Hepatocellular carcinoma (HCC) is the fifth most
common type of malignancy worldwide and is associated with the
third highest number of cancer-related mortalities (1). Approximately 626,000 new cases are
diagnosed each year, a large proportion of which are fatal
(2). Although the diagnosis and
treatment of HCC have improved over the years, the mortality rate
of patients with HCC continues to be high (3). Tumor metastasis and recurrence remain
the major obstacles to achieving higher long-term survival rates
(4). It is therefore clinically
important to elucidate the molecular pathogenesis of HCC, as this
could assist in revealing effective therapeutic strategies and,
ultimately, aid patients with HCC to achieve a favorable
prognosis.
Conventional wisdom holds that protein-coding genes
play dominant roles in gene regulation on the basis of the genetic
central dogma: DNA encodes mRNA, which encodes protein. However,
technological advances have revealed that only 1.5% of the human
genome is protein coding, while the remaining ~98% is transcribed
into RNAs that do not produce any proteins, termed non-coding RNAs
(ncRNAs) (5). The ncRNAs are divided
into long ncRNAs (lncRNAs; >200 nucleotides) and small ncRNAs
(sncRNAs; <200 nucleotides) (6).
sncRNAs such as microRNAs (miRNAs) have important roles in the
initiation and development of various malignancies (such as breast
cancer, lung cancer and gastric cancer) (7–10) and
other diseases, including systemic lupus erythematosus, rheumatoid
arthritis and multiple sclerosis (11–13).
Recently, the association between human disease, including cancer,
and lncRNAs has attracted increasing attention. Numerous
investigations center around the functions of lncRNAs in the
initiation and progression of HCC (2,14–19). These studies suggested that
dysregulation of several lncRNAs was tightly associated with
metastasis, disease recurrence and poorer clinical outcomes in
patients with HCC (Table I) (2,14,19,20–34). HOX
transcript antisense RNA (HOTAIR), a 2,158-nucleotide lncRNA
derived from the antisense strand of homeobox gene C cluster (HOXC)
(35), has been identified as an
HCC-related lncRNA. In the present review, the expression and
function of HOTAIR in HCC is investigated. Additionally, the
clinical potential of HOTAIR as a biomarker for predicting HCC
prognosis, and whether it could serve as a novel target for HCC
therapy, is discussed.
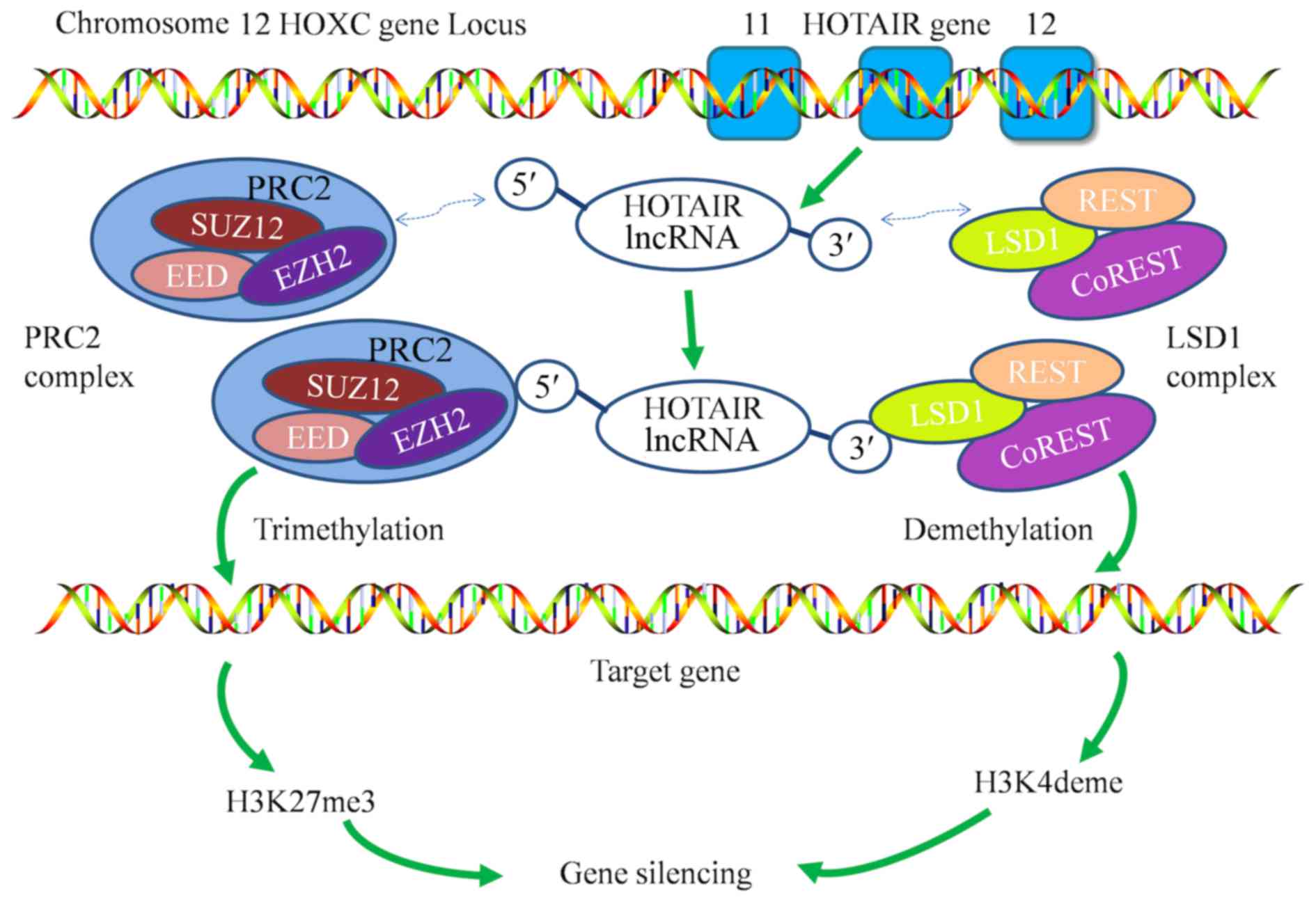
The human HOTAIR gene contains six exons; exons 1 to
5 (range, 33–140 bp) are short, whereas exon 6 (1,817 bp) is long
and is classified into domains A and B (37). Although the overall sequences of
HOTAIR are poorly conserved, the 5′ and 3′ binding domains of
HOTAIR, which bind to PRC2 and LSD1/CoREST/REST complexes,
respectively, may possess relatively constant sequences and
structures (40). The PRC2 complex is
made up of suppressor of zeste 12 homolog (SUZ12), enhancer of
zeste homolog 2 (EZH2) and embryonic ectoderm development (14). EZH2 is a methyltransferase that
requires the presence of the other subunits to be functional
(41). EZH2 mediates the
transcriptional silencing via the trimethylation of histone H3Lys27
(giving H3K27me3) (42). Within the
LSD1/CoREST/REST complex, LSD1 also acts as a transcriptional
repressor. However, LSD1 also functions as a histone demethylase
that mediates transcriptional repression via the demethylation of
dimethyl histone H3 Lys4 (H3K4me2) (Fig.
1) (43). In brief, HOTAIR
coordinates the functions of the two chromatin-modification
complexes (44) and alters the
expression of multiple genes that are associated with diverse
biological functions (35).
The expression of HOTAIR in tumor tissue is markedly
higher than in the adjacent healthy tissue in HCC patients
(2,19,35,45). The
abnormal expression of HOTAIR in HCC is associated with lymph node
metastasis, larger tumor size, tumor recurrence after liver
transplantation (LT) and shorter disease-free survival (DFS) after
surgical resection or LT (2,14,19,21).
It is well known that the expression of the v-myc
avian myelocytomatosis viral oncogene homolog (MYC) oncogene, a
powerful cancer-promoting gene, affects the expression of HOTAIR.
MYC is implicated in tumorigenesis in a diverse range of tissues,
regulating gene expression and cell proliferation (46). For instance, a previous experimental
study demonstrated that expression of HOTAIR is stimulated by c-Myc
through interaction with a putative E-box element located upstream
of the HOTAIR gene in gallbladder cancer cells (47). Since c-Myc has a pivotal role in
hepatocarcinogenesis (47,48), the part it plays in HCC necessitates
investigation.
A recent study reported that HOTAIR is regulated by
IκB kinase (IKK), made up of IKKα, IKKβ and IKKγ subunits, in a
H3k27me3-dependent manner (49). The
three subunits of IKK affected the growth of liver cancer stem
cells (LCSCs) through regulation of HOTAIR expression.
Specifically, IKKα and IKKβ upregulated the expression of HOTAIR,
whereas IKKγ downregulated it. A reverse interaction was also
observed, with the carcinogenic effects of three core IKK subunits
being activated by HOTAIR (49).
More recent studies have demonstrated that miRNAs
serve notable roles in HOTAIR regulation (50,51).
Unlike HOTAIR, miR-141 exerts a tumor suppressive function that
inhibits the malignancy of cancer cells in several types of cancer
(35). HOTAIR expression levels were
found to be inversely associated with those of miR-141 in renal
carcinoma cells (51). miR-141
suppressed the expression of HOTAIR in an argonaute-2-dependent
manner, which ultimately resulted in reduced cell proliferation and
tumor invasion (51). Although these
findings were observed in other cancer types, the
miRNA-141-mediated degradation of HOTAIR in HCC necessitates
further investigation (52–55).
Osteopontin (OPN) is a phosphoglycoprotein that has
been reported to be able to upregulate HOTAIR in cancer cells, with
the knockdown of OPN markedly diminishing the level of HOTAIR
expression (56). The CD44 receptor
is a positive regulator of OPN that is also implicated in the
regulation of OPN-mediated HOTAIR expression. Previously considered
to be a tumor suppressor, expression of interferon regulatory
factor-1 (IRF1) was verified to be elevated in cancer cells, with
its overexpression decreasing the levels of HOTAIR expression
(56). In fact, OPN promoted HOTAIR
expression via the inhibition of IRF1 and its signaling pathway
(56). Since OPN has been identified
as a promoter of HCC progression and metastasis (57,58), the
mechanism of OPN-induced HOTAIR expression in HCC requires further
investigation.
Transforming growth factor-β (TGF-β) stimulates
expression of HOTAIR and triggers EMT (59). Similarly, type I collagen (Col-1),
which is often overexpressed in lung cancer, could also promote
HOTAIR expression (60). Since TGF-β
and Col-1 are powerful triggers of EMT in HCC (61,62), their
further investigation may lead to novel insights into the
regulatory role of HOTAIR in HCC.
HOTAIR enhancement is associated with invasion,
progression, metastasis and poorer clinical outcomes in HCC
patients (Table I) (2,14,19–21). In
HCC cells, HOTAIR modulates various genes and molecules that play
critical roles in cancer migration and invasion. A recent
experimental study found that knockdown of HOTAIR by short
interfering RNAs upregulated RNA binding motif protein 38 (RBM38)
expression in HCC cells (16).
Moreover, the study also reported that the level of RBM38
expression in HCC tissue was markedly lower than that in healthy
tissue in the same patients. RBM38-knockdown could restore the
inhibition of malignancy that was mediated by the downregulation of
HOTAIR (16). Therefore, it could be
concluded that HOTAIR mediates the migratory and invasive
phenotypes of HCC cells through the suppression of RBM38,
indicating that HOTAIR and RBM38 serve notable roles in HCC
development (16).
A recent study observed that HOTAIR exerted
oncogenic effects in LCSCs. A negative correlation between the
expression of HOTAIR and SET domain-containing 2 (SETD2) was also
found in this study (63).
Furthermore, the study demonstrated that SETD2 abrogates the
oncogenic activity of HOTAIR, thus exerting a tumor suppressive
function. Taking these observations together, HOTAIR serves an
oncogenic role through decreasing the expression of SETD2 in LCSCs
(63).
It is well accepted that autophagy exerts important
effects on the initiation and development of human cancer (64). A recent study suggested that HOTAIR
was overexpressed in HCC and that its dysregulation could promote
autophagy (65). Moreover, it was
also observed that increases in the expression of autophagy-related
gene 3 (ATG3) and ATG7 were accompanied by HOTAIR overexpression.
In summary, overexpression of HOTAIR triggers autophagy through the
upregulation of ATG3 and ATG7, thus eventually facilitating HCC
progression (65).
HOTAIR exerts biological functions through
recruiting the PRC2 and LSD1 complexes to the target genes and
repressing their expression (35).
The PRC2 complex has been observed to be essential for silencing
certain tumor suppressive genes (45). Several studies concerning HCC
demonstrated that the subunits of the PRC2 complex are
hyperexpressed in HCC and could mediate hepatocarcinogenesis
(66–68). EZH2 was found to be upregulated in HCC
cells; its overexpression could inactivate several tumor
suppressive miRNAs. Moreover, high expression of EZH2 was closely
associated with a negative clinical outcome in patients with HCC
(66,67). SUZ12, another component of the PRC2
complex, serves an important role in H3-K27 methylation and gene
silencing (68). SUZ12 was also found
to be upregulated in liver tumors and has been considered as a
novel target for HCC therapy (68).
Besides the PRC2 complex, HOTAIR also facilitates the progression
of HCC through LSD1. LSD1 is an important regulator of hepatic
carcinogenesis whose upregulation is closely associated with
advanced stages of HCC (69). B-cell
lymphoma 2 (Bcl-2) and c-Myc are oncogenes that can stimulate the
initiation and development of cancer. Data suggest that the
downregulation of Bcl-2 and c-Myc is followed by LSD1 silencing,
indicating that LSD1 may function as a tumor promoter through the
stimulation of Bcl-2 and c-Myc expression (69). Further evidence has suggested that
LSD1 overexpression is a poor prognostic predictor for patients
with primary HCC (70).
The invasive and metastatic phenotypes of HCC are
attributed to the progression of EMT (62). A recent study observed that HOTAIR
overexpression in normal liver stem cells (NLSCs) could result in
their malignant transformation (71).
Ye et al (71) suggested that
HOTAIR could promote the initiation of HCC by inducing EMT. During
EMT, HOTAIR promoted tumor progression by regulating EMT-related
genes (72,73). For example, HOTAIR stimulated EMT by
suppressing miR-331-3p, which is an inhibitor of the human
epidermal growth factor receptor 2 (HER2)/AKT/heat-shock factor
1/Slug pathway (74). HOTAIR has also
been reported to stimulate EMT through suppression of the EMT
inhibitor Wnt inhibitory factor 1 in esophageal squamous cell
carcinoma cells (75,76). HOTAIR has been shown to downregulate
miR-7, a tumor suppressor that has been reported to reverse EMT in
breast cancer cells (77). In
addition to suppressing EMT inhibitors, HOTAIR triggers EMT by
facilitating the expression of EMT effector molecules. For
instance, HOTAIR may be involved in the expression of matrix
metalloproteinases (MMPs), which are critical regulators of the
invasive and metastatic phenotypes of HCC cells (19,78–80).
However, future investigation is required to support this
viewpoint.
HOTAIR serves a considerable role in hepatitis B
virus (HBV)-mediated hepatic tumorigenesis by enhancing the
ubiquitination of SUZ12 and zinc finger protein 198 (ZNF198) in the
presence of polo-like kinase 1 (Plk1) (81). SUZ12 is an important component of the
PRC2 complex that can repress transcription. Another complex that
represses transcription, LSD1/Co-REST/histone deacetylase 1, is
stabilized by ZNF198. In cells infected with HBV, negative
regulation of ZNF198 and SUZ12 results in the epigenetic
reprogramming of the cell. In brief, HOTAIR facilitates the
ubiquitination of Plk1-phosphorylated SUZ12 and ZNF198 and
accelerates their proteasomal degradation during HBV-induced liver
carcinogenesis (81).
HOTAIR promotes tumorigenesis via interaction with
miRNAs. As aforementioned, the level of HOTAIR expression is
modulated by several miRNAs with tumor suppressor effects,
including miR-141 (51). However,
HOTAIR also modulates miRNA levels, recognizing and then degrading
target miRNAs (82). For instance, a
miR-130a binding site has been found in the HOTAIR lncRNA and was
proven to be important for the HOTAIR-mediated regulation of
miR-130a (47). In gallbladder cancer
tissue, miR-130a expression was found to be negatively associated
with HOTAIR expression. This suggests that HOTAIR partly exerts its
carcinogenic effect by downregulating miR-130a (47). A previous study demonstrated that
HOTAIR functions as a competitive endogenous RNA (ceRNA), competing
with HER2 for miR-331-3p binding sites and thus ultimately
alleviating the miR-331-3p-mediated repression of oncogenic HER2
expression in gastric cancer cells (83). Another, more recent, study suggested
that the dysregulated expression of HOTAIR is also involved in
hematological malignancies (84).
HOTAIR was again found to act as a ceRNA, binding miR-193a and
thereby regulating the expression of proto-oncogene c-KIT in acute
myeloid leukemia cells (84).
Although these findings have not all been reported in HCC, the
interaction between HOTAIR and miRNAs should be investigated in
HCC, as miR-130a and miR-193a-3p serve as tumor suppressors in the
disease (85,86).
HOTAIR is considered to be an effective molecular
marker in HCC: its reinforced expression in HCC tissues is closely
associated with lymph node metastasis, larger tumor size, tumor
recurrence after LT and inferior DFS after surgical resection or LT
(Table I). In a cohort of 50 patients
with HCC that underwent surgical resection, 3-year recurrence-free
survival rates in patients with low HOTAIR expression were
noticeably higher than those in patients with high HOTAIR
expression. The study also suggested that HOTAIR could be employed
as a marker for predicting lymph node metastasis in HCC (19). In another study of 64 patients with
HCC, the overall survival rate of the 13 patients with high HOTAIR
expression was markedly lower than that of the 51 patients with low
HOTAIR expression. A positive association between hyperexpression
of HOTAIR and larger tumor size was also observed in this study
(14). In addition, a recent study of
60 patients with HCC who underwent LT demonstrated that patients
who overexpressed HOTAIR were more susceptible to tumor recurrence
after LT. Yang et al (2)
proposed that HOTAIR can be employed as a predictive biomarker for
predicting tumor relapse after LT.
As with cancer-specific miRNAs, HOTAIR, as an
lncRNA, is stable and detectable in various sorts of biological
specimens, including serum and urine (3,87). lncRNAs
can be determined at low cost and levels are easily assessed by
simple methodologies such as quantitative PCR, which are already in
routine clinical practice (87).
Therefore, lncRNAs, including HOTAIR, could be potentially employed
as fluid-based non-invasive markers for clinical use (3).
An increasing number of studies have reported that
HOTAIR is a promising therapeutic target in HCC. For example, under
the positive regulation of HOTAIR, vascular endothelial growth
factor (VEGF) and MMP-9 could promote the progression of HCC.
Therefore, treatments aimed at HOTAIR could inhibit HCC growth
(19,45). Drugs directly targeting VEGF or MMP-9
may also be effective for HCC therapy (45). As aforementioned, HOTAIR mediates
migratory and invasive phenotypes of HCC cells via the inhibition
of RBM38, with downregulation of HOTAIR leading to a marked
reduction in cell motility. These observations indicate that HOTAIR
is a potential target for HCC therapy and that RMB38 serves as a
suppressed target of HOTAIR (16).
Moreover, in a previous study, inhibiting HOTAIR function decreased
the viability and invasiveness of HCC cells and increased their
sensitivity to TNF-α-mediated apoptosis and chemotherapeutic drugs
(2). An independent study has shown
that HOTAIR plays a considerable role in hepatic tumorigenesis by
downregulating miR-218 expression and inactivating the
P14ARF and P16Ink4a signal pathways (88). HOTAIR silenced the expression of
miR-218 by directly recruiting EZH2 to its promoter. Antitumor
drugs for HCC could therefore be designed to directly disrupt the
HOTAIR-EZH2-miR-218 negative regulatory axis (88). As has been discussed, PRC2 and LSD1
complexes were overexpressed in HCC and acted as partners of HOTAIR
in the development of HCC. They could therefore be considered to be
latent targets of HCC treatment (66,69).
HCC is one of the most pressing health problems
around the world; it is aggressively malignant and has a high risk
of recurrence. Despite the use of diverse treatment methods,
including chemotherapy, surgery and LT, the clinical prognosis
remains poor. Improvements in the speed of diagnosis and the
discovery of effective therapeutic targets for HCC are therefore
essential if this prognosis is to be improved. As has been
mentioned, HOTAIR is abnormally expressed in HCC and serves a key
role in the progression of HCC. HOTAIR recruits the PRC2 and LSD1
complexes to their target gene promoters, inducing H3K27
trimethylation and H3K4me2 demethylation, which eventually leads to
gene silencing (Fig. 1). HOTAIR
expression is modulated by various molecules, including miR-141,
c-Myc, IKK and OPN. HOTAIR promotes invasive and aggressive
phenotypes of HCC cells by diverse mechanisms, which include
suppression of RBM38, reduction of SETD2 expression, stimulation of
autophagy, induction of EMT and interaction with different tumor
suppressor miRNAs, including miR-130a.
Numerous studies have demonstrated that HOTAIR can
be employed as a novel prognostic molecular marker in HCC, as its
enforced expression is strongly associated with lymph node
metastasis, larger tumor size, tumor recurrence following LT and
inferior DFS after surgical resection or LT. In addition, HOTAIR
expression levels can be easily determined non-invasively, making
its clinical application feasible.
As a promising therapeutic target of HCC, HOTAIR
could be blocked in diverse ways. Firstly, drugs that mask the
certain binding sites could disrupt the interplay between HOTAIR
and its molecule partners, which include the PCR2 and
LSD1/CoREST/REST complexes, thus silencing HOTAIR. Moreover, HOTAIR
could be targeted for degradation by specific miRNAs such as
miR-141. Finally, antitumor agents could be designed that are
targeted at molecules that participate in the HOTAIR pathway in
HCC, such as VEGF and MMP-9. This could be an indication of the
future directions of novel drug development in HCC therapy.
In summary, HOTAIR is emerging as a novel prognostic
molecular marker and as an efficient therapeutic target for HCC.
However, further investigation of the underlying molecular
mechanism behind dysregulated HOTAIR expression, and how it drives
HCC progression, is required to maximize the clinical potential of
HOTAIR.
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. LY15H160021) and the
Traditional Chinese Medicine Scientific Research Fund Project of
Zhejiang Province (grant no. 2017ZA079).
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Chen J, Zhang K, Feng B, Wang R and
Chen L: Progress and prospects of long noncoding RNAs (lncRNAs) in
hepatocellular carcinoma. Cell Physiol Biochem. 36:423–434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao K, Luk JM, Lee NP, Mao M, Zhang C,
Ferguson MD, Lamb J, Dai H, Ng IO, Sham PC and Poon RT: Predicting
prognosis in hepatocellular carcinoma after curative surgery with
common clinicopathologic parameters. BMC cancer. 9:3892009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Song YX, Ma B, Wang JJ, Sun JX,
Chen XW, Zhao JH, Yang YC and Wang ZN: Regulatory roles of
non-coding RNAS in colorectal cancer. Int J Mol Sci.
16:19886–19919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: MiRNA-26b inhibits proliferation by targeting PTGS2 in
breast cancer. Cancer Cell Int. 13:72013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Wu J, Zheng J, Li Y, Yang T, Hu G,
Dai J, Yang Q, Dai L and Jiang Y: Altered miRNA expression profiles
and miR-1a associated with urethane-induced pulmonary
carcinogenesis. Toxicol Sci. 135:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Zhang X, Li H, Yu J and Ren X: The
role of miRNA-29 family in cancer. Eur J Cell Biol. 92:123–128.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X,
Huang K and Tong Q: miRNA-145 targets v-ets erythroblastosis virus
E26 oncogene homolog 1 to suppress the invasion, metastasis, and
angiogenesis of gastric cancer cells. Mol Cancer Res. 11:182–193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CP, Choi YJ, Hicks GG and He L: The
emerging functions of the p53-miRNA network in stem cell biology.
Cell cycle. 11:2063–2072. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roderburg C, Luedde M, Cardenas D Vargas,
Vucur M, Mollnow T, Zimmermann HW, Koch A, Hellerbrand C,
Weiskirchen R, Frey N, et al: miR-133a mediates TGF-β-dependent
derepression of collagen synthesis in hepatic stellate cells during
liver fibrosis. J Hepatol. 58:736–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh RP, Massachi I, Manickavel S, Singh
S, Rao NP, Hasan S, Mc Curdy DK, Sharma S, Wong D, Hahn BH and
Rehimi H: The role of miRNA in inflammation and autoimmunity.
Autoimmun Rev. 12:1160–1165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishibashi M, Kogo R, Shibata K, Sawada G,
Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, et
al: Clinical significance of the expression of long non-coding RNA
HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 29:946–950.
2013.PubMed/NCBI
|
|
15
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding C, Cheng S, Yang Z, Lv Z, Xiao H, Du
C, Peng C, Xie H, Zhou L, Wu J and Zheng S: Long non-coding RNA
HOTAIR promotes cell migration and invasion via down-regulation of
RNA binding motif protein 38 in hepatocellular carcinoma cells. Int
J Mol Sci. 15:4060–4076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toffanin S, Villanueva A and Llovet JM:
miRNA delivery: Emerging therapy for hepatocellular carcinoma.
Gastroenterology. 138:1202–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borel F, Konstantinova P and Jansen PL:
Diagnostic and therapeutic potential of miRNA signatures in
patients with hepatocellular carcinoma. J Hepatol. 56:1371–1383.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao JZ, Li J, Du JL and Li XL: Long
non-coding RNA HOTAIR is a marker for hepatocellular carcinoma
progression and tumor recurrence. Oncol Lett. 11:1791–1798.
2016.PubMed/NCBI
|
|
21
|
Zhao J, Greene CM, Gray SG and Lawless MW:
Long noncoding RNAs in liver cancer: What we know in 2014. Expert
Opin Ther Targets. 18:1207–1218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsang WP and Kwok TT: Riboregulator H19
induction of MDR1-associated drug resistance in human
hepatocellular carcinoma cells. Oncogene. 26:4877–4881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Yang F, Yuan JH, Yuan SX, Zhou
WP, Huo XS, Xu D, Bi HS, Wang F and Sun SH: Epigenetic activation
of the MiR-200 family contributes to H19-mediated metastasis
suppression in hepatocellular carcinoma. Carcinogenesis.
34:577–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie H, Ma H and Zhou D: Plasma HULC as a
promising novel biomarker for the detection of hepatocellular
carcinoma. Biomed Res Int. 2013:1361062013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du Y, Kong G, You X, Zhang S, Zhang T, Gao
Y, Ye L and Zhang X: Elevation of highly up-regulated in liver
cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell
proliferation via down-regulating p18. J Biol Chem.
287:26302–26311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, Zhang H, Wan X, Yang X, Zhu C, Wang
A, He L, Miao R, Chen S and Zhao H: Long noncoding RNA plays a key
role in metastasis and prognosis of hepatocellular carcinoma.
Biomed Res Int. 2014:7805212014.PubMed/NCBI
|
|
28
|
Huang J, Zhang X, Zhang M, Zhu JD, Zhang
YL, Lin Y, Wang KS, Qi XF, Zhang Q, Liu GZ, et al: Up-regulation of
DLK1 as an imprinted gene could contribute to human hepatocellular
carcinoma. Carcinogenesis. 28:1094–1103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang F, Huo XS, Yuan SX, Zhang L, Zhou WP,
Wang F and Sun SH: Repression of the long noncoding RNA-LET by
histone deacetylase 3 contributes to hypoxia-mediated metastasis.
Mol Cell. 49:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang
Y, Tang GN, Zhou WP and Sun SH: Hepatitis B virus X protein
(HBx)-related long noncoding RNA (lncRNA) down-regulated expression
by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by
targeting the intermediate filament protein vimentin. Hepatology.
57:1882–1892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang F, Zhang L, Huo XS, Yuan JH, Xu D,
Yuan SX, Zhu N, Zhou WP, Yang GS, Wang YZ, et al: Long noncoding
RNA high expression in hepatocellular carcinoma facilitates tumor
growth through enhancer of zeste homolog 2 in humans. Hepatology.
54:1679–1689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma
D, Yi Z and Chen F: Mdig de-represses H19 large intergenic
non-coding RNA (lincRNA) by down-regulating H3K9me3 and
heterochromatin. Oncotarget. 4:1427–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hajjari M and Salavaty A: HOTAIR: An
oncogenic long non-coding RNA in different cancers. Cancer Biol
Med. 12:1–9. 2015.PubMed/NCBI
|
|
36
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He S, Liu S and Zhu H: The sequence,
structure and evolutionary features of HOTAIR in mammals. BMC Evol
Biol. 11:1022011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaneko S, Li G, Son J, Xu CF, Margueron R,
Neubert TA and Reinberg D: Phosphorylation of the PRC2 component
Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA.
Gene Dev. 24:2615–2620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu Y, Zhang L, Wang Y, Li H, Ren X, Wei F,
Yu W, Wang X, Zhang L, Yu J and Hao X: Long noncoding RNA HOTAIR
involvement in cancer. Tumour Biol. 35:9531–9538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu H, Zeng H, Dong A, Li F, He H,
Senisterra G, Seitova A, Duan S, Brown PJ, Vedadi M, et al:
Structure of the catalytic domain of EZH2 reveals conformational
plasticity in cofactor and substrate binding sites and explains
oncogenic mutations. PLoS One. 8:e837372013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kirmizis A, Bartley SM, Kuzmichev A,
Margueron R, Reinberg D, Green R and Farnham PJ: Silencing of human
polycomb target genes is associated with methylation of histone H3
Lys 27. Gene Dev. 18:1592–1605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi Y, Lan F, Matson C, Mulligan P,
Whetstine JR, Cole PA, Casero RA and Shi Y: Histone demethylation
mediated by the nuclear amine oxidase homolog LSD1. Cell.
119:941–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao Y, Li J and Wang L: Large intervening
non-coding RNA HOTAIR is an indicator of poor prognosis and a
therapeutic target in human cancers. Int J Mol Sci. 15:18985–18999.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zimonjic DB and Popescu NC: Role of DLC1
tumor suppressor gene and MYC oncogene in pathogenesis of human
hepatocellular carcinoma: Potential prospects for combined targeted
therapeutics (review). Int J Oncol. 41:393–406. 2012.PubMed/NCBI
|
|
47
|
Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD,
Qin YY, Gong W and Quan ZW: Long non-coding RNA HOTAIR, a c-Myc
activated driver of malignancy, negatively regulates miRNA-130a in
gallbladder cancer. Mol Cancer. 13:1562014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang L, Zhang X, Jia LT, Hu SJ, Zhao J,
Yang JD, Wen WH, Wang Z, Wang T, Zhao J, et al: c-Myc-mediated
epigenetic silencing of MicroRNA-101 contributes to dysregulation
of multiple pathways in hepatocellular carcinoma. Hepatology.
59:1850–1863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
An J, Wu M, Xin X, Lin Z, Li X, Zheng Q,
Gui X, Li T, Pu H, Li H and Lu D: Inflammatory related gene IKKα,
IKKβ, IKKγ cooperates to determine liver cancer stem cells
progression by altering telomere via heterochromatin protein
1-HOTAIR axis. Oncotarget. 7:50131–50149. 2016.PubMed/NCBI
|
|
50
|
Chiyomaru T, Yamamura S, Fukuhara S,
Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y,
Enokida H, et al: Genistein inhibits prostate cancer cell growth by
targeting miR-34a and oncogenic HOTAIR. PLoS One. 8:e703722013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chiyomaru T, Fukuhara S, Saini S, Majid S,
Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, et
al: Long non-coding RNA HOTAIR is targeted and regulated by miR-141
in human cancer cells. J Biol Chem. 289:12550–12565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu SM, Ai HW, Zhang DY, Han XQ, Pan Q, Luo
FL and Zhang XL: MiR-141 targets ZEB2 to suppress HCC progression.
Tumour Biol. 35:9993–9997. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Ding Y, Huang J, Wang S, Ni W, Guan
J, Li Q, Zhang Y, Ding Y, Chen B and Chen L: MiR-141 suppresses the
migration and invasion of HCC cells by targeting Tiam1. PLoS One.
9:e883932014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin L, Liang H, Wang Y, Yin X, Hu Y, Huang
J, Ren T, Xu H, Zheng L and Chen X: microRNA-141 inhibits cell
proliferation and invasion and promotes apoptosis by targeting
hepatocyte nuclear factor-3β in hepatocellular carcinoma cells. BMC
cancer. 14:8792014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang G, Zhang S, Gao F, Liu Z, Lu M, Peng
S, Zhang T and Zhang F: Osteopontin enhances the expression of
HOTAIR in cancer cells via IRF1. Biochim Biophys Acta.
1839:837–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qin L: Osteopontin is a promoter for
hepatocellular carcinoma metastasis: A summary of 10 years of
studies. Front Med. 8:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei
J, Sheng Y, Zheng Y, Yu J, Xie L, et al: Osteopontin promotes
epithelial-mesenchymal transition of hepatocellular carcinoma
through regulating vimentin. Oncotarget. 7:12997–13012.
2016.PubMed/NCBI
|
|
59
|
Alves C Padua, Fonseca AS, Muys BR, de
Barros E Lima Bueno R, Bürger MC, De Souza JE, Valente V, Zago MA
and Silva WA Jr: Brief report: The lincRNA Hotair is required for
epithelial-to-mesenchymal transition and stemness maintenance of
cancer cell lines. Stem cells. 31:2827–2832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao W, An Y, Liang Y and Xie XW: Role of
HOTAIR long noncoding RNA in metastatic progression of lung cancer.
Eur Rev Med Pharmacol Sci. 18:1930–1936. 2014.PubMed/NCBI
|
|
61
|
Yang MC, Wang CJ, Liao PC, Yen CJ and Shan
YS: Hepatic stellate cells secretes type I collagen to trigger
epithelial mesenchymal transition of hepatoma cells. Am J Cancer
Res. 4:751–763. 2014.PubMed/NCBI
|
|
62
|
van Zijl F, Zulehner G, Petz M, Schneller
D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H and Mikulits
W: Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu
H and Lu D: LncRNA HOTAIR promotes human liver cancer stem cell
malignant growth through downregulation of SETD2. Oncotarget.
6:27847–27864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Green DR and Levine B: To be or not to be?
How selective autophagy and cell death govern cell fate. Cell.
157:65–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang L, Zhang X, Li H and Liu J: The long
noncoding RNA HOTAIR activates autophagy by upregulating ATG3 and
ATG7 in hepatocellular carcinoma. Mol Biosyst. 12:2605–2612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Au SL, Wong CC, Lee JM, Fan DN, Tsang FH,
Ng IO and Wong CM: Enhancer of zeste homolog 2 epigenetically
silences multiple tumor suppressor microRNAs to promote liver
cancer metastasis. Hepatology. 56:622–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gao SB, Xu B, Ding LH, Zheng QL, Zhang L,
Zheng QF, Li SH, Feng ZJ, Wei J, Yin ZY, et al: The functional and
mechanistic relatedness of EZH2 and menin in hepatocellular
carcinoma. J Hepatol. 61:832–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kirmizis A, Bartley SM and Farnham PJ:
Identification of the polycomb group protein SU(Z)12 as a potential
molecular target for human cancer therapy. Mol Cancer Ther.
2:113–121. 2003.PubMed/NCBI
|
|
69
|
Zhao ZK, Dong P, Gu J, Chen L, Zhuang M,
Lu WJ, Wang DR and Liu YB: Overexpression of LSD1 in hepatocellular
carcinoma: A latent target for the diagnosis and therapy of
hepatoma. Tumour Biol. 34:173–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao ZK, Yu HF, Wang DR, Dong P, Chen L,
Wu WG, Ding WJ and Liu YB: Overexpression of lysine specific
demethylase 1 predicts worse prognosis in primary hepatocellular
carcinoma patients. World J Gastroenterol. 18:6651–6656. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye P, Wang T, Liu WH, Li XC, Tang LJ and
Tian FZ: Enhancing HOTAIR/MiR-10b drives normal liver stem cells
toward a tendency to malignant transformation through inducing
epithelial- to-mesenchymal transition. Rejuvenation Res.
18:332–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW,
Jin HY, Zhang Y, Li Q and Hua KQ: Overexpression of long non-coding
RNA HOTAIR predicts poor patient prognosis and promotes tumor
metastasis in epithelial ovarian cancer. Gynecol Oncol.
134:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ono H, Motoi N, Nagano H, Miyauchi E,
Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N and
Ishikawa Y: Long noncoding RNA HOTAIR is relevant to cellular
proliferation, invasiveness, and clinical relapse in small-cell
lung cancer. Cancer Med. 3:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou X, Chen J and Tang W: The molecular
mechanism of HOTAIR in tumorigenesis, metastasis, and drug
resistance. Acta Biochim Biophys Sin (Shanghai). 46:1011–1015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7:902014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang H, Cai K, Wang J, Wang X, Cheng K,
Shi F, Jiang L, Zhang Y and Dou J: MiR-7, inhibited indirectly by
lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of
breast cancer stem cells by downregulating the STAT3 pathway. Stem
Cells. 32:2858–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garcia-Irigoyen O, Latasa MU, Carotti S,
Uriarte I, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini
S, Benito P, Ladero JM, et al: Matrix metalloproteinase 10
contributes to hepatocarcinogenesis in a novel crosstalk with the
stromal derived factor 1/C-X-C chemokine receptor 4 axis.
Hepatology. 62:166–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Y, Shen Y, Cao B, Yan A and Ji H:
Elevated expression levels of androgen receptors and matrix
metalloproteinase-2 and −9 in 30 cases of hepatocellular carcinoma
compared with adjacent tissues as predictors of cancer invasion and
staging. Exp Ther Med. 9:905–908. 2015.PubMed/NCBI
|
|
80
|
Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ,
Huang L, Yu PF and Cheng XD: Knockdown of long non-coding RNA
HOTAIR suppresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang H, Diab A, Fan H, Mani SK, Hullinger
R, Merle P and Andrisani O: PLK1 and HOTAIR accelerate proteasomal
degradation of SUZ12 and ZNF198 during Hepatitis B virus-induced
liver carcinogenesis. Cancer Res. 75:2363–2374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
83
|
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB,
Yin DD, Kong R, Xia R, Lu KH, Li JH, et al: Lnc RNA HOTAIR
functions as a competing endogenous RNA to regulate HER2 expression
by sponging miR-331-3p in gastric cancer. Mol Cancer. 13:922014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY, Bin
Zhou, Wu JB, Tang LY and Gao SM: Long non-coding RNA HOTAIR
modulates c-KIT expression through sponging miR-193a in acute
myeloid leukemia. FEBS Lett. 589:1981–1987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu Y, Ren F, Luo Y, Rong M, Chen G and
Dang Y: Down-regulation of MiR-193a-3p dictates deterioration of
HCC: A Clinical real-time qRT-PCR study. Med Sci Monit.
21:2352–2360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kladi-Skandali A, Michaelidou K, Scorilas
A and Mavridis K: Long noncoding RNAs in digestive system
malignancies: A novel class of cancer biomarkers and therapeutic
targets? Gastroenterol Res Pract. 2015.3198612015.PubMed/NCBI
|
|
88
|
Fu WM, Zhu X, Wang WM, Lu YF, Hu BG, Wang
H, Liang WC, Wang SS, Ko CH, Waye MM, et al: Hotair mediates
hepatocarcinogenesis through suppressing miRNA-218 expression and
activating P14 and P16 signaling. J Hepatol. 63:886–895. 2015.
View Article : Google Scholar : PubMed/NCBI
|