Introduction
Although the incidence of gastric cancer has
decreased worldwide, particularly in Western countries, it remains
the fourth most common type of cancer and the second-most common
cause of cancer-associated mortality worldwide (1,2).
Peritoneal carcinomatosis (PC) is the most frequent mode of
recurrence and is responsible for ~60% of gastric cancer-associated
mortalities (3). Patients with
gastric cancer exhibiting PC are considered not to be curable and
are usually treated with systemic chemotherapy without surgical
resection (4,5). Patients with PC and ascites have very
poor prognoses, with a median survival of 3–6 months, and there are
no long-term survivors (4,5). PC is a common event in the natural
history of gastrointestinal tract cancers, with a high 6-month
mortality rate (6). Malignant
ascites, one of the most frequent comorbid conditions complicating
PC (7), may severely affect patient
quality of life, and its symptoms may be particularly painful and
life threatening (7,8).
PC from gastrointestinal cancers has been considered
an incurable condition, for which the role of surgical intervention
is limited (9). Systemic chemotherapy
is the treatment of choice, with significant survival benefits
compared with best-supportive care, but even systemic chemotherapy
is relatively ineffective against PC due to the blood-peritoneal
barrier, which consists of a monolayer of mesothelial cells and
submesothelial connective tissue ~90 µm thick between the basement
membrane and vasculature (10).
Intraperitoneal (IP) chemotherapy is designed to
increase the dose and exposure time of intra-abdominal cancer cells
to anticancer drugs, while minimizing systemic toxic effects
(11,12). Prolonged retention in the peritoneal
cavity and clearance from the systemic circulation are regarded as
key attributes for IP chemotherapy drugs (11,12). Heat
has been exhibited to be synergistic with the antitumoral effects
of mitomycin C, cisplatin (CDDP) and oxaliplatin (13,14).
Drugs regarded as ideal for IP administration are
those that maintain a high concentration in the peritoneum, exhibit
high penetration into the tumors and have low systemic
concentrations; CDDP and carboplatin have been shown to penetrate
1–2 mm from the surface of PC nodules (15). These two drugs are easily absorbed
from the peritoneal cavity and are suitable for IP chemotherapy
(15). CDDP, an alkylating agent used
for treating gastric cancer, ovarian cancer and diffuse malignant
peritoneal mesothelioma, is the most common agent used during
heat-enhanced IP chemotherapy (HIPC) (16). A combination of systemic and
loco-regional chemotherapy may be effective in patients with
carcinomatosis from gastric cancer, particularly in patients with
small volumes of disease and symptomatic ascites (16).
The present study describes the use of bidirectional
chemotherapy in the treatment of gastric cancer with PC, using
newly developed response criteria regarding the treatment of
malignant ascites. The present study also investigated the
association between effusion response and patient survival.
Materials and methods
Patients
A total of 41 patients were enrolled in the present
study, including 23 (56.1%) males and 18 (43.9%) females, with a
median age of 42 (range, 31–69) years. The trial was conducted by
the Department of Oncology, The Third Affiliated Hospital, Soochow
University (Changzhou, China), between June 2010 and May 2014.
Patients were eligible if they met the following criteria:
Histopathological confirmation of gastric cancer; tumor cells in
ascites, pathological findings of peritoneal metastasis or
macroscopic PC diagnosed by laparoscopy; the absence of
non-curative factors, including distant metastasis to the liver or
lungs, with the exception of metastases to the peritoneum; Eastern
Cooperative Oncology Group (ECOG) (17) performance status <2; age <75
years; no prior treatment; adequate bone marrow function (leukocyte
count >3,000/ml and platelet count >100,000/ml); adequate
liver function (serum bilirubin level <1.5 mg/dl and serum
transaminase levels <2 times the upper normal limit); adequate
renal function (serum creatinine level <1.5 mg/dl); no other
severe medical conditions, including symptomatic infectious
disease, intestinal pneumonia, active hemorrhage/bleeding or
obstructive bowel disease; not pregnant or lactating; provision of
written informed consent in accordance with hospital regulations;
and an expected survival time >3 months. Gastric cancer ascites
was observed with a light microscope (BX51; Olympus Corporation,
Tokyo, Japan) with ×200 magnification.
The present study was approved by the Ethics
Committee of Soochow University. All patients provided written
informed consent regarding their involvement in the study, in
accordance with institutional guidelines.
Bidirectional chemotherapy and
HIPC
IP catheters, ARROWg+ and blue central venous
catheters (Arrow International, Inc., Reading, PA, USA) were
inserted under local anesthesia prior to therapy. On day 1 of every
3-week cycle, heated (43.0±0.5°C) CDDP perfusion solution (60 µg/ml
saline for a dose of 75 mg/m2) was infused into the
peritoneal cavity of all patients through an inflow tube using an
automatic hyperthermia chemotherapy perfusion device (HGG-Z102:
Hejia Medical Treatment Information Industry Co., Ltd., Zhuhai,
China). Subsequent to perfusion, to allow the solution to
distribute itself throughout the entire peritoneal surface, the
tilt of the patient was changed at 15 min intervals during
continuous perfusion over 2 h as follows: (1) level; (2)
Trendelenburg + left tilt; (3)
Trendelenburg + right tilt; (4)
level; (5) reverse Trendelenburg +
left tilt; and (6) reverse
Trendelenburg + right tilt. Approximately 30 min was required to
deliver a volume of 1l. Additionally, all patients were infused
intravenously for 1 h with docetaxel (75 mg/m2) on day 1 of the 3
week cycle.
Evaluation and determination of
efficacy
Baseline evaluations included patient history,
physical examination, measurement of the serum concentration of
carcinoembryonic antigen (CEA) (18)
and an examination of the abdomen using B-mode ultrasound within 1
week prior to therapy. CEA and a B-mode ultrasound inspection of
the abdomen were repeated prior to each cycle of chemotherapy.
Malignant ascites is considered a non-evaluable
lesion, since it is difficult to detect by conventional
radiological examinations. The present study used a new response
criterion to assess the effect of treatment of PC: B-mode
ultrasound examination above the bladder using longitudinal
sections prior to and following therapy. Complete response (CR) was
defined as complete absence of effusion on B-mode ultrasound or CT
scans; partial response (PR) as a ≥50% reduction in ascites depth
on B-mode ultrasound; and non-PR (nPR) as a <50% reduction or an
increase in ascites depth. Patients were assessed by B-mode
ultrasound following every second treatment cycle. The majority of
patients received 6 cycles of HIPC and systemic chemotherapy.
Statistical analysis
Survival time was calculated from the initial date
of treatment to the date of the most recent follow-up visit of the
event of patient mortality, using the Kaplan-Meier method.
Univariate analysis was performed using the log-rank test, and
multivariate analysis was performed using a Cox proportional
hazards model. P<0.05 was considered to indicate a statistically
significant difference. The primary study endpoint was overall
survival (OS) rate, and the secondary endpoints were efficacy and
serious adverse events, defined as severe local and/or systemic
infection, intestinal occlusion or mortality associated with the
procedure. The statistical analyses were performed using SPSS
version 16.0. (SPSS Inc., Chicago, IL USA).
Results
Between June 2010 and May 2014, 41 patients were
enrolled in the present study, including 17 with primary tumors and
peritoneal dissemination, and 24 with peritoneal recurrence
(Table I). The 41 patients consisted
of 23 (56.1%) males and 18 (43.9%) females, with a median age of 42
(range, 31–69) years. All patients had an ECOG performance status
score of 0 or 1. Metastatic sites included the peritoneum (41/41;
100%), lymph nodes (13/41; 31.7%), liver (12/41; 12.2%) and lungs
(2/41; 4.9%), with 34 patients (82.9%) having positive peritoneal
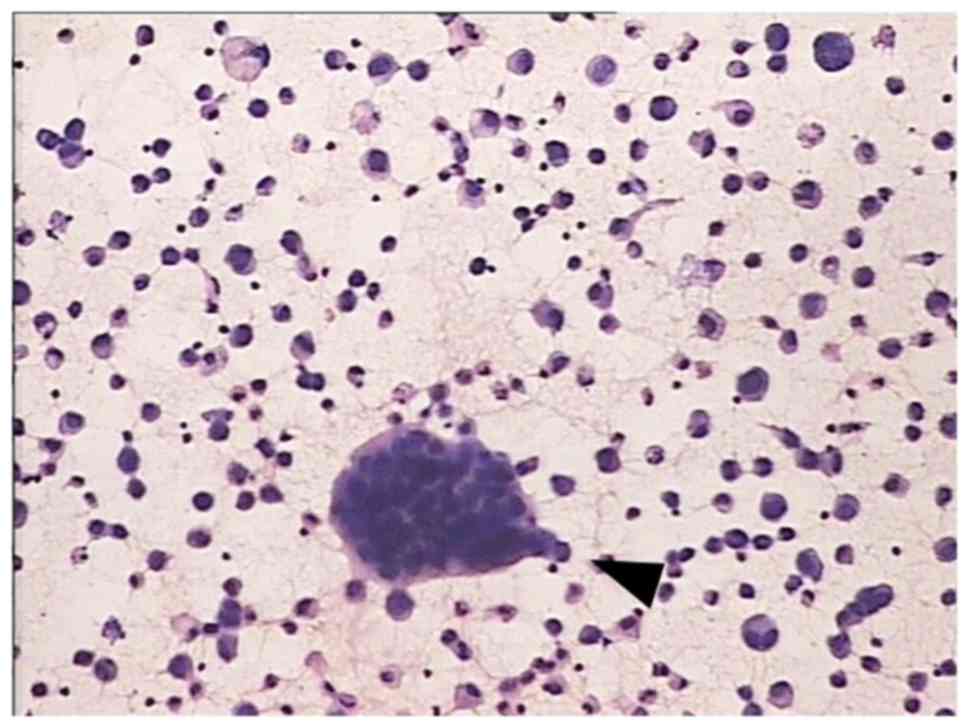
cytology. Fig. 1 shows typical cancer
cells in gastric cancer ascites. All patients presented with
malignant ascites.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Variables | Patients, n (%) |
|---|
| Age, years |
42.1±13.2a
(31–69) |
| Sex |
| Male | 23 (56.1) |
|
Female | 18 (43.9) |
| ECOG performance
status |
| 0 | 2 (4.9) |
| 1 | 13 (31.7) |
| 2 | 16 (39.0) |
| Ascites |
| No | 0 (0.0) |
|
Yes | 41 (100.0) |
| Peritoneal lavage
cytology |
|
Negative | 7 (17.1) |
|
Positive | 34 (82.9) |
| Primary or
recurrence |
|
Primary | 17 (41.5) |
|
Recurrence | 24 (58.5) |
| Cycles of
hyperthermic intraperitoneal perfusion chemotherapy | 2.2b (1–4) |
| Total | 41 (100.0) |
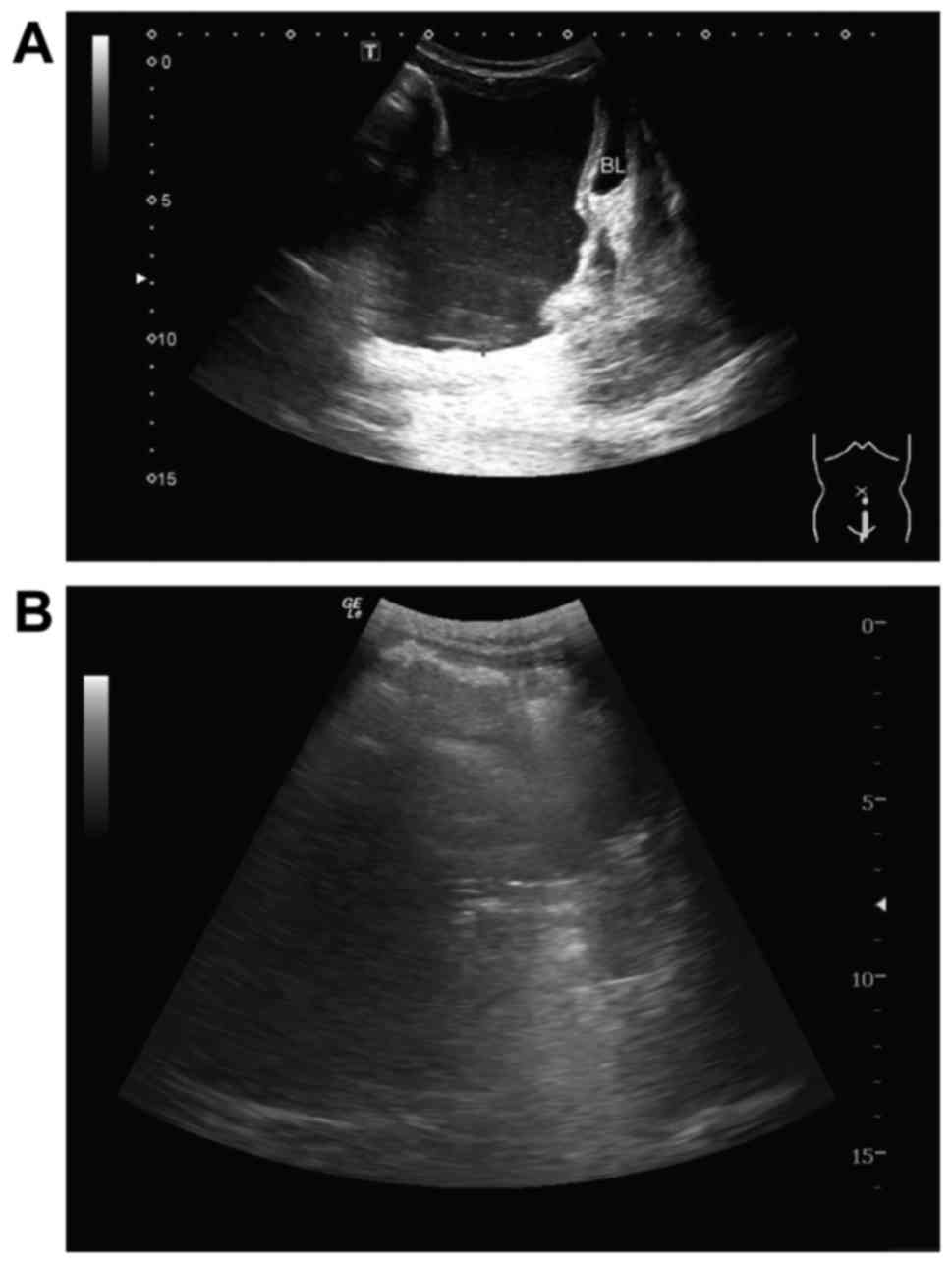
Fig. 2 shows a typical
peritoneal effusion response imaged using B-mode ultrasound. Prior
to treatment, numerous abdominal effusions were observed above the
bladder (Fig. 2A). Following two
cycles of treatment, the peritoneal effusions had disappeared above
the bladder (Fig. 2B), indicating
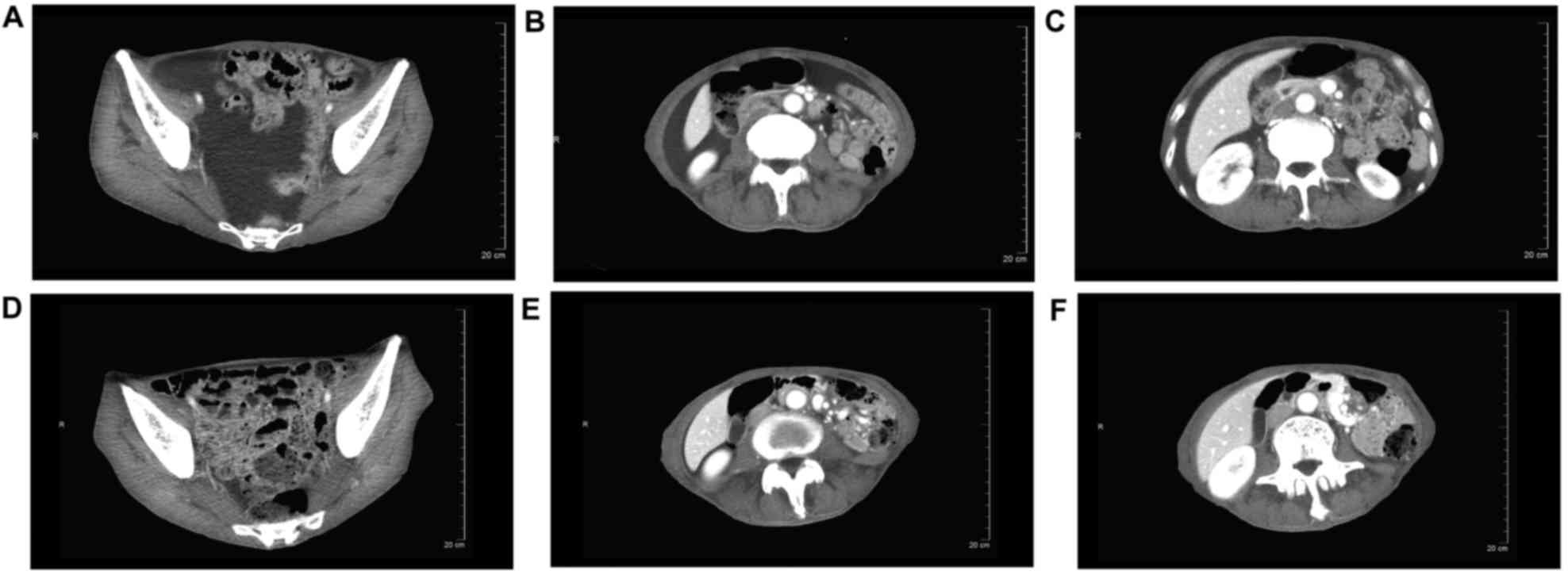
that this patient had achieved CR. CT of another patient also
showed a typical peritoneal effusion response during therapy
(Fig. 3); this patient was also
regarded as having achieved CR.
Table II shows the
effects of therapy on peritoneal dissemination. The majority of
patients showed clinical regression of ascites and associated
symptoms. The peritoneal effusion response rate (RR=CR+PR) was
73.2%, including 7 patients (17.1%) who achieved CR and 23 (56.1%)
who achieved PR, with the remaining 11 patients (26.8%) achieving
nPR. There were no mortalities associated with the procedure. At a
median follow-up of 11.4 months, the mean survival time (MST) of
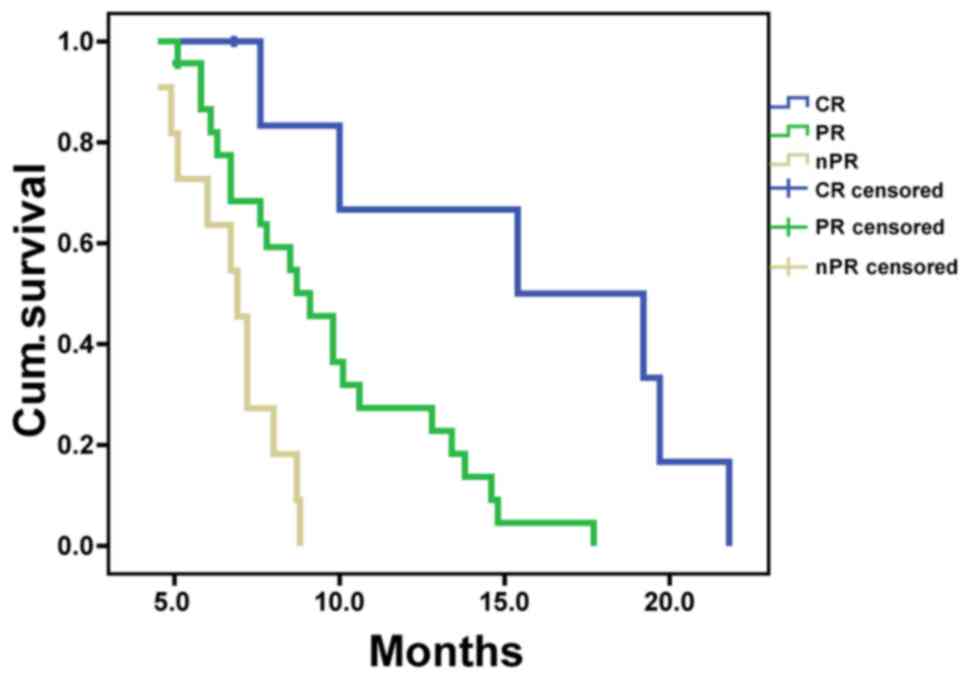
all 41 patients enrolled in the present study was 8.5±0.7 months.
MSTs in CR, PR and nPR groups were 15.4±5.6, 9.1±0.9 and 6.9±0.5
months, respectively (Table II),
with significant differences among the 3 groups (P<0.05;
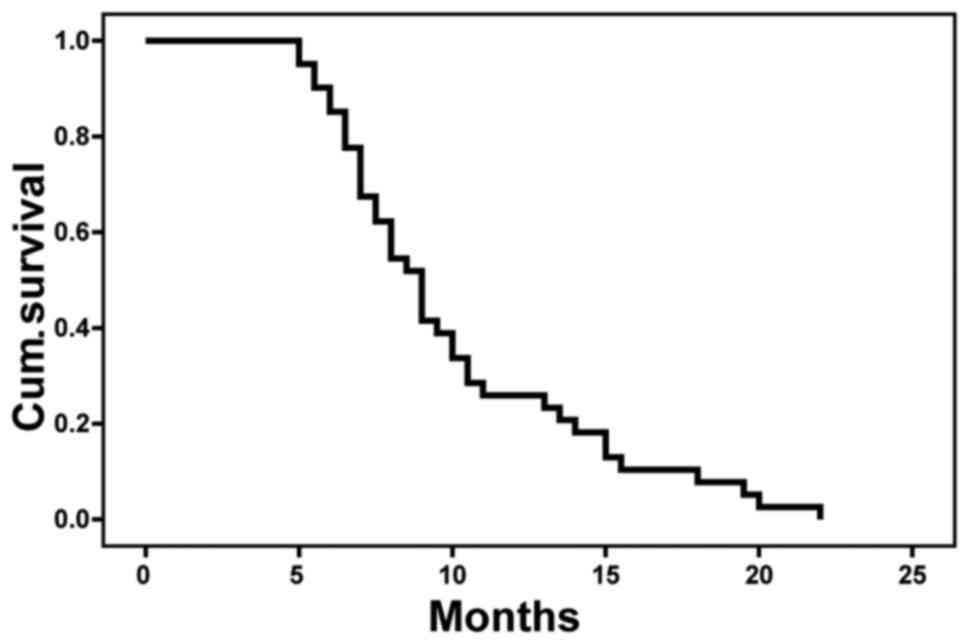
Fig. 4). Kaplan-Meier analysis showed
that the 1-year OS rate was 24.4% (Fig.
5).
 | Table II.Effects of therapy on peritoneal
dissemination. |
Table II.
Effects of therapy on peritoneal
dissemination.
| Response | Patients, n
(%) | Mean survival time
(months) | 95% confidence
interval |
|---|
| Disappeared
(complete response) | 7
(17.07) | 15.4±5.6 |
4.358–26.442 |
| Decrease of ascites
≥50% (partial response) | 23 (56.10) |
9.1±0.9 |
7.263–10.937 |
| Decrease of ascites
<50% or increase (non-partial response) | 11 (26.83) |
6.9±0.5 | 5.929–7.871 |
| Overall | 41
(100.00) |
8.5±0.7 | 7.175–9.825 |
Discussion
Peritoneal metastasis frequently occurs in patients
with recurrent gastrointestinal malignancies (19). The most serious condition that may
develop in peritoneal metastasis is PC, which has an extremely poor
prognosis (6,20,21). PC
has been estimated to be responsible for 60% of all gastric
cancer-associated mortalities, with peritoneal metastases in these
patients considered terminal (6,20,21). Therapy consists mainly of palliative
chemotherapy; long-term survival is considered poor, since systemic
chemotherapy agents are unlikely to reach cytotoxic concentrations
in peritoneal nodules (22–27).
Although IP chemotherapy may deliver high-dose
intensity treatment to the peritoneal cavity, drug penetration deep
into the peritoneal surface is limited (28). Hyperthermia treatment may augment the
penetration distance of anticancer drugs by up to 2,000 µm, as well
as altering the permeability of tumor cell membranes to enhance
uptake of chemotherapeutic drugs (28). In addition, the combination of
hyperthermic treatment and chemotherapeutic drugs, including
mitomycin C, etoposide and CDDP have shown synergistic cytotoxicity
towards cancer cells (29). Thus,
combinations of systemic and loco-regional chemotherapy may be
considered in patients with carcinomatosis from gastric cancer.
These combinations may be particularly effective for patients with
small volumes of disease and symptomatic ascites. A phase I/II
trial revealed that IP docetaxel plusS-1 was safe and effective in
patients with gastric cancer with PC (30).
The present study analyzed the effectiveness of a
combination of systemic chemotherapy (intravenous docetaxel) and
loco-regional chemotherapy (IP CDDP), with the two administered on
day 1 of every 3-week cycle. The present findings indicated that
this therapeutic protocol was feasible and useful, and achieved
satisfactory clinical outcomes. Another study reported that
palliative treatment of ascites with IP instillation of
mitoxantrone achieved an average decrease in ascites of ≥50%
(31).
In daily clinical practice, CT was used to measure
solid tumors, and the Response Evaluation Criteria in Solid Tumors
1.1 criteria (32) was used to
determine the response to treatment. Malignant ascites were
considered to be a non-evaluable lesion in the present study, since
it is difficult to detect using conventional radiological
examinations. Therefore, a simple method of estimating residual
peritoneal ascites was established, and new response criteria were
developed using B-mode ultrasound examination above the bladder in
the longitudinal section. Therapeutic outcomes were categorized as
CR, PR and nPR, allowing its use in clinical settings.
The prognosis is poor for patients with macroscopic
PC, which is responsible for ~60% of all gastric cancer-associated
mortalities (33,34). Two prospective studies assessed
outcomes in patients with PC from non-gynecologic malignancies:
Gastric, colorectal and pancreatic cancer (6,35). The
presence of ascites was associated with poor survival of patients
with gastric or pancreatic carcinoma. Analyses of factors
prognostic for survival showed that differentiation of the primary
tumor did not affect the prognosis of patients with PC. Mean and
median OS times in these patients with PC from non-gynecologic
malignancies were 6.0 and 3.1 months, respectively (6,35).
In contrast to these earlier studies, which analyzed
factors associated with survival in untreated patients, the
patients in the present study received HIPC and intravenous
chemotherapy. MST in all treated patients was 8.5±0.7 months, being
15.4±5.6 months in the CR group, 9.1±0.9 months in the PR group and
6.9±0.5 months in the nPR group, with significant differences
between each pair of groups. These results provided evidence that
the presence of ascites was associated with poor survival.
In conclusion, the present study indicated that
B-mode ultrasound was effective and feasible in evaluating
malignant ascites in patients with gastric cancer, as well as in
determining response to treatment. The present study also
demonstrated that the combination of HIPC and intravenous
chemotherapy was effective and safe in patients with gastric cancer
with malignant ascites. The overall response rate was 73.2%, and
patients who achieved CR exhibited significantly longer survival
compared with those who achieved PR or nPR. Additional studies in
larger populations are required to evaluate the efficacy and safety
of this combination in carefully selected patients with gastric
cancer with peritoneal metastases.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 nnual report
of the JGCA nationwide registry. Gastric Cancer. 14:301–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadeghi B, Arvieux C, Glehen O, Beaujard
AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL,
Faure JL, et al: Peritoneal carcinomatosis from non-gynecologic
malignancies: Results of the EVOCAPE 1 multicentric prospective
study. Cancer. 88:358–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McQuellon RP, Loggie BW, Fleming RA,
Russell GB, Lehman AB and Rambo TD: Quality of life after
intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal
carcinomatosis. Eur J Surg Oncol. 27:65–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garofalo A, Valle M, Garcia J and
Sugarbaker PH: Laparoscopic intraperitoneal hyperthermic
chemotherapy for palliation of debilitating malignant ascites. Eur
J Surg Oncol. 32:682–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glockzin G, Ghali N, Lang SA, Agha A,
Schlitt HJ and Piso P: Peritoneal carcinomatosis. Surgical
treatment, including hyperthermal intraperitoneal chemotherapy.
Chirurg. 78(1100): 1102–1106, 1108-1110. 2007.(In German).
|
|
10
|
Roth AD, Fazio N, Stupp R, Falk S,
Bernhard J, Saletti P, Köberle D, Borner MM, Rufibach K, Maibach R,
et al: Docetaxel, cisplatin, and fluorouracil; docetaxel and
cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic
treatment for advanced gastric carcinoma: A randomized phase II
trial of the Swiss Group for Clinical Cancer Research. J Clin
Oncol. 25:3217–3223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dedrick RL, Myers CE, Bungay PM and DeVita
VT Jr: Pharmacokinetic rationale for peritoneal drug administration
in the treatment of ovarian cancer. Cancer Treat Rep. 62:1–11.
1978.PubMed/NCBI
|
|
12
|
Markman M: Intraperitoneal antineoplastic
drug delivery: Rationale and results. Lancet Oncol. 4:277–283.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Detroz B, Laurent S, Honoré P, Blaffart F,
Limet R and Meurisse M: Rationale for hyperthermic intraperitoneal
chemotherapy (HIPEC) in the treatment or prevention of peritoneal
carcinomatosis. Acta Chir Belg. 104:377–383. 2004.PubMed/NCBI
|
|
14
|
González-Moreno S, González-Bayón LA and
Ortega-Pérez G: Hyperthermic intraperitoneal chemotherapy:
Rationale and technique. World J Gastrointest Oncol. 2:68–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van der Speeten K, Stuart OA and
Sugarbaker PH: Using pharmacologic data to plan clinical treatments
for patients with peritoneal surface malignancy. Curr Drug Discov
Technol. 6:72–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan TD, Cao CQ and Munkholm-Larsen S: A
pharmacological review on intraperitoneal chemotherapy for
peritoneal malignancy. World J Gastrointest Oncol. 2:109–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tozzoli R, Basso SM, D'Aurizio F, Metus P
and Lumachi F: Evaluation of predictive value of pleural CEA in
patients with pleural effusions and histological findings: A
prospective study and literature review. Clin Biochem.
49:1227–1231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yonemura Y, Endou Y, Sasaki T, Hirano M,
Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M and Li Y:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan TD, Black D, Savady R and Sugarbaker
PH: Systematic review on the efficacy of cytoreductive surgery
combined with perioperative intraperitoneal chemotherapy for
peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol.
24:4011–4019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chua TC, Robertson G, Liauw W, Farrell R,
Yan TD and Morris DL: Intraoperative hyperthermic intraperitoneal
chemotherapy after cytoreductive surgery in ovarian cancer
peritoneal carcinomatosis: Systematic review of current results. J
Cancer Res Clin Oncol. 135:1637–1645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glehen O, Gilly FN, Arvieux C, Cotte E,
Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F and Elias
D: Association Française de Chirurgie: Peritoneal carcinomatosis
from gastric cancer: A multi-institutional study of 159 patients
treated by cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy. Ann Surg Oncol. 17:2370–2377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JY and Bae HS: A controlled clinical
study of serosa-invasive gastric carcinoma patients who underwent
surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP).
Gastric Cancer. 4:27–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glehen O, Schreiber V, Cotte E,
Sayag-Beaujard AC, Osinsky D, Freyer G, François Y, Vignal J and
Gilly FN: Cytoreductive surgery and intraperitoneal
chemohyperthermia for peritoneal carcinomatosis arising from
gastric cancer. Arch Surg. 139:20–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 Study
Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scaringi S, Kianmanesh R, Sabate JM,
Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM, Flamant Y and
Msika S: Advanced gastric cancer with or without peritoneal
carcinomatosis treated with hyperthermic intraperitoneal
chemotherapy: A single western center experience. Eur J Surg Oncol.
34:1246–1252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yonemura Y, Endou Y, Shinbo M, Sasaki T,
Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Mizuno M, et
al: Safety and efficacy of bidirectional chemotherapy for treatment
of patients with peritoneal dissemination from gastric cancer:
Selection for cytoreductive surgery. J Surg Oncol. 100:311–316.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Los G, van Vugt MJ and Pinedo HM: Response
of peritoneal solid tumours after intraperitoneal chemohyperthermia
treatment with cisplatin or carboplatin. Br J Cancer. 69:235–241.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yonemura Y: Hyperthermo-chemotherapy for
treatment of peritoneal disseminationPeritoneal Dissemination.
Yonemura Y: Kanazawa: Maeda Shoten; pp. 237–260. 1998
|
|
30
|
Fushida S, Kinoshita J, Kaji M, Hirono Y,
Goda F, Yagi Y, Oyama K, Sudo Y, Watanabe Y and Fujimura T: Society
for Study of Peritoneal Carcinomatosis in Gastric Cancer: Phase
I/II study of intraperitoneal docetaxel plus S-1 for the gastric
cancer patients with peritoneal carcinomatosis. Cancer Chemother
Pharmacol. 71:1265–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Link KH, Roitman M, Holtappels M,
Runnebaum I, Urbanzyk H, Leder G and Staib L: Intraperitoneal
chemotherapy with mitoxantrone in malignant ascites. Surg Oncol
Clin N Am. 12:865–872, xvi-xvii. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bando E, Yonemura Y, Takeshita Y,
Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T,
Nishimura G and Miwa K: Intraoperative lavage for cytological
examination in 1,297 patients with gastric carcinoma. Am J Surg.
178:256–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugarbaker PH and Yonemura Y: Clinical
pathway for the management of resectable gastric cancer with
peritoneal seeding: Best palliation with a ray of hope for cure.
Oncology. 58:96–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in nongynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989. View Article : Google Scholar : PubMed/NCBI
|