Introduction
Previous studies have reported that high expression
of basic fibroblast growth factor (bFGF) is associated with tumor
genesis, growth and prognosis (1,2). bFGF may
affect the proliferation and differentiation of a number of cell
types in vitro, including neural stem cells (3). The bFGF is a type of polypeptide growth
factor, which are produced via cell proliferation in the mesoderm
and neural ectoderm (4). The most
significant biological function of bFGF reported is to promote
proliferation (5). In pathological
cases, bFGF is involved in tumor genesis, growth and promoting the
repair of damaged issues (5). bFGF
and its receptors in tumor tissue are potential targets for
antitumor therapy (6). Targeting
anti-bFGF antibodies and fibroblast growth factor receptor (FGFR)
antagonists may inhibit tumor cell proliferation and metastasis as
well as block the blood supply for tumor growth and metastasis
(6).
bFGF is reported to be involved in tumor genesis and
growth through two mechanisms as follows: i) Promotion of
overproliferation of tumor cells in an autocrine or paracrine
manner; and ii) promotion of tumor angiogenesis to provide
nutrients for tumor cell growth, which is the main route by which
bFGF is involved in the pathological process of tumors (7). bFGF is a major pro-angiogenic factor
during tumor angiogenesis. In normal tissues, bFGF expression is
absent, or it is detected at low levels. By contrast, the level of
bFGF expression level is high in tumor issues (8). bFGF is also an important factor involved
in tumor growth and invasion, and is involved in the genesis and
growth of a variety of malignant tumors, including breast cancer,
lung cancer and prostate cancer (9,10).
Previous studies have investigated the expression
level of bFGF in advance NSCLC cell lines (11,12).
However, the limitations of these studies were evident. These
studies used relatively small sample sizes and investigated only a
single type of tumor. In addition, sex-based differences were not
taken into consideration. Therefore, in the present study, 508
female patients with malignant tumors [103 non-small cell lung
cancer (NSCLC), 147 colon cancer, 206 breast cancer and 52
melanoma] were recruited to study the level of bFGF expression in
tumor cells, and to investigate the association between expression
level of bFGF protein and clinicopathological characteristics of
malignant tumors.
Patients and methods
Female patients
Prior to recruiting female patients, approval to
conduct the present study was obtained from the Ethics Committee of
Henan University of Science and Technology (Henan, China). Written
informed consent was obtained from all patients. Female patients
with one of the following malignant tumors were included between
March 2008 and May 2015: NSCLC, colon cancer, breast cancer and
melanoma. Patients that received radiotherapy, chemotherapy or
traditional Chinese medicine treatment prior to surgery were
excluded. All patients were diagnosed by pathological or clinical
method. Finally, a total of 103 patients with NSCLC, 147 patients
with colon cancer, 206 patients with breast cancer and 52 patients
with melanoma were recruited in the present study.
Immunohistochemical staining
The tumor tissues from the female patients were
obtained by standard method (routine examination) and embedded in
paraffin (4% paraformaldehyde for 24 h at room temperature). The
paraffin-embedded tumor tissues were serially cut for histological
examination. A total of two sections (thickness, 5 µm) were
randomly selected from each patient. Hematoxylin-eosin staining was
performed at room temperature for 24 h on one section for
histomorphometric observations. The tissue sections were subjected
to dewaxing, dehydration (descending alcohol series) and washing
with PBS, and subsequently treated with hematoxylin for 10–15 min
at 60°C, 5% hydrochloric acid in ethanol for several sec subsequent
to washing in running water. The tissue sections were re-stained
with eosin for 5 min at room temperature, dehydrated by graded
alcohol and treated with mounting medium (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Olympus PM 20 (Olympus Corporation,
Tokyo, Japan) was used to capture images (magnification, ×100). The
immunohistochemical peroxidase-conjugated streptavidin method was
performed on the other section to detect the expression level of
bFGF protein in tumor tissues using an SP immunohistochemistry kit
(rabbit) supplied by Nanjing KeyGen Biotech Co., Ltd. PBS was used
as the negative control to replace the primary antibody. Goat serum
(KGIHC008; Nanjing KeyGen Biotech Co., Ltd.) was applied at 4°C
overnight to block non-specific binding. The anti-bFGF rabbit
polyclonal antibody (dilution, 1:200; KGIHC009; Nanjing KeyGen
Biotech Co., Ltd.) was used at 4°C overnight. Then a horseradish
peroxidase-conjugated sheep anti-rabbit secondary antibody
(dilution, 1:200; KGIHC011; Nanjing KeyGen Biotech Co., Ltd.) was
applied at room temperature for 1 h. The Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) was used to measure the
density.
Scoring of immunostaining
Positive bFGF protein expression in tumor tissues
was identified by the presence of yellow-brown granules in the
tumor cell cytoplasm. The immunoreactivity was semi-quantitatively
scored using the intensity of the immunostaining and the percentage
of tumor cells with positive bFGF expression, as described
previously (13). Positive percentage
was graded as 0 (<10% positive tumor cells), 1 (10–24%), 2
(25–49%), 3 (50–75%) and 4 (>75%). The intensity of the
immunostaining was graded as 0 (no immunoreactivity), 1 (weak
intensity), 2 (moderate intensity) and 3 (strong intensity). The
two scores were subsequently added and scored as follows: >5,
strong expression (+++); 4–5, as moderate expression (++); 2–3,
weak expression (+); and 0–1, absent expression (−).
Statistical analysis
The continuous data are expressed as the mean ±
standard deviation, and the numerical data are expressed as the
number and percentage. Student's t-test and one-way analysis of
variance were used when appropriate. Ranked data were evaluated by
Kruskal-Wallis test for multiple group comparisons and Nemenyi test
for comparisons between two groups. If a significant difference was
found, a Bonferroni post hoc test was performed. P<0.05 was
considered to indicate a statistically significant difference. SPSS
version 19.0 was used to perform all analysis (IBM Corp., Armonk,
NY, USA).
Results
bFGF protein expression in NSCLC
In total, 103 patients with NSCLC were included in
the present study. The mean age of these patients was 54.3 years
(range, 35–75 years). Of these patients, ~70% were married, 62.3%
were non-smokers and 66.4% received ≤9 years education. Among the
married patients, the percentage of smoking in husbands of these
patients was 85.4%. According to the tumor-node-metastasis (TNM)
stage (14), a total of 24 patients
were in stage I, 25 were in stage II and 54 were in stage III/IV.
Among these patients, there were 71 patients with lymph node
metastasis, and 64 patients with poor differentiation. The total
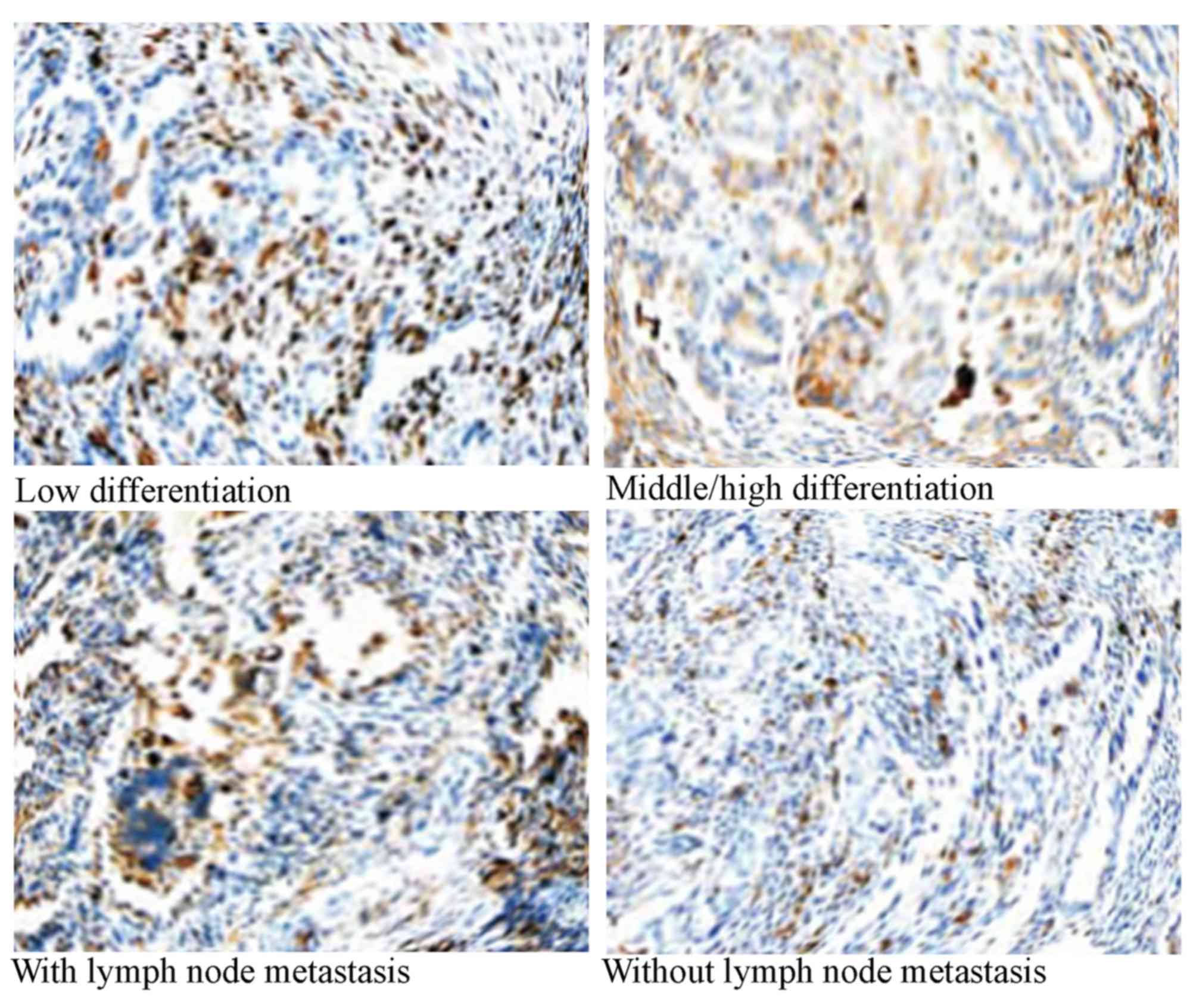
rate of positive bFGF staining in NSCLC tissues was 64.1% (66/103).
The rate of positive bFGF staining in NSCLC with poor
differentiation tissues was significantly increased compared with
patients with moderately/well differentiated NSCLC (P=0.00023).
Compared with patients without lymph node metastasis, patients with
lymph node metastasis had a significantly increased rate of
positive bFGF staining (P=0.00025; Table
I; Fig. 1).
 | Table I.bFGF protein expression in patients
with non-small cell lung cancer. |
Table I.
bFGF protein expression in patients
with non-small cell lung cancer.
|
|
| bFGF expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | ++ | +++ | Positive rate, % | P-valuea |
|---|
| Differentiation |
|
|
|
|
|
| 0.00023 |
| Poor | 64 | 13 | 10 | 25 | 16 | 79.7 |
|
|
Moderate/well | 39 | 24 | 9 | 5 | 1 | 38.5 |
|
| TNM stage |
|
|
|
|
|
| 0.77827 |
| I | 24 | 10 | 8 | 4 | 2 | 58.3 |
|
| II | 25 | 9 | 8 | 6 | 2 | 64.0 |
|
|
III/IV | 54 | 18 | 20 | 11 | 5 | 66.7 |
|
| Lymph node
metastasis |
|
|
|
|
|
| 0.00025 |
| Yes | 71 | 16 | 9 | 21 | 25 | 77.5 |
|
| No | 32 | 21 | 8 | 3 | 0 | 34.4 |
|
bFGF protein expression in colon
cancer
A total of 147 patients with colon cancer were
included in the present study. The mean age of these patients was
46.3 years (range, 33–61 years). Of these patients, ~73% were
married, 69.5% were non-smoker patients and 60.3% received ≤9 years
education. According to the TNM staging system (14), 44 patients were in stage I, 55 were in
stage II and 48 were in stage III/IV. Among these patients, there
were 105 patients with lymph node metastasis and 80 patients with
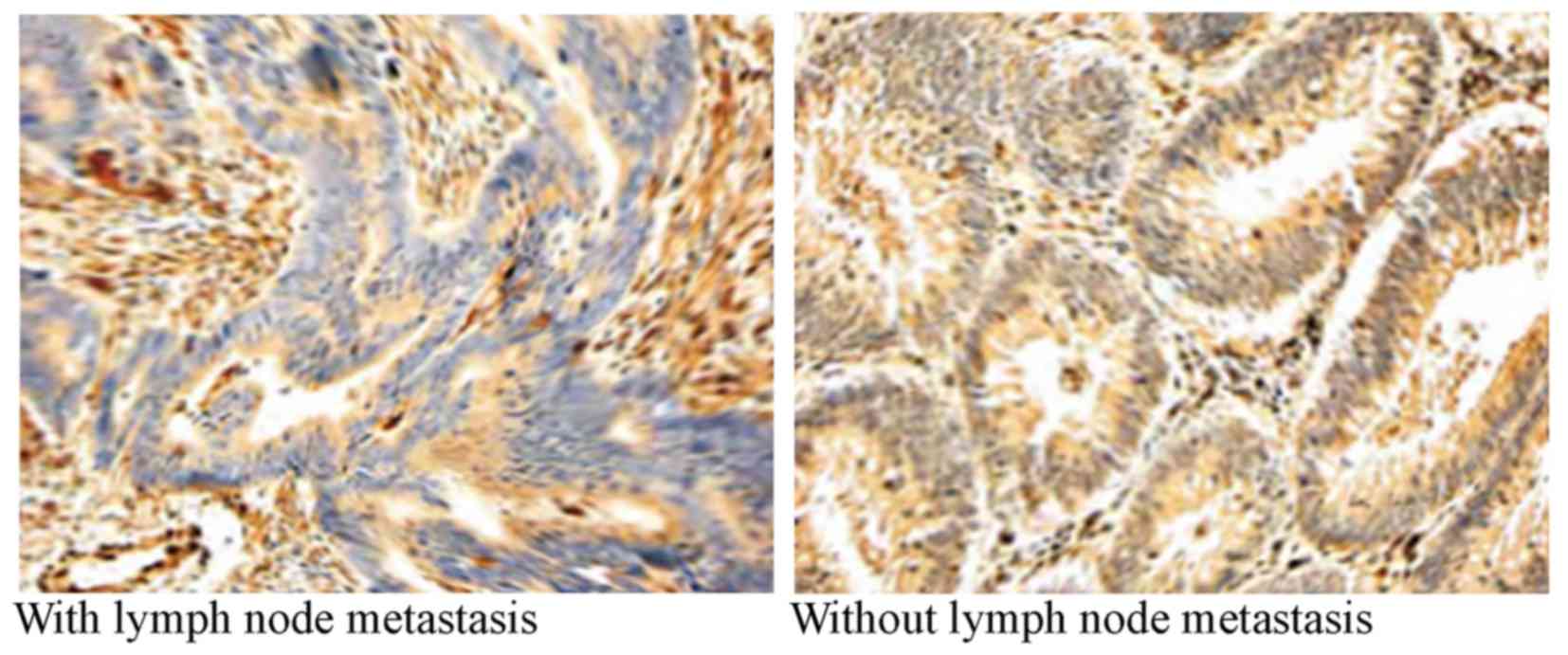
poor differentiation. The rate of positive bFGF staining in
patients with colon cancer was 60.5% (89/147). The rate of positive
bFGF staining in patients with colon cancer with lymph node
metastasis was significantly higher compared with patients without
lymph node metastasis (P=0.00001; Table
II; Fig. 2).
 | Table II.bFGF protein expression in patients
with colon cancer. |
Table II.
bFGF protein expression in patients
with colon cancer.
|
|
| bFGF expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | ++ | +++ | Positive rate, % | P-valuea |
|---|
| Differentiation |
|
|
|
|
|
| 0.22727 |
| Poor | 80 | 28 | 13 | 27 | 12 | 65.0 |
|
|
Moderate/well | 67 | 30 | 8 | 21 | 8 | 55.2 |
|
| TNM stage |
|
|
|
|
|
| 0.83647 |
| I | 44 | 18 | 8 | 14 | 4 | 59.1 |
|
| II | 55 | 20 | 11 | 18 | 6 | 63.6 |
|
|
III/IV | 48 | 20 | 7 | 16 | 5 | 58.3 |
|
| Lymph node
metastasis |
|
|
|
|
|
| 0.00001 |
|
Yes | 105 | 29 | 19 | 25 | 32 | 72.4 |
|
| No | 42 | 28 | 9 | 5 | 0 | 33.3 |
|
bFGF protein expression in breast
cancer
In total, 206 patients with breast cancer were
included in the present study. The mean age of these patients was
52.6 years (range, 38–56 years). Of these patients, ~62.7% were
married, 65.3% were non-smoker patients and 63.8% received ≤9 years
education. According to the TNM staging system (14), 59 patients were in stage I, 48 were in
stage II and 99 were in stage III/IV. Among these patients, there
were 134 patients with lymph node metastasis and 118 patients with
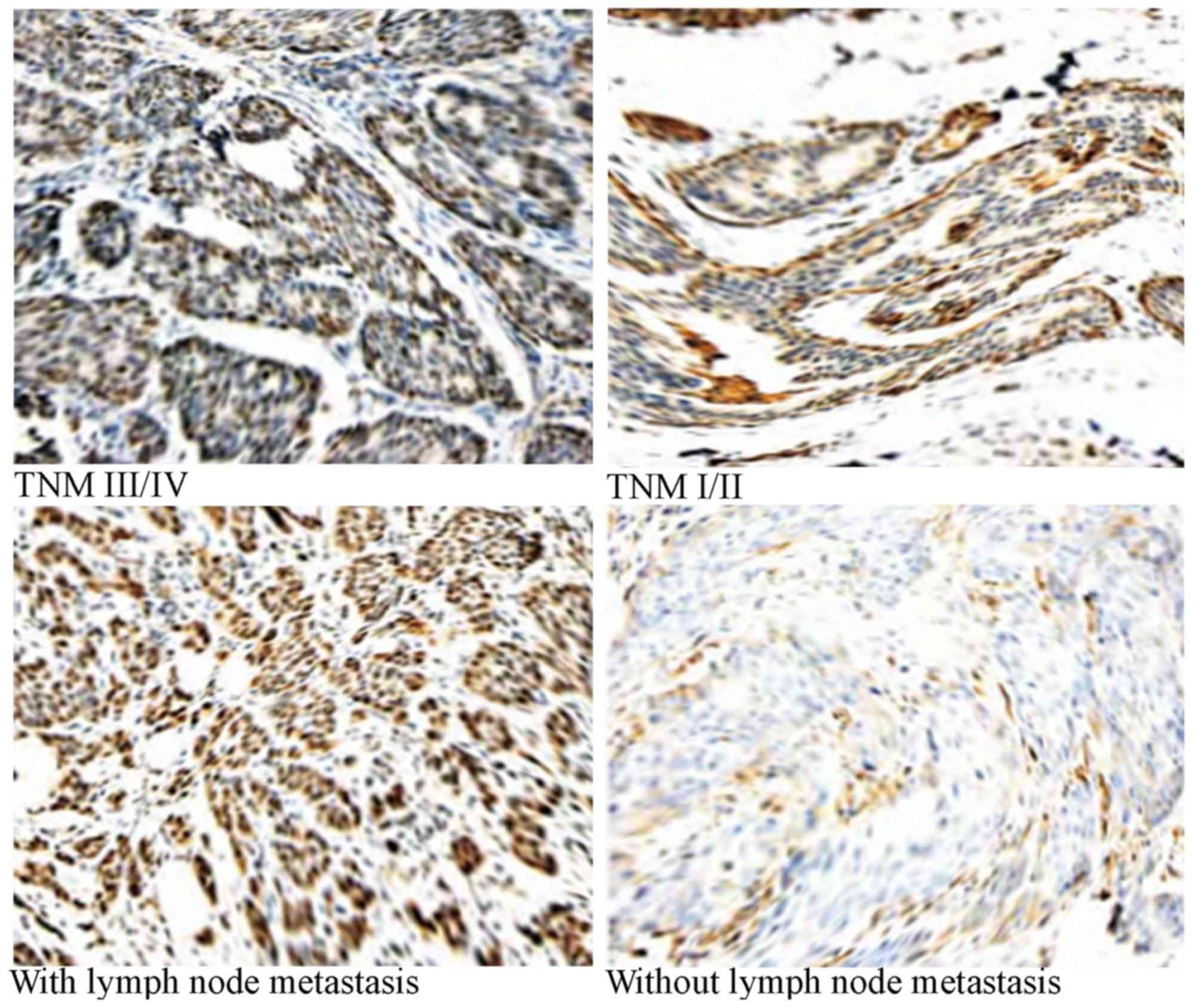
poor differentiation. The total rate of positive bFGF staining in
breast cancer was 71.8% (148/206). The positive rate in patients
with breast cancer with lymph node metastasis was significantly
higher compared with patients without lymph node metastasis
(P<0.00001). Compared with patients in stage I–II, patients in
stage III/IV had a significantly higher rate of positive staining
(P=0.00001; Table III; Fig. 3).
 | Table III.bFGF protein expression in patients
with breast cancer. |
Table III.
bFGF protein expression in patients
with breast cancer.
|
|
| bFGF expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | ++ | +++ | Positive rate,
% |
P-valuea |
|---|
|
Differentiation |
|
|
|
|
|
| 0.31327 |
|
Poor | 118 | 30 | 41 | 34 | 13 | 74.6 |
|
|
Moderate/well | 88 | 28 | 30 | 25 | 5 | 68.2 |
|
| TNM stage |
|
|
|
|
|
| 0.00001 |
| I | 59 | 27 | 17 | 10 | 5 | 54.2 |
|
| II | 48 | 18 | 15 | 9 | 6 | 62.5 |
|
|
III/IV | 99 | 13 | 22 | 38 | 26 | 86.8 |
|
| Lymph node
metastasis |
|
|
|
|
|
| <0.00001 |
|
Yes | 134 | 118 | 19 | 25 | 32 | 88.1 |
|
| No | 72 | 30 | 9 | 5 | 0 | 41.7 |
|
bFGF protein expression in
melanoma
In total, 52 patients with melanoma were included in
the present study. The average age of these patients was 55.8 years
(range, 40–63 years). Of these patients, ~71.0% were married, 69.4%
were non-smoker patients and 68.4% received ≤9 years education.
Additionally, ~73.5% patients or one of their family members
presented with dysplastic nevus. According to the TNM staging
system (14), 22 patients were in
stage I, 16 were in stage II and 14 were in stage III/IV. Among
these patients, there were 32 patients with lymph node metastasis
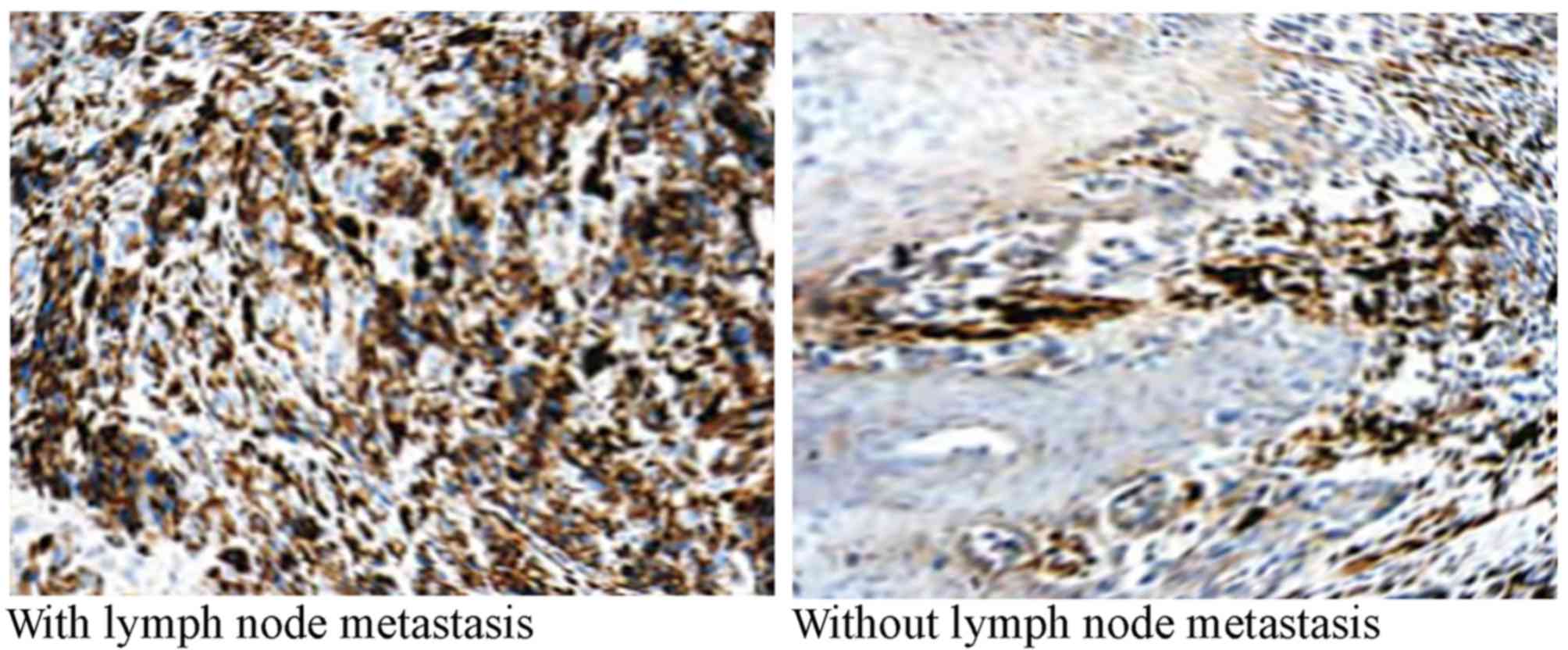
and 23 patients with poor differentiation. The total rate of
positive bFGF staining in melanoma was 59.6% (31/52). The rate of
positive bFGF staining in patients with melanoma with lymph node
metastasis was significantly higher compared with patients without
lymph node metastasis (P=0.00006; Table
IV; Fig. 4).
 | Table IV.bFGF protein expression in patients
with melanoma. |
Table IV.
bFGF protein expression in patients
with melanoma.
|
|
| bFGF expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | − | + | ++ | +++ | Positive rate,
% |
P-valuea |
|---|
|
Differentiation |
|
|
|
|
|
| 0.68627 |
|
Poor | 23 | 10 | 7 | 6 | 0 | 56.5 |
|
|
Moderate/well | 29 | 11 | 6 | 10 | 2 | 62.1 |
|
| TNM stage |
|
|
|
|
|
| 0.90318 |
| I | 22 | 9 | 7 | 5 | 1 | 59.1 |
|
| II | 16 | 7 | 4 | 4 | 1 | 56.3 |
|
|
III/IV | 14 | 5 | 3 | 4 | 2 | 64.2 |
|
| Lymph node
metastasis |
|
|
|
|
|
| 0.00006 |
|
Yes | 32 | 6 | 7 | 14 | 5 | 81.3 |
|
| No | 20 | 15 | 3 | 2 | 0 | 25.0 |
|
Discussion
Invasion and metastasis are the essential biological
characteristics of malignant tumors, as well as the primary causes
of cancer-associated mortality (15).
Tumor angiogenesis and lymph node metastasis are the prerequisite
of malignant tumor invasion and metastasis (15). Therefore, the aim of antitumor therapy
is to inhibit vascular endothelial cell proliferation and lymph
node metastasis (16).
As a cell mitogen and angiogenic factor, bFGF is a
member of the fibroblast growth factor family (17). The bFGF is able to promote malignant
tumor invasion and metastasis by secreting a number of proteolytic
enzymes and collagenase, including matrix metalloproteinase-14
(15).
The present study revealed that the level of bFGF
expression was markedly increased in patients with NSCLC. The rate
of positive bFGF staining was significantly higher in
low-differentiated NSCLC compared with moderate/well NSCLC
(Table I). In addition, the rate of
positive bFGF staining was significantly higher in patients with
NSCLC with lymph node metastasis compared with in patients with
NSCLC without lymph node metastasis. These results indicated that
the differentiation stage and lymph node metastasis had effects on
the level of bFGF expression, which was consistent with a previous
study (18). A number of previous
studies also reported that the expression of bFGF and FGFR was
increased in advanced NSCLC cell lines compared with in normal cell
lines (11,12). Therefore, bFGF and its receptor may be
novel targets for NSCLC treatment.
The present study also revealed that the level of
bFGF expression in colon cancer tumor tissues was increased, and
the rate of positive bFGF staining in patients with colon cancer
with lymph node metastasis was significantly increased compared
with that of patients without lymph node metastasis. This finding
indicated that bFGF may serve a role in enabling colon cancer
metastasis, which was similar to the findings of a previous study
(19). In addition, there was a
non-significant increased rate of positive bFGF staining in tissues
obtained from patients with poorly differentiated colon cancer
compared with tissues from patients with
moderately/well-differentiated colon cancer (65.0 vs. 55.2%), which
may indicate that bFGF may be involved in promoting angiogenesis
and proliferation of cells in poorly-differentiated colon cancer.
Landriscina et al (20) also
revealed that the bFGF tumour concentration was not associated with
the stage of disease. Therefore, bFGF may be a potential biomarker
for colon cancer diagnosis and prognosis.
In the present study, immunohistochemical results
indicated that the rate of positive bFGF staining in breast cancer
is 71.8%, which was similar with the results of a previous
long-term follow-up study (21). This
finding indicated that the high level of bFGF expression was
associated with breast cancer. In the present study, the rate of
positive bFGF staining was demonstrated to be significantly higher
in patients with stage III/IV breast cancer (86.8%) compared with
stage I–II breast cancer (57.9%). In addition, there was a
significantly increased rate of positive bFGF staining in breast
cancer patients with lymph node metastasis compared with patients
without lymph node metastasis, which indicated that the high level
of bFGF expression was associated with tumor invasion (22,23). A
previous study also reported that patients with breast cancer
without lymph node metastasis and positive bFGF expression
exhibited a longer median overall survival time than patients with
lymph node metastasis (21).
Therefore, monitoring the change in bFGF expression may be helpful
for clinicians and patients with breast cancer to predict the
efficacy of treatment.
Previous studies reported that the rate of positive
bFGF expression in patients with melanoma was 30–90% (24,25). In
the present study, the rate of positive bFGF staining in patients
with melanoma was demonstrated to be 59.6%. Straume and Akslen
(24) reported that the high level of
bFGF expression in melanoma may be associated with the high density
of small vessels in melanoma cell. Compared with patients without
lymph node metastasis, patients with lymph node metastasis had
significantly increased rate of positive bFGF staining. This may be
due to the expression level of bFGF being increased along with an
increase in degree of malignancy of melanoma cells. Birck et
al (25) indicated that the level
of bFGF expression in melanoma was increased by 36% compared with
the level in premalignant lesion (nevi). These results demonstrated
that bFGF was an important factor involved in melanoma genesis and
growth.
The present study had several limitations. The
sample size was relatively small. Therefore, large-scale studies
are required to validate the results. All of the patients were
female, so whether the conclusion of the study can be applied to
males was unclear. Additionally, only four types of malignant
tumors were investigated, so whether the level of bFGF protein can
be a potential biomarker for diagnosing other types of
female-specific cancer, including cervical cancer and ovarian
cancer, requires future studies.
In conclusion, the present study indicated that the
level of bFGF expression was associated with malignant tumor growth
and metastasis. bFGF protein may be a potential biomarker for
diagnosing malignant tumor metastasis in women. The present results
may be helpful for the clinical application of targeted anti-bFGF
antibody and FGFR antagonists.
References
|
1
|
Attoub S, Arafat K, Gélaude A, Al Sultan
MA, Bracke M, Collin P, Takahashi T, Adrian TE and De Wever O:
Frondoside a suppressive effects on lung cancer survival, tumor
growth, angiogenesis, invasion, and metastasis. PLoS One.
8:e530872013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chao Y, Li CP, Chau GY, Chen CP, King KL,
Lui WY, Yen SH, Chang FY, Chan WK and Lee SD: Prognostic
significance of vascular endothelial growth factor, basic
fibroblast growth factor, and angiogenin in patients with
resectable hepatocellular carcinoma after surgery. Ann Surg Oncol.
10:355–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gospodarowicz D: Fibroblast growth factor.
Chemical structure and biologic function. Clin Orthop Rel Res.
257:231–248. 1990.
|
|
4
|
Montero A, Okada Y, Tomita M, Ito M,
Tsurukami H, Nakamura T, Doetschman T, Coffin JD and Hurley MM:
Disruption of the fibroblast growth factor-2 gene results in
decreased bone mass and bone formation. J Clin Invest.
105:1085–1093. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei X: Research advances of basic
fibroblast growth factor in tissue repair. Orthop J Chin.
19:1108–1110. 2011.
|
|
6
|
Presta M, Dell'Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo CF, Hong HL, Lu YL, Wang H and Liu MQ:
Expression of bFGF and PTEN in cervical carcinoma and their
clinical significance. Zhonghua Zhong Liu Za Zhi. 32:533–538.
2010.(In Chinese). PubMed/NCBI
|
|
8
|
Trojan L, Thomas D, Knoll T, Grobholz R,
Alken P and Michel MS: Expression of pro-angiogenic growth factors
VEGF, EGF and bFGF and their topographical relation to
neovascularisation in prostate cancer. Urol Res. 32:97–1031. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cucina A, Borrelli V, Lucarelli M,
Sterpetti AV, Cavallaro A, Strom R, Santoro-D'Angelo L and Scarpa
S: Autocrine production of basic fibroblast growth factor
translated from novel synthesized mRNA mediates thrombin-induced
mitogenesis in smooth muscle cells. Cell Biochem Funct. 20:39–46.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polnaszek N, Kwabi-Addo B, Peterson LE,
Ozen M, Greenberg NM, Ortega S, Basilico C and Ittmann M:
Fibroblast growth factor 2 promotes tumor progression in an
autochthonous mouse model of prostate cancer. Cancer Res.
63:5754–5760. 2003.PubMed/NCBI
|
|
11
|
Coldren CD, Helfrich BA, Witta SE, Sugita
M, Lapadat R, Zeng C, Barón A, Franklin WA, Hirsch FR, Geraci MW
and Bunn PA Jr: Baseline gene expression predicts sensitivity to
gefitinib in non-small cell lung cancer cell lines. Mol Cancer Res.
4:521–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marek L, Ware KE, Fritzsche A, Hercule P,
Helton WR, Smith JE, McDermott LA, Coldren CD, Nemenoff RA, Merrick
DT, et al: Fibroblast growth factor (FGF) and FGF receptor-mediated
autocrine signaling in non-small-cell lung cancer cells. Mol
Pharmacol. 75:196–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu TM, Xin Y, Cui MH, Jiang X and Gu LP:
Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide
on growth and angiogenesis of ovarian cancer. Chin Med J (Engl).
120:584–588. 2007.PubMed/NCBI
|
|
14
|
Sobin LH, Hermanek P and Hutter RV: TNM
classification of malignant tumors. A comparison between the new
(1987) and the old editions. Cancer. 61:2310–2314. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song S, Wientjes MG, Gan Y and Au JL:
Fibroblast growth factors: An epigenetic mechanism of broad
spectrum resistance to anticancer drugs. Proc Natl Acad Sci USA.
97:pp. 8658–8663. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amino N, Ideyama Y, Yamano M, Kuromitsu S,
Tajinda K, Samizu K, Hisamichi H, Matsuhisa A, Shirasuna K, Kudoh M
and Shibasaki M: YM-359445, an orally bioavailable vascular
endothelial growth factor receptor-2 tyrosine kinase inhibitor, has
highly potent antitumor activity against established tumors. Clin
Cancer Res. 12:1630–1638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keegan K, Johnson DE, Williams LT and
Hayman MJ: Isolation of an additional member of the fibroblast
growth factor receptor family, FGFR-3. Proc Natl Acad Sci USA.
88:pp. 1095–1099. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joensuu H, Anttonen A, Eriksson M,
Mäkitaro R, Alfthan H, Kinnula V and Leppä S: Soluble syndecan-1
and serum basic fibroblast growth factor are new prognostic factors
in lung cancer. Cancer Res. 62:5210–5217. 2002.PubMed/NCBI
|
|
19
|
Elagoz S, Egilmez R, Koyuncu A,
Muslehiddinoglu A and Arici S: The intratumoral microvessel density
and expression of bFGF and nm23-H1 in colorectal cancer. Pathol
Oncol Res. 12:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Landriscina M, Cassano A, Ratto C, Longo
R, Ippoliti M, Palazzotti B, Crucitti F and Barone C: Quantitative
analysis of basic fibroblast growth factor and vascular endothelial
growth factor in human colorectal cancer. Br J Cancer. 78:765–770.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Faridi A, Rudlowski C, Biesterfeld S,
Schuh S, Rath W and Schröder W: Long-term follow-up and prognostic
significance of angiogenic basic fibroblast growth factor (bFGF)
expression in patients with breast cancer. Pathol Res Pract.
198:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeda M, Mikami T, Numata Y, Okamoto M
and Okayasu I: Papillary thyroid carcinoma with heterotopic
ossification is a special subtype with extensive progression. Am J
Clin Pathol. 139:587–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chikazawa M, Inoue K, Fukata S, Karashima
T and Shuin T: Expression of angiogenesis-related genes regulates
different steps in the process of tumor growth and metastasis in
human urothelial cell carcinoma of the urinary bladder.
Pathobiology. 75:335–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Straume O and Akslen LA: Importance of
vascular phenotype by basic fibroblast growth factor, and influence
of the angiogenetic factors basic fibroblast growth
factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on
melanoma progression. Am J Pathol. 160:1009–1019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Birck A, Kirkin AF, Zeuthen J and
Hou-Jensen K: Expression of basic fibroblast growth factor and
vascular endothelial growth factor in primary and metastatic
melanoma from the same patients. Melanoma Res. 9:375–381. 1999.
View Article : Google Scholar : PubMed/NCBI
|