Because α-taxilin levels in adenoma cells are already as high as
those in adenocarcinoma cells, upregulation of α-taxilin alone
cannot be a marker of malignant potential of colorectal neoplasms.
In contrast, except for a few cases, no such common precancerous
lesion has been reported for HCC and RCC. The majority of both
tumors may develop de novo, and therefore α-taxilin
upregulation could be a marker of malignancy. Second, we selected a
relatively homogenous group of CRCs to investigate only the effect
of α-taxilin expression on prognosis. We enrolled only cases of
histologically proven differentiated adenocarcinoma in the
left-sided colon with anatomic stage II and/or III that underwent
surgical resection. However, this selection might have resulted in
selection bias because α-taxilin levels might have affected pT and
nodal/distant metastasis. Analysis of all consecutive CRC cases in
our regional cohort during the study period might have resulted in
a different conclusion. This is definitely a limitation to this
trial, in part because patients with stage I disease would undergo
endoscopic treatment and those with stage IV disease may not
undergo surgical resection. Third, histological structure differs
greatly between CRC and HCC/RCC; invasive CRCs are relatively
stroma-rich tumors whereas both HCCs and RCCs are deficient in
fibrous stroma. In the present study, as well as in previous
studies, α-taxilin expression was also noted in the stromal cells
such as fibroblasts. Previous studies suggested fibroblasts as the
cellular origin of matrix metalloproteinase (MMP)-9 in CRCs
(20,21). Increased MMP-2 and MMP-9 expression
has been associated with worse outcome of CRC (22–24). The
fact that α-taxilin preferentially binds to free syntaxins and
thereby prevents formation of the SNARE complex suggests that
α-taxilin might act as a negative regulator of t-SNARE formation,
leading to impaired intracellular vesicular transport (8,9). SNAREs
have been reported to be involved in secretion of MMP-2 and MMP-9
(25), therefore α-taxilin expression
in fibroblasts might cause decreased MMP-9 secretion, leading to a
favorable prognosis of CRCs. α-taxilin expression in both cancer
cells and stromal cells may offset the prognostic effect in
stroma-rich CRCs.
In conclusion, this is the first report of α-taxilin
expression in human colorectal tumors. In both adenoma and
adenocarcinoma, α-taxilin was significantly associated with cell
proliferation activity. However, α-taxilin expression levels were
not significantly associated with local invasiveness or
recurrence-free/overall survival of advanced CRCs. Taken together,
our findings indicate that α-taxilin is a cell proliferation marker
in colorectal epithelial neoplasms, but cannot be a marker of
malignancy, and its expression does not affect prognosis of
CRC.
Acknowledgements
We are greatly indebted to Ms. Chiaki Matsuyama and
Ms. Ayako Shimizu for their excellent technical assistance. This
study was supported in part by JSPS KAKENHI (JP16K08695) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
References
|
1
|
World Cancer Research Fund/American
Institute for Cancer Research, . Food, Nutrition, Physical Activity
and the Prevention of Cancer: A Global Perspective. AICR;
Washington DC: pp. 280–288. 2007
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strömberg S, Agnarsdóttir M, Magnusson K,
Rexhepaj E, Bolander A, Lundberg E, Asplund A, Ryan D, Rafferty M,
Gallagher WM, et al: Selective expression of Syntaxin-7 protein in
benign melanocytes and malignant melanoma. J Proteome Res.
8:1639–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto S, Onodera Y, Hashimoto A,
Tanaka M, Hamaguchi M, Yamada A and Sabe H: Requirement for Arf6 in
breast cancer invasive activities. Proc Natl Acad Sci USA. 101:pp.
6647–6652. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bascom JL, Fata JE, Hirai Y, Sternlicht MD
and Bissell MJ: Epimorphin overexpression in the mouse mammary
gland promotes alveolar hyperplasia and mammary adenocarcinoma.
Cancer Res. 65:8617–8621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YA and Scheller RH: SNARE-mediated
membrane fusion. Nat Rev Mol Cell Biol. 2:98–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nogami S, Satoh S, Nakano M, Shimizu H,
Fukushima H, Maruyama A, Terano A and Shirataki H: Taxilin; a novel
syntaxin-binding protein that is involved in Ca2+-dependent
exocytosis in neuroendocrine cells. Genes Cells. 8:17–28. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nogami S, Satoh S, Nakano M, Terano A and
Shirataki H: Interaction of taxilin with syntaxin which does not
form the SNARE complex. Biochem Biophys Res Commun. 311:797–802.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horii Y, Sakane H, Nogami S, Ohtomo N,
Tomiya T and Shirataki H: Expression of α-taxilin in the murine
gastrointestinal tract: Potential implication in cell
proliferation. Histochem Cell Biol. 141:165–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sai XB, Makiyama T, Sakane H, Horii Y,
Hiraishi H and Shirataki H: TSG101, a tumor susceptibility gene,
bidirectionally modulates cell invasion through regulating MMP-9
mRNA expression. BMC Cancer. 15:9332015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oba-Shinjo SM, Bengtson MH, Winnischofer
SM, Colin C, Vedoy CG, De Mendonça Z, Marie SK and Sogayar MC:
Identification of novel differentially expressed genes in human
astrocytomas by cDNA representational difference analysis. Brain
Res Mol Brain Res. 140:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohtomo N, Tomiya T, Tanoue Y, Inoue Y,
Nishikawa T, Ikeda H, Seyama Y, Kokudo N, Shibahara J, Fukayama M,
et al: Expression of α-taxilin in hepatocellular carcinoma
correlates with growth activity and malignant potential of the
tumor. Int J Oncol. 37:1417–1423. 2010.PubMed/NCBI
|
|
14
|
Mashidori T, Shirataki H, Kamai T,
Nakamura F and Yoshida K: Increased alpha-taxilin protein
expression is associated with the metastatic and invasive potential
of renal cell cancer. Biomed Res. 32:103–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamilton SR, Bosman FT, Boffetta P, Ilyas
M, Morreau H, Nakamura SI, Quirke P, Riboli E and Sobin LH:
Carcinoma of the colon and rectumWHO Classification of Tumours of
the Digestive System. 4th. Bozman FT, Carneiro F, Hruban RH and
Theise ND: IARC Press; Lyon: pp. 134–146. 2010
|
|
16
|
American Joint Committee on Cancer (AJCC),
. Colon and Rectum Cancer Staging. 7th. AJCC; Chicago, IL: 2009,
https://cancerstaging.org/references-tools/quickreferences/Documents/ColonMedium.pdfMay
25–2017
|
|
17
|
Imai Y: Poorly differentiated
adenocarcinoma of the colon: Subsite location and clinicopathologic
features. Int J Colorectal Dis. 30:187–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakakibara S, Nakadate K, Tanaka-Nakadate
S, Yoshida K, Nogami S, Shirataki H and Ueda S: Developmental and
spatial expression pattern of alpha-taxilin in the rat central
nervous system. J Comp Neurol. 511:65–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fodde R, Smits R and Clevers H: APC,
signal transduction and genetic instability in colorectal cancer.
Nat Rev Cancer. 1:55–67. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roeb E, Dietrich CG, Winograd R, Arndt M,
Breuer B, Fass J, Schumpelick V and Matern S: Activity and cellular
origin of gelatinases in patients with colon and rectal carcinoma
differential activity of matrix metalloproteinase-9. Cancer.
92:2680–2691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behrens P, Mathiak M, Mangold E, Kirdorf
S, Wellmann A, Fogt F, Rothe M, Florin A and Wernert N: Stromal
expression of invasion-promoting, matrix-degrading proteases MMP-1
and −9 and the Ets 1 transcription factor in HNPCC carcinomas and
sporadic colorectal cancers. Int J Cancer. 107:183–188. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Said AH, Raufman JP and Xie G: The role of
matrix metalloproteinases in colorectal cancer. Cancers (Basel).
6:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng ZS, Huang Y, Cohen AM and Guillem JG:
Prediction of colorectal cancer relapse and survival via tissue RNA
levels of matrix metalloproteinase-9. J Clin Oncol. 14:3133–3140.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kean MJ, Williams KC, Skalski M, Myers D,
Burtnik A, Foster D and Coppolino MG: VAMP3, syntaxin-13 and SNAP23
are involved in secretion of matrix metalloproteinases, degradation
of the extracellular matrix and cell invasion. J Cell Sci.
122:4089–4098. 2009. View Article : Google Scholar : PubMed/NCBI
|