Introduction
Choroid plexus tumors (CPTs) are rare intracranial
neoplasms that derive from the choroid plexus epithelium of the
ventricles (1,2). The lesions are commonly located in the
lateral and the fourth ventricles, and rarely in the
cerebellopontine angle and the third ventricle (3,4). The
majority of CPTs arise with clinical symptoms that are associated
with hydrocephalus as a result of direct mechanical obstruction of
the flow of cerebrospinal fluid (CSF) and arachnoid granulation
blockage from hemorrhage and overproduction of CSF (1,5).
Treatment of CPTs is currently based on histological
diagnosis. According to the World Health Organization (WHO)
classification scheme, CPTs are diagnosed as choroid plexus
papilloma (CPP, WHO grade I), atypical choroid plexus papilloma
(ACPP, WHO grade II) and choroid plexus carcinoma (CPC, WHO grade
III) (3). For all classifications,
the general treatment strategy is surgical resection unless this is
not possible due to tumor location, for example. Gross total
resection (GTR) is the indicated course of treatment for CPP as
well as for ACPP. CPP is a benign neoplasm and recurrence following
GTR is rare, therefore adjuvant radiotherapy and chemotherapy are
not generally recommended (2,3,6,7). ACPP cases exhibit atypical histological
features along with increased mitotic activity; however, patients
with this type of tumor also exhibit a good prognosis following
GTR. Patients with ACPP generally only require close observation
and follow-up; however, chemotherapy is necessary when GTR is not
performed or there is tumor dissemination or recurrence. CPC,
however, is hypervascular and infiltrative, making GTR difficult to
achieve. Patients with CPC may therefore require more comprehensive
treatment, regardless of the extent of resection (8–12).
Radiotherapy and chemotherapy effectively prolong
overall survival times in patients with CPC (10,13,14).
Radiotherapy, however, is only suitable for patients >3 years
(2) and it remains unclear which
patients may benefit from adjuvant therapy based on histological
examination alone. Therefore, in the present study it was analyzed
whether biomarkers may be used to predict and distinguish
distinctions in prognosis between CPT cases of distinct grades. The
association between the expression of progenitor and stem cell
markers neuron glia antigen-2 (NG2) and sex-determining region
Y-box 2 (SOX2) in CPTs and tumor grade was determined.
Materials and methods
Ethical statement
The present study was approved by the Ethics
Committee of Qilu Hospital, Shandong University (Jinan, China) and
all procedures were conducted according to the World Medical
Association Declaration of Helsinki Ethical Principles for Medical
Research Involving Human Subjects (2013). Written informed consent
was obtained from all study participants.
Patients
Patients (n=34) treated for pathologically confirmed
CPT in the Department of Neurosurgery, Qilu Hospital of Shandong
University, between January 2003 and December 2013 were included in
the present study. Tumors were resected using microscopic surgery,
with the surgical approach based on tumor location and size in
order to avoid damage to cortical functional areas and tracts.
Patients received routine post-surgical care and follow-up.
Surgical specimens were formalin-fixed, paraffin-embedded,
sectioned and stained with hematoxylin and eosin for histological
examination by a neuropathologist. Patient characteristics are
provided in Table I.
 | Table I.Clinicopathological characteristics of
34 patients with choroid plexus tumors of various grades. |
Table I.
Clinicopathological characteristics of
34 patients with choroid plexus tumors of various grades.
| Characteristic | All | CPP | ACPP | CPC |
|---|
| No. of patients | 34 | 25 | 5 | 4 |
| Age, years |
| Mean | 31.1 | 30.5 | 32.5 | 28.8 |
|
Range | 1–63 | 1–63 | 2–61 | 1–53 |
| Sex |
| Male | 21 | 15 | 4 | 2 |
|
Female | 13 | 10 | 1 | 2 |
| Location of
tumor |
| Lateral
ventricle | 14 | 7 | 4 | 3 |
| Third
ventricle | 1 | 1 | 0 | 0 |
| Fourth
ventricle | 15 | 13 | 1 | 1 |
|
Cerebellopontine angle | 4 | 4 | 0 | 0 |
Immunohistochemistry
Paraffin-embedded specimens were sectioned (3 µm),
deparaffinized in xylene and rehydrated in a graded series of
alcohol. Antigen retrieval was carried out by heating sections in a
microwave oven in citrate buffer (pH 6.0; Thermo Fisher Scientific,
Inc., Waltham, MA, USA; 005000) at 97°C for 20 min and then
allowing them to cool to room temperature (RT). Endogenous
peroxidase activity was inhibited by incubation at RT with 3%
H2O2 for 20 min and subsequently incubated at
4°C with primary antibodies overnight (anti-SOX2; ab97959; 1:1,000;
anti-NG2; ab50009; 1:50; Abcam, Cambridge, MA, USA). Positive and
negative controls were included in parallel. Detection was
performed by incubating slides at RT for 30 min with a biotinylated
secondary antibody followed by streptavidin-biotin-horseradish
peroxidase complex (SP9000 kit; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijng, China; anti-mouse/rat/rabbit,
Beijing, China). All washes in between incubations were performed
with PBS and diaminobenzidine (DAB) was used as the chromogenic
substrate for visualization. Sections were counterstained with
hematoxylin and eosin, followed by differentiation in hydrochloric
acid-ethanol and bluing in ammonia water. Slides were dehydrated in
an ethanol series, cleared with xylene, mounted in neutral balsam
and examined under a light microscope (Leica Application Suite
version 4, Wetzlar, Germany). Slides were scored between 0 and 3+
on the basis of the proportion of NG2- or SOX2-positive neoplastic
cells (0, no staining; 1+, <25; 2+, 26 to 50; and 3+,
>50%).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0 for Windows; IBM Corp., Armonk, NY, USA).
The association between age and tumor grade was analyzed using
Student's t-test and Fisher's exact test. The association between
sex, NG2/SOX2 labeling index and tumor grade were examined using
χ2 or Fisher's exact tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinicopathological features
Clinical characteristics of patients are presented
in Table I. A total of 34 patients
(21 males and 13 females) were included in the present study and
the mean age was 31.1 years (range, 1–63 years). All patients
underwent surgical resection and all tumor grades were included in
the cohort [CPP (n=25), ACPP (n=5) and CPC (n=4)]. Atypical
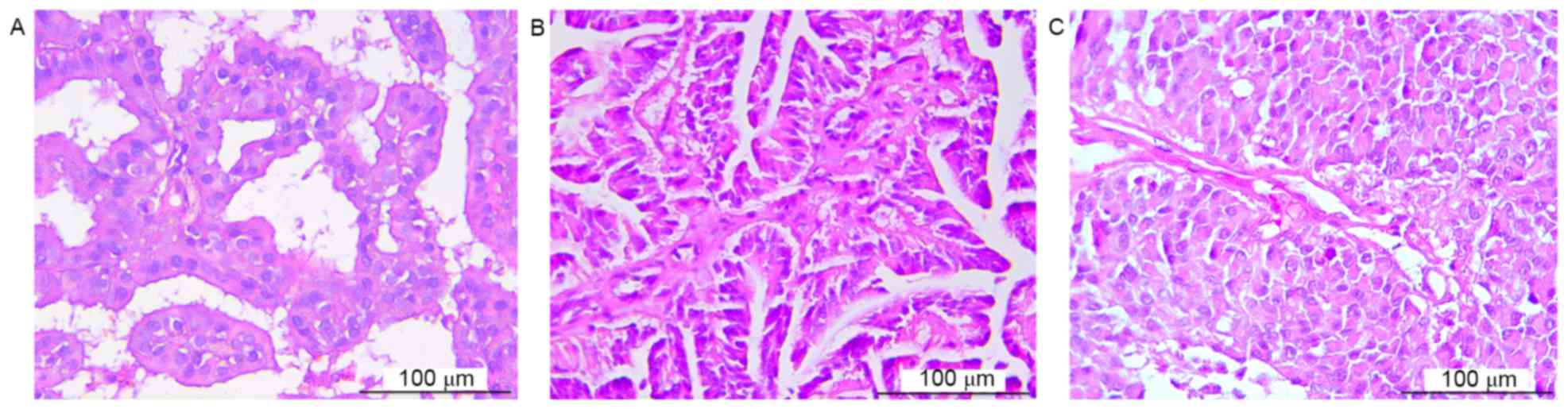
morphology was observed only in high-grade CPTs (Fig. 1). Tumor location was determined on the
basis of radiographic studies and surgical results, and was
distributed as follows: Lateral ventricle (n=14), third ventricle
(n=1), fourth ventricle (n=5) and cerebellopontine angle (n=4).
Notably, no association was identified between tumor grade and age
or any other clinicopathological factor examined.
Overexpression of NG2 and SOX2 is
associated with higher grade in CPT
Immunohistochemistry was performed to determine
whether CPT cases expressed NG2 or SOX2 and, if so, whether
expression was associated with the tumor grade. The results of the
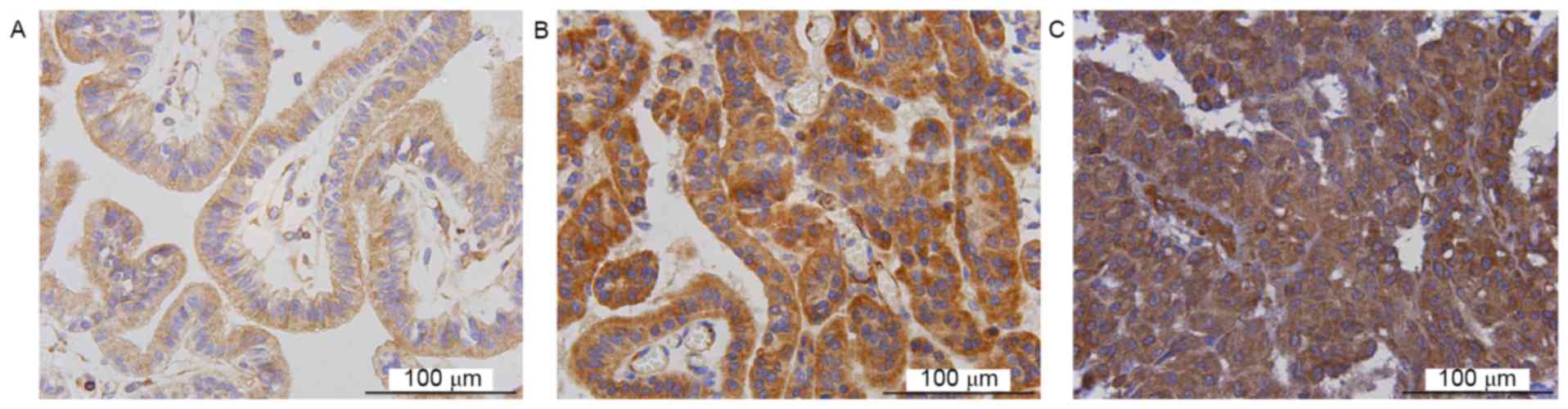
present study demonstrated that NG2 and SOX2 were expressed in CPT.
SOX2 and NG2 expression was identified in 34 and 33 cases,
respectively (Figs. 2 and 3, respectively). Staining for NG2 and SOX2
were scored for each slide. The mean labeling indices for CPP, ACPP
and CPC were 1.12, 1.80 and 2.75, respectively, for NG2, and 1.20,
2.00 and 3.00, respectively, for SOX2.
A score ≥2 for immunohistochemistry was considered
to indicate increased expression. The distribution of cases with
scores ≥2 for NG2 and SOX2 was as follows: CPP, 3/25 and 4/25;
ACPP, 4/5 and 4/5; and CPC, 4/4 and 4/4, respectively. Almost all
the cases exhibiting decreased expression of NG2 or SOX2 were CPP
and a χ2 test was therefore used to identify the
association between expression and tumor grade, in order to
determine their utility as biomarkers in pathological diagnosis.
Statistically significant associations were identified between CPP
and ACPP for NG2 and SOX2 (P=0.001 and 0.003, respectively) in
addition to CPP and CPC (P<0.001). However, analysis for the
comparison between ACPP and CPC cases were not statistically
significant (P=0.343 for NG2 and SOX2). These results demonstrated
that expression of NG2 and SOX2 were significantly associated with
tumor grade and that increased expression may be used to
distinguish between grades of CPP and ACPP and between CPP and CPC
(Table II).
 | Table II.Comparison of immunohistochemical
score between various grades of patients with choroid plexus
tumors. |
Table II.
Comparison of immunohistochemical
score between various grades of patients with choroid plexus
tumors.
|
| WHO grade |
|
|---|
|
|
|
|
|---|
| Variable | I | II | III | P-value |
|---|
| NG2 | 0.001a |
| 0 | 1 | 0 | 0 | 0.343b |
| 1 | 21 | 1 | 0 |
<0.001c |
| 2 | 2 | 4 | 1 |
|
| 3 | 1 | 0 | 3 |
|
|
Expression score ≥2 | 3 | 4 | 4 |
|
| Mean
score | 1.12 | 1.80 | 2.75 |
|
| SOX2 |
| 0 | 0 | 0 | 0 |
|
| 1 | 21 | 1 | 0 | 0.003a |
| 2 | 3 | 3 | 0 | 0.343b |
| 3 | 1 | 1 | 4 |
<0.001c |
|
Expression score ≥2 | 4 | 4 | 4 |
|
| Mean
score | 1.20 | 2.00 | 3.00 |
|
Discussion
Molecular markers serve an integral role in current
cancer diagnosis, providing a basis for distinguishing between the
clinical behavior of tumors that may otherwise appear
pathologically similar. In the present study, the expression of
progenitor and stem cell markers NG2 and SOX2 in distinct grades of
CPTs were examined using immunohistochemical staining. The results
of the present study revealed a statistically significant
association between increased expression of NG2 and SOX2 and
high-grade CPT. These results indicate that NG2 and SOX2 may refine
the diagnosis and development of treatment strategies of affected
patients.
To the best of our knowledge there are no previous
studies investigating NG2 or SOX2 expression levels in CPTs but
they have been previously associated with cancer development
(15–22). Increased expression of NG2 has been
demonstrated in a variety of head and neck cancers, glioma and
pituitary cells (15–18). SOX2 overexpression or gene
amplification has been associated with the development of lung,
esophageal, breast and gastric cancers (19–22).
NG2 expression is typically increased in the
developing and adult central nervous system (CNS). It is a type-I
membrane protein expressed by diverse cell types within the CNS
during development and differentiation. NG2 has been identified to
be expressed during development in extraneural progenitor cell
types, including mesenchymal stem cells, chondroblasts,
osteoblasts, immature keratinocytes, muscle progenitors and
melanocytes (23). Additionally, NG2
serves a role in cell viability and angiogenesis (24). NG2 deficiency during early development
results in the loss of pericyte-endothelium association and
defective formation of basement membranes in blood vessels
(25). NG2 has been postulated to
function as a cell adhesion molecule, which was supported by the
observation that the extracellular region between the transmembrane
domain and the laminin G/neurexin/sex-hormone-binding globulin
domains binds to integrins and collagen V and VI (26). Previous studies have revealed a more
defined role for NG2 in brain homeostasis including intracellular
functions in oligodendrocyte progenitor cells, which modulate
migration, gene expression and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
clustering (15,24,27).
SOX2 is classified in the SoxB1 family due to the
similarity of the amino acid sequence of the high-mobility group
box domain (28,29). SOX2 is a transcription factor that
serves a primary role in maintaining stem cells in adult tissues,
including the CNS. During development of the peripheral nervous
system, SOX2 is expressed in neural crest stem cells and regulates
differentiation into dorsal root ganglion neurons. In addition,
SOX2 is expressed in immature Schwann cells and inhibits
myelination (30) and is re-expressed
in undifferentiated myelinating Schwann cells following nerve
injury and regulates their sorting. Finally, it is one of the
transcription factors initially used to generate induced
pluripotent stem cells from fibroblast cells (31). Thus, NG2 and SOX2 function in the
maintenance of progenitor and stem cells, which may contribute to
the development of cancer.
NG2 and SOX2 exhibit increased expression in
higher-grade CPTs. Although additional studies are required to
elucidate the functional roles of NG2 and SOX2 in the development
of CPTs, the results of the present study demonstrate that
expression levels of these proteins may be helpful for determining
tumor grade in histologically controversial cases. In addition, NG2
and SOX2 may assist in identifying cases where aggressive or novel
treatment may be necessary for improved patient outcomes.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81402060 and 81572487), the
Shandong Provincial Natural Science Foundation (grant nos.
BS2014YY033 and BS2012YY016), the Special foundation for Taishan
Scholars (grant nos. ts20110814 and tshw201502056), the Fundamental
Research Funds of Shandong University, the Department of Science
and Technology of Shandong Province (grant nos. 2015GGE27101 and
2015ZDXX0801A01), the China Postdoctoral Science Foundation (grant
no. 2014M551916), the University of Bergen, Helse Bergen, Norway,
and the Norwegian Centre for International Cooperation in Education
(SIU) (grant no. UTF-2014/10047).
References
|
1
|
Strojan P, Popović M, Surlan K and Jereb
B: Choroid plexus tumors: A review of 28-year experience.
Neoplasma. 51:306–312. 2004.PubMed/NCBI
|
|
2
|
Sun MZ, Oh MC, Ivan ME, Kaur G, Safaee M,
Kim JM, Phillips JJ, Auguste KI and Parsa AT: Current management of
choroid plexus carcinomas. Neurosurg Rev. 37:179–192; discussion
192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koh EJ, Wang KC, Phi JH, Lee JY, Choi JW,
Park SH, Park KD, Kim IH, Cho BK and Kim SK: Clinical outcome of
pediatric choroid plexus tumors: Retrospective analysis from a
single institute. Childs Nerv Syst. 30:217–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krishnan S, Brown PD, Scheithauer BW,
Ebersold MJ, Hammack JE and Buckner JC: Choroid plexus papillomas:
A single institutional experience. J Neurooncol. 68:49–55. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buxton N and Punt J: Choroid plexus
papilloma producing symptoms by secretion of cerebrospinal fluid.
Pediatr Neurosurg. 27:108–111. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samuel TA, Parikh J, Sharma S, Giller CA,
Sterling K, Kapoor S, Pirkle C and Jillella A: Recurrent adult
choroid plexus carcinoma treated with high-dose chemotherapy and
syngeneic stem cell (bone marrow) transplant. J Neurol Surg A Cent
Eur Neurosurg. 74 Suppl 1:e149–e154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolff JE, Sajedi M, Brant R, Coppes MJ and
Egeler RM: Choroid plexus tumours. Br J Cancer. 87:1086–1091. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bettegowda C, Adogwa O, Mehta V, Chaichana
KL, Weingart J, Carson BS, Jallo GI and Ahn ES: Treatment of
choroid plexus tumors: A 20-year single institutional experience. J
Neurosurg Pediatr. 10:398–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubicky CD, Sahgal A, Chang EL and Lo SS:
Rare primary central nervous system tumors. Rare Tumors.
6:54492014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McEvoy AW, Harding BN, Phipps KP, Ellison
DW, Elsmore AJ, Thompson D, Harkness W and Hayward RD: Management
of choroid plexus tumours in children: 20 Years experience at a
single neurosurgical centre. Pediatr Neurosurg. 32:192–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rickert CH and Paulus W: Tumors of the
choroid plexus. Microsc Res Tech. 52:104–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turkoglu E, Kertmen H, Sanli AM, Onder E,
Gunaydin A, Gurses L, Ergun BR and Sekerci Z: Clinical outcome of
adult choroid plexus tumors: Retrospective analysis of a single
institute. Acta Neurochir (Wien). 156:1461–1478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kourbeti IS, Jacobs AV, Koslow M,
Karabetsos D and Holzman RS: Risk factors associated with
postcraniotomy meningitis. Neurosurgery. 60:317–325; discussion
325–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Malley MR and Haynes DS: Assessment and
management of meningitis following cerebellopontine angle surgery.
Curr Opin Otolaryngol Head Neck Surg. 16:427–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farnedi A, Rossi S, Bertani N, Gulli M,
Silini EM, Mucignat MT, Poli T, Sesenna E, Lanfranco D,
Montebugnoli L, et al: Proteoglycan-based diversification of
disease outcome in head and neck cancer patients identifies
NG2/CSPG4 and syndecan-2 as unique relapse and overall survival
predicting factors. BMC Cancer. 15:3522015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicolosi PA, Dallatomasina A and Perris R:
Theranostic impact of NG2/CSPG4 proteoglycan in cancer.
Theranostics. 5:530–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tateno T, Nakano-Tateno T, Ezzat S and Asa
SL: NG2 targets tumorigenic Rb inactivation in Pit1-lineage
pituitary cells. Endocr Relat Cancer. 23:445–456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yadavilli S, Hwang EI, Packer RJ and
Nazarian J: The role of NG2 proteoglycan in glioma. Transl Oncol.
9:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrasco-Garcia E, Santos JC, Garcia I,
Brianti M, García-Puga M, Pedrazzoli J Jr, Matheu A and Ribeiro ML:
Paradoxical role of SOX2 in gastric cancer. Am J Cancer Res.
6:701–713. 2016.PubMed/NCBI
|
|
20
|
Li Q, Liu F, Zhang Y, Fu L, Wang C, Chen
X, Guan S and Meng X: Association of SOX2 and nestin DNA
amplification and protein expression with clinical features and
overall survival in non-small cell lung cancer: A systematic review
and meta-analysis. Oncotarget. 7:34520–34531. 2016.PubMed/NCBI
|
|
21
|
Schaefer T, Wang H, Mir P, Konantz M,
Pereboom TC, Paczulla AM, Merz B, Fehm T, Perner S, Rothfuss OC, et
al: Molecular and functional interactions between AKT and SOX2 in
breast carcinoma. Oncotarget. 6:43540–43556. 2015.PubMed/NCBI
|
|
22
|
van Olphen S, Biermann K, Spaander MC,
Kastelein F, Steyerberg EW, Stoop HA, Bruno MJ and Looijenga LH:
SOX2 as a novel marker to predict neoplastic progression in
Barrett's esophagus. Am J Gastroenterol. 110:1420–1428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stallcup WB: The NG2 proteoglycan: Past
insights and future prospects. J Neurocytol. 31:423–435. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakry D and Trotter J: The role of the NG2
proteoglycan in OPC and CNS network function. Brain Res.
1638:161–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang FJ, You WK, Bonaldo P, Seyfried TN,
Pasquale EB and Stallcup WB: Pericyte deficiencies lead to aberrant
tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol.
344:1035–1046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukushi J, Makagiansar IT and Stallcup WB:
NG2 proteoglycan promotes endothelial cell motility and
angiogenesis via engagement of galectin-3 and alpha3beta1 integrin.
Mol Biol Cell. 15:3580–3590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brekke C, Lundervold A, Enger PØ, Brekken
C, Stålsett E, Pedersen TB, Haraldseth O, Krüger PG, Bjerkvig R and
Chekenya M: NG2 expression regulates vascular morphology and
function in human brain tumours. Neuroimage. 29:965–976. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albright JE, Stojkovska I, Rahman AA,
Brown CJ and Morrison BE: Nestin-positive/SOX2-negative cells
mediate adult neurogenesis of nigral dopaminergic neurons in mice.
Neurosci Lett. 615:50–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rizzino A and Wuebben EL: Sox2/Oct4: A
delicately balanced partnership in pluripotent stem cells and
embryogenesis. Biochim Biophys Acta. 1859:780–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le N, Nagarajan R, Wang JY, Araki T,
Schmidt RE and Milbrandt J: Analysis of congenital hypomyelinating
Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell
differentiation and myelination. Proc Natl Acad Sci USA. 102:pp.
2596–2601. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|