Introduction
Nasopharyngeal carcinoma (NPC) is a cancer that
arises from the epithelium of nasopharynx. This cancer is uncommon
in Europe and America, and its incidence is higher in Africa and
Southeast Asia (1). Its unique
epidemiology, pathogenesis and association with the Epstein-Barr
virus make it distinct from other types of head and neck cancer.
Although NPC is reported to respond well to radiation therapy,
chemotherapy also has a function in the current treatment protocol
(2). For cases that have spread
beyond the nasopharynx (i.e., cancer stages II, III, IVA and IVB),
the addition of chemotherapy is common. Typically, platinum based
agents, including cisplatin or carboplatin are used to treat NPC
alongside fluorouracil (3). However,
these medications also harm normal cells, causing side effects that
include a decrease in white blood cells, anemia, kidney toxicity
and nausea.
Apoptosis, the process of programmed cell death, is
a crucial self-defense mechanism for the human body to counteract
cancerous cells. A number of existing chemotherapeutic agents aim
to initiate the apoptosis cascade for an anti-tumor effect
(4,5).
However, as the disease progresses, certain cancer cell populations
become resistant to chemotherapeutic agents and the efficacy of
chemotherapy gradually deteriorates. Therefore, developing novel
chemotherapeutic agents against NPC is crucial.
Tripterygium wilfordii, also known as thunder
god vine, is an herb traditionally used in Asia to treat various
diseases, including tissue inflammation, arthritis, certain
rheumatoid diseases, and neurodegenerative diseases (6). Previous studies have revealed that
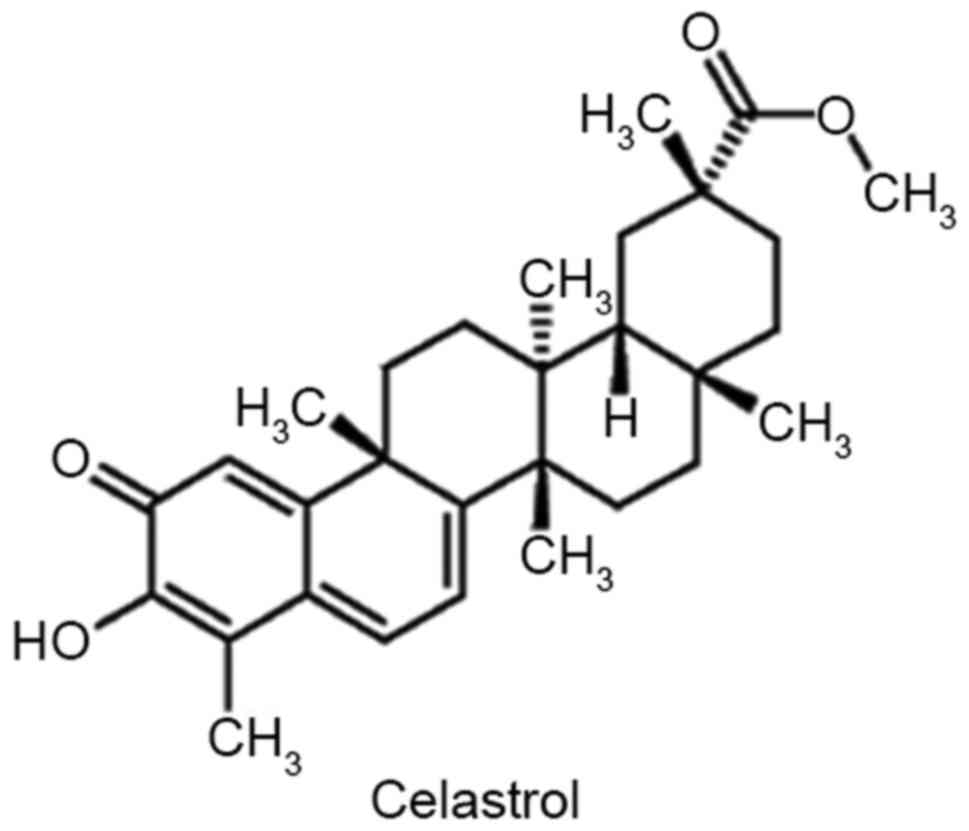
celastrol, a bioactive isolate from the root of T.
wilfordii, has potential as a cancer treatment. The chemical
structure of celastrol is depicted in Fig. 1. Several studies have produced
promising results using celastrol against various cancer cell lines
in vitro, including gastric cancer (7), prostate cancer (8), hepatocellular carcinoma (9), breast cancer (10), esophageal cancer (11), lung cancer (12) and osteosarcoma (13). However, the therapeutic effects of
celastrol on nasopharyngeal cancer have yet to been studied
thoroughly. As natural products have been increasingly utilized in
chemotherapy, the present study aimed to assess the cytotoxic
effect of celastrol on NPC cells, identify the mechanism for the
effect, and therefore, evaluate the potential for the utilization
of this traditional herb as a novel agent against NPC.
Materials and methods
Reagents
Celastrol was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) as ≥98% powder. Stock
solutions of celastrol were prepared with concentrations of 1, 2
and 4 mM in DMSO, and stored at −20°C. The final concentration of
DMSO for all treatments was consistently <0.1%. MTT, U0126,
SB203580 and DAPI reagents were obtained from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). Antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
The human NPC cell lines HONE-1 and NPC-039 were
purchased from American Type Culture Collection (Manassas, VA,
USA), and cultured in Gibco RPMI-1640 Medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 0.1 mM non-essential amino acids, 1 mM glutamine, 1%
penicillin/streptomycin, 1.5 g/l sodium bicarbonate, and 1 mM
sodium pyruvate (Sigma-Aldrich; Merck KGaA). The cell cultures were
maintained at 37°C in a humidified atmosphere of 5%
CO2.
In vitro cytotoxicity MTT assay
The influence of celastrol on cell growth was
assayed by the MTT method. A total of 5×104 HONE-1 and
NPC-039 cells were seeded in each well of 24-well plates. Celastrol
(0, 1, 2 or 4 µM) was then added to the culture media and incubated
at 37°C for 24 h. Following celastrol treatment, MTT was added to
each well to a 0.5 mg/ml final concentration, and the cells were
incubated for a further 4 h. The viable cell number was directly
proportional to the production of formazan following solubilization
with isopropanol, as detected by the optical density at 595 nm.
Cell cycle analysis
To determine the effect of celastrol on the cell
cycle, 1×106 cells were cultured in 1 ml serum-free
RPMI-1640 medium for 18 h to induce starvation, and then incubated
with 1, 2 or 4 µM celastrol at 37°C for 24 h. Following the
harvesting of cells by centrifugation (300 × g at room temperature
for 5 min), washing in PBS and fixing with 70% ethanol overnight,
they were incubated for 30 min in the dark at room temperature,
with propidium iodide (PI) buffer (4 g/ml PI, 1% Triton X-100, 0.5
mg/ml RNase A (Affymetrix, Inc., Santa Clara, CA, USA) in PBS). The
cells were filtered with a Falcon 40 µm nylon cell strainer
(Corning Incorporated, Corning, NY, USA). The cell cycle
distribution of 3,000 cells was analyzed by Muse Cell Analyzer flow
cytometry (EMD Millipore, Billerica, MA, USA) and analysis data by
Muse Cell Soft V1.4.0.0 Analyzer Assays (EMD Millipore).
Annexin V/PI double staining
To detect apoptosis in cells following celastrol
exposure, a Muse Annexin V and Dead Cell Assay kit was used to
quantify the number of cells at each stage of cell death (EMD
Millipore). Briefly, 1×105 cells were suspended in 100
µl Muse reagent. Following incubation for 20 min at room
temperature, the cell suspension was gently mixed and analyzed by
the Muse Cell Analyzer flow cytometer (EMD Millipore). The data was
processed with Muse Cell Soft V1.4.0.0 Analyzer Assays (EMD
Millipore).
DAPI staining
Cells (3×105) that had been incubated
with 0, 1, 2 or 4 µM celastrol at 37°C for 24 h were fixed with 4%
paraformaldehyde for 15 min at room temperature, then at 4°C
overnight. The plates were washed twice with PBS and the cell
nuclei from the plates were stained with 100 ng/ml DAPI for 15 min
in the dark. Following 3 washes with tap water, the cells were
examined under a fluorescence microscope. Cells that appeared to
exhibit a rough surface and darkly-stained nuclei with fragmented
chromosomes were considered to be apoptotic.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (EMD Millipore) containing protease inhibitor cocktail and
phosphatase inhibitor cocktail (EMD Millipore). Cells were
harvested by centrifugation (12,000 × g, 4°C, 10 min). Protein
concentration was determined by the Pierce bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). Cell lysates
(total 20 µg) were separated on a 10 or 15% polyacrylamide gel and
transferred onto a PVDF membrane (EMD Millipore). The blot was
subsequently incubated with 3–5% skimmed milk in PBS at room
temperature for 1 h to block non-specific binding, and probed with
the following antibodies: Cleaved caspase-3 (cat. no. 9664;
dilution, 1:1,000); cleaved caspase-8 (cat. no. 9496; dilution,
1:1,000); cleaved caspase-9 (cat. no. 9505; dilution, 1:1,000);
poly (ADP-ribose) polymerase (PARP) (cat. no. 556494; dilution,
1:1,000); cyclin A (cat. no. 4656; 1:1,000); cyclin B (cat. no.
12231; 1:1,000); cyclin-dependent kinase (CDK)1 (cat. no. 9116;
dilution, 1:1,000); CDK2 (cat. no. 2546; dilution, 1:1,000); CDK
inhibitor 1A (p21Cip; cat. no, 2947; dilution, 1:1,000);
CDK inhibitor 1B (p27Kip; cat. no. 3686; dilution,
1:1,000); Bcl-2 (cat. no. 2870; dilution, 1:1,000); B-cell
lymphoma-extra-large (Bcl-xL; cat. no. 2764; dilution, 1:1,000);
Bcl-2 associated X, apoptosis regulator (Bax; cat. no. 5023;
dilution, 1:1,000); Bcl-2 antagonist/killer 1 (Bak; cat. no. 12105;
dilution, 1:1,000); Bcl-2-like 11 (Bim; cat. no. 2933; dilution,
1:1,000); BH3-interacting death agonist (Bid; cat. no. 2002;
dilution, 1:1,000); Fas (cat. no. 4233; dilution, 1:1,000),
Fas-associated via death domain (FADD; cat. no. 2782; dilution,
1:1,000); TNFRSF1A-associated via death domain (TRADD; cat. no.
3684; dilution, 1:1,000); TNF receptor superfamily member 1A
(TNF-R1; cat. no. 3736; dilution, 1:1,000); TNF receptor
superfamily member 10b (DR5; cat. no. 8074; dilution, 1:1,000);
protein kinase B (AKT; cat. no. 4298; dilution, 1:1,000);
phosphorylated (p-)AKT (cat. no. 4060; dilution, 1:1,000);
mitogen-activated protein kinase 14 (p38; cat. no. 9212; dilution,
1:1,000); p-p38 (cat. no. 9211; dilution, 1:1,000); extracellular
signal-regulated kinase 1/2 (ERK1/2; cat. no. 4695; dilution,
1:1,000); p-ERK1/2 (cat. no. 4370; dilution, 1:1,000); c-Jun
N-terminal kinase 1/2 (JNK1/2; cat. no. 9258; dilution, 1:1,000);
p-JNK1/2 (cat. no. 4668; dilution, 1:1,000); and β-actin (cat. no.
NB600-501; dilution, 1:5,000). Membranes were incubated with
primary antibodies overnight at 4°C, and then with an appropriate
peroxidase-conjugated secondary antibody at room temperature for 1
h [anti-mouse Ig; dilution, 1:3,000 (cat. no. 7076); anti-rabbit
IgG; dilution, 1:3,000 (cat. no. 7074)]. Following the final wash,
the immunoreactive signal was detected with an enhanced
chemiluminescence detection system (WBKLS0500; EMD Millipore), and
the relative density was quantitated with gel documentation and
analysis (AlphaImager 2000; Alpha Innotech Corporation, San
Leandro, CA, USA).
Mitochondrial membrane potential (Δψm)
measurement
The reduction in Δψm was detected with a Muse
Mitopotential Assay kit (EMD Millipore). Briefly, 1×105
cells which had been treated with celastrol were washed with PBS.
The cells were suspended in Muse MitoPotential working solution and
incubated at 37°C for 20 min. Following incubation, 5 µl Muse 7-AAD
was added, and incubated at room temperature for 5 min. The
reaction volume was thoroughly mixed, run on Muse Cell Analyzer
flow cytometry (EMD Millipore), and analyzed by Muse Cell Soft
V1.4.0.0 Analyzer Assays (EMD Millipore).
Statistical analysis
All values included represent the mean ± standard
deviation of three repetitions per procedure. Statistical analyses
of >3 groups were performed with a one-way analysis of variance
test followed by Tukey's post hoc test. Comparisons between two
groups were performed with a Student's t-test. Statistical tests
were performed using SigmaStat 2.0 software (Systat Software, Inc.,
San Jose, CA, USA). P<0.05 was considered to represent a
statistically significant difference.
Results
Celastrol has cytotoxic effects on NPC
cell lines
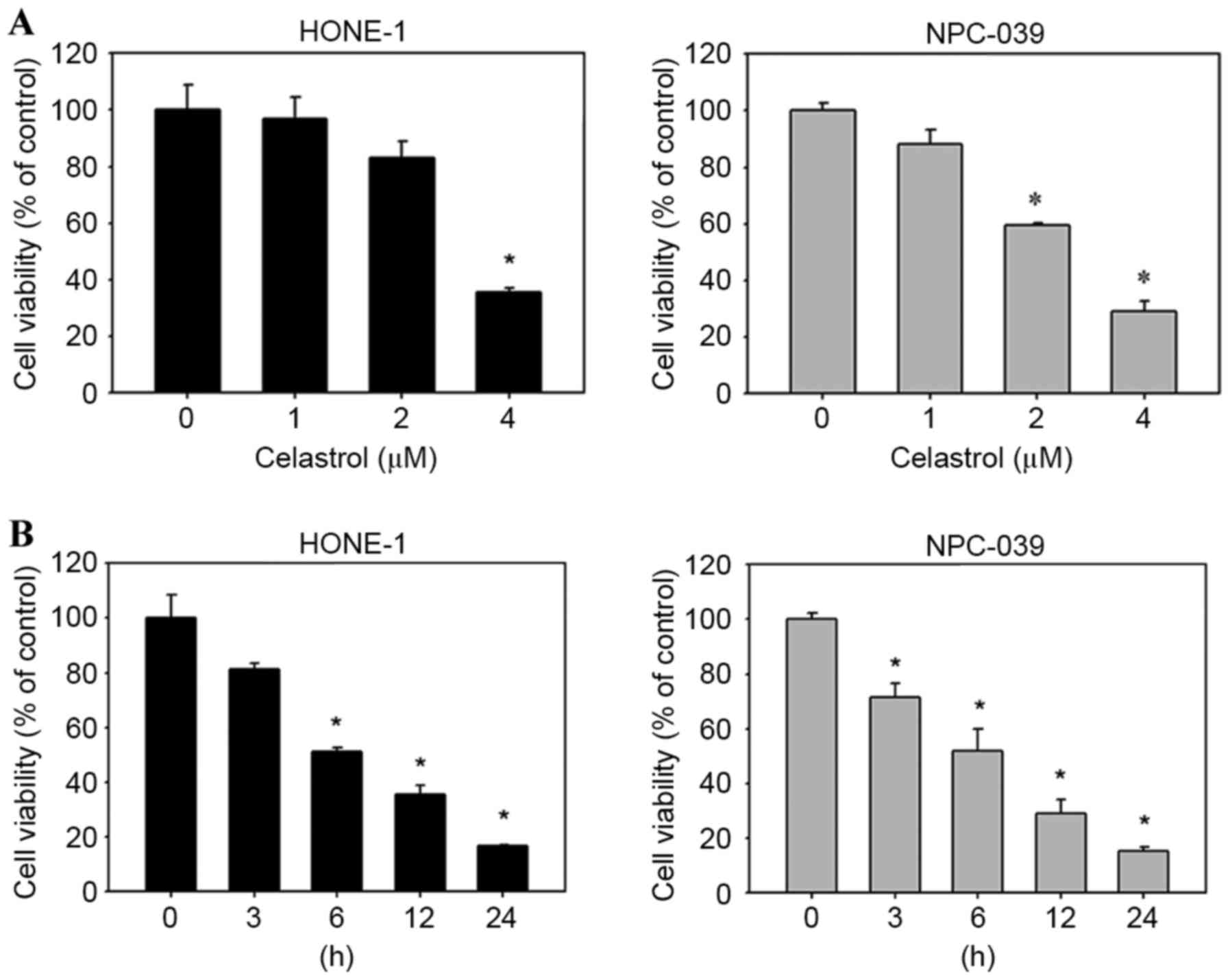
To evaluate the effects of celastrol on cell
viability, HONE-1 and NPC-039 cells were treated with celastrol for
varying concentrations and durations, and viability was assessed
with an MTT assay. The result demonstrated that celastrol decreased
the viability of HONE-1 and NPC-039 cells, compared with untreated
cells, in a dose-(Fig. 2A) and
time-(Fig. 2B) dependent manner.
Celastrol induces cell cycle arrest
and apoptosis in human NPC cell lines
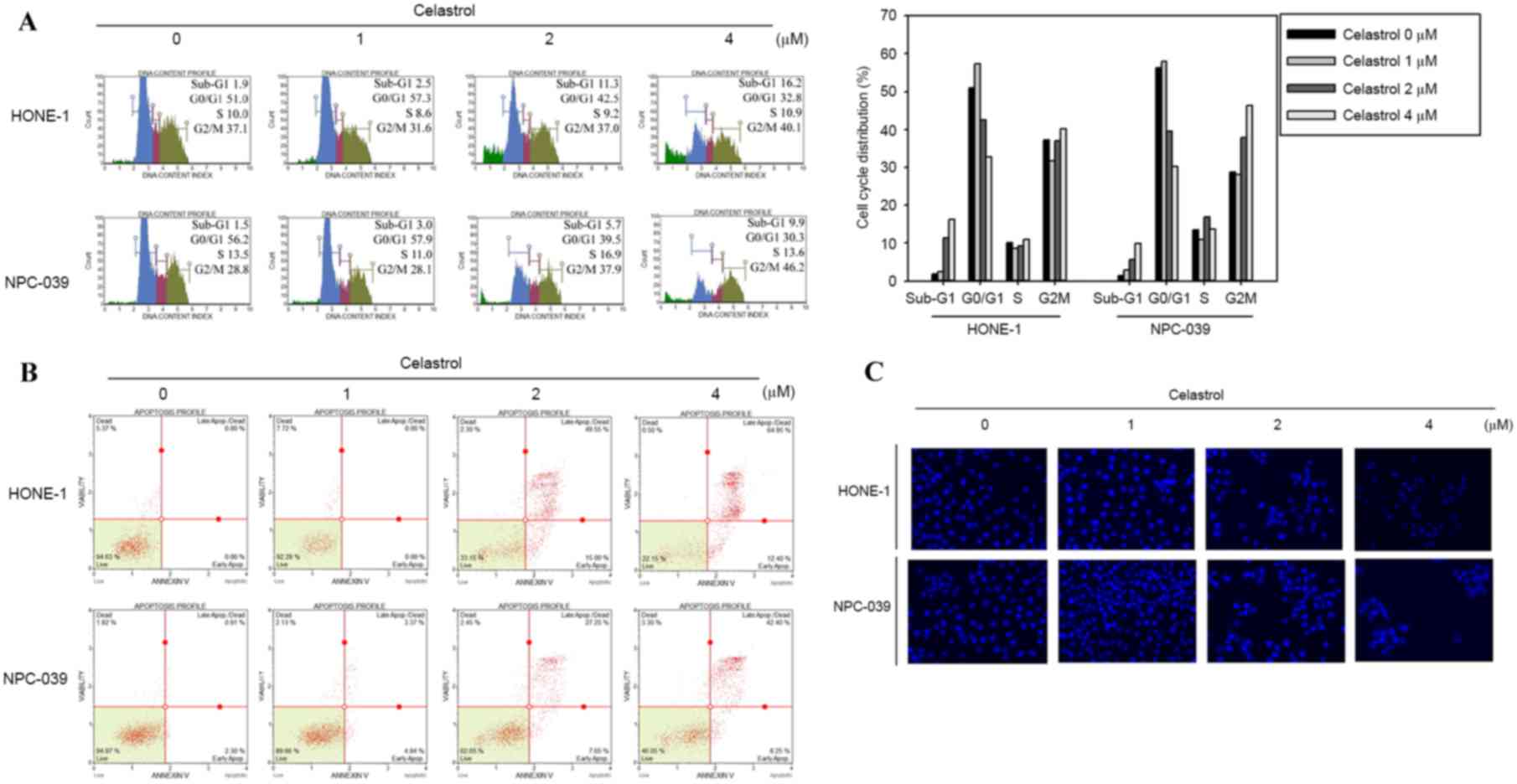
To determine whether the inhibitory effect on cell
viability produced by celastrol was associated with the induction
of apoptosis, HONE-1 and NPC-039 cells were treated with 0, 1, 2 or
4 µM of celastrol for 24 h and analyzed for cell cycle distribution
analysis using flow cytometry. There was an accumulation of sub-G1
phase cells in HONE-1 cells (Fig.
3A). In NPC-039 cells, the treatment induced an increase of
cells in the Sub-G1 and G2/M phases compared with the untreated
control, in a dose dependent manner (Fig.
3A). The appearance of a sub-G1 population indicated apoptotic
cells. These results suggested that the reduction in cell viability
associated with celastrol may involve the induction of G2/M phase
arrest and apoptosis. To further verify whether celastrol-induced
cell death was associated with apoptosis, Annexin V/PI double
staining was performed to quantify the level of apoptosis. A
dose-dependent increase in apoptotic cells following 24 h of
celastrol treatment was demonstrated for HONE-1 and NPC-039 cells
(Fig. 3B). In addition, DAPI staining
was applied to observe changes in nuclear morphology. In the two
cell types, the condensed and fragmented nuclei in the treated
cells was smaller compared with the control, which was observed
following 24 h of 4 µM celastrol treatment (Fig. 3C).
Celastrol inhibits the cyclin-CDK
checkpoint and induces activation of caspase-3, −8 and −9 in HONE-1
cells
To clarify whether caspase activation occurred in
celastrol-induced apoptosis, apoptosis-associated molecules were
examined with western blot analysis. Following treatment with
celastrol at doses of 1–4 µM for 24 h, the levels of cleaved
fragments of caspases-3, −8, and −9 and PARP increased in HONE-1
cells vs. untreated cells (Fig. 4A).
A celastrol treatment of 4 µM for 24 h significantly increased the
level of cleaved caspases-3, −8, −9 and PARP, by 52, 72, 30 and
42%, respectively, compared with the control (Fig. 4B). In addition, celastrol treatment
significantly decreased the level of cyclin A and B and CDK1 and 2,
and increased p21Cip and p27Kip levels,
compared with the control (Fig. 4C and
D).
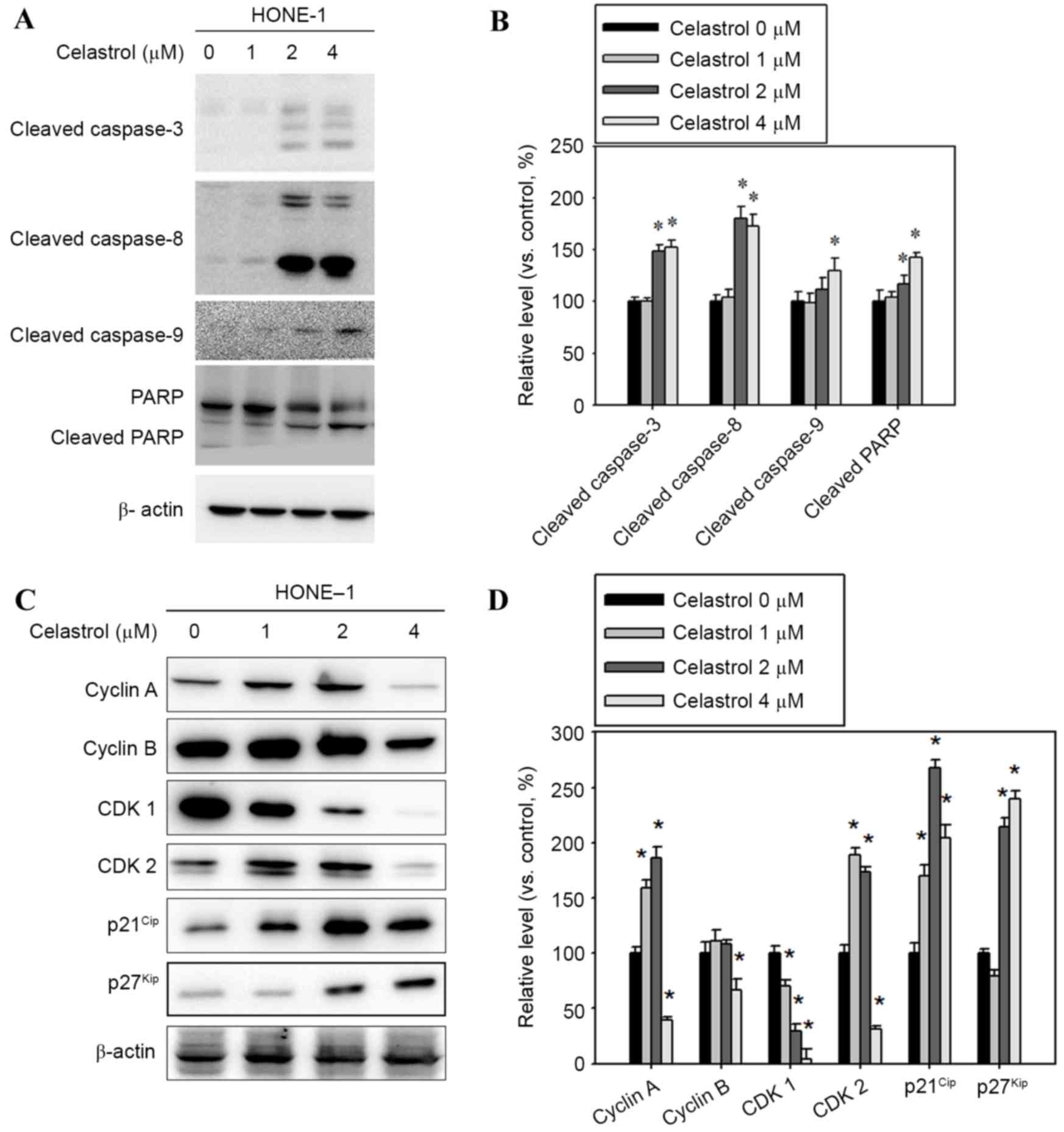 | Figure 4.Effect of celastrol on cell
cycle-associated proteins, and caspase and PARP cleavage. Cells
were treated with celastrol, at doses of 1, 2 or 4 µM, for 24 h.
(A) Western blot analysis detected an increase in cleaved
caspase-3, −8, −9 and PARP levels. (B) The western blot was
quantified, with detected protein levels normalized to β-actin
using a densitometer. (C) Western blot analysis was used to detect
the expression level change in cyclin A, cyclin B, CDK1, CDK2,
p21Cip and p27Kip (D) The western blot was
quantified, with detected protein levels normalized to β-actin with
a densitometer. *P<0.05 vs. 0 µM control. PARP, poly
(ADP-ribose) polymerase 1; CDK, cyclin-dependent kinase;
p21Cip, cyclin-dependent kinase inhibitor 1A;
p27Kip, cyclin-dependent kinase inhibitor 1B. |
Bcl-2 family expression is altered in
celastrol-treatment HONE-1 cells
Bcl-2 family proteins are associated with the
regulation of apoptosis, therefore, western blot analysis was
performed to determine whether Bcl-2 family members were involved
in the process of apoptosis that was previously observed. The
expression levels of the pro-apoptotic proteins Bax, Bak, t-Bid and
BimS were increased, whereas the anti-apoptotic Bcl-2
and Bcl-xL decreased (Fig. 5A).
Celastrol treatment at 4 µM for 24 h significantly increased the
expression levels of Bax, Bak, t-Bid and BimS by 24,
135, 93 and 23% respectively, compared with the control (Fig. 5B). The 4 µM celastrol treatment also
significantly decreased the expression levels of Bcl-2 and Bcl-xL
by 30 and 35%, respectively, compared with the control (Fig. 5B).
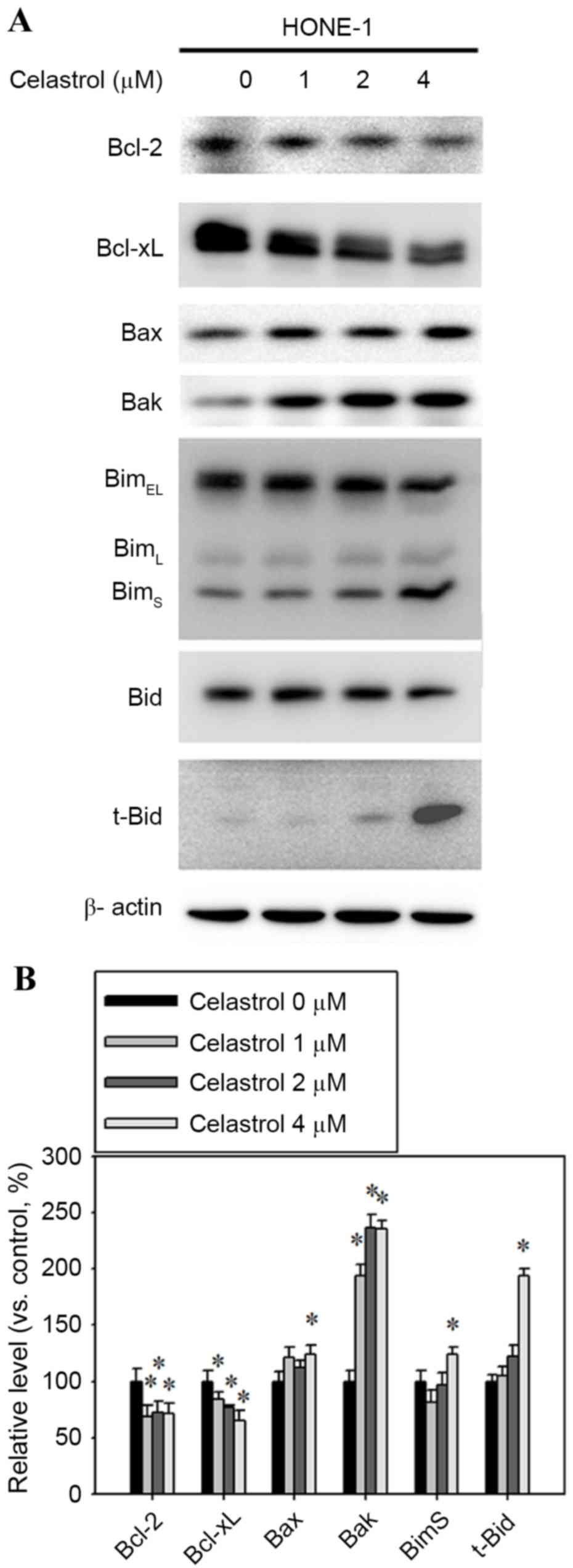 | Figure 5.Effect of celastrol on the expression
of Bcl-2, Bcl-xL, Bax, Bak, Bim and t-Bid. Cells were treated with
celastrol, at doses of 1, 2 or 4 µM, for 24 h. (A) Western blot
analysis detected the change in the expression level of Bcl-2,
Bcl-xL, Bax, Bak, Bim and t-Bid. (B) The western blot was
quantified, with detected protein levels normalized to β-actin with
a densitometer. *P<0.05 vs. 0 µM control. Bcl-xL, B-cell
lymphoma-extra large; Bax, Bcl-2 associated X, apoptosis regulator;
Bak, Bcl-2 antagonist/killer 1; Bid, BH3-interacting death agonist;
t-, truncated; BimEL, Bcl-2-like 11 isoform EL;
BimL, Bcl-2-like 11 isoform L; BimS,
Bcl-2-like 11 isoform S. |
Celastrol-induced apoptosis is
specifically mediated by the mitochondrial and Fas-mediated
pathways
To determine the molecular mechanism by which
celastrol induced the apoptosis of HONE-1 cells, the mitochondrial
membrane potential and the protein levels of death receptor
proteins were assessed. The mitochondrial membrane potential was
reduced in celastrol-treated HONE-1 cells (Fig. 6A). In addition, celastrol also
increased Fas, FADD, TRADD, TNF-R1 and DR5 expression, as assessed
with a western blot analysis (Fig.
6B). A 24 h, 4 µM celastrol treatment significantly increased
the expression levels of Fas, FADD, TRADD, TNF-R1 and DR5 by 39,
51, 103, 126 and 88%, respectively, compared with the control
(Fig. 6C).
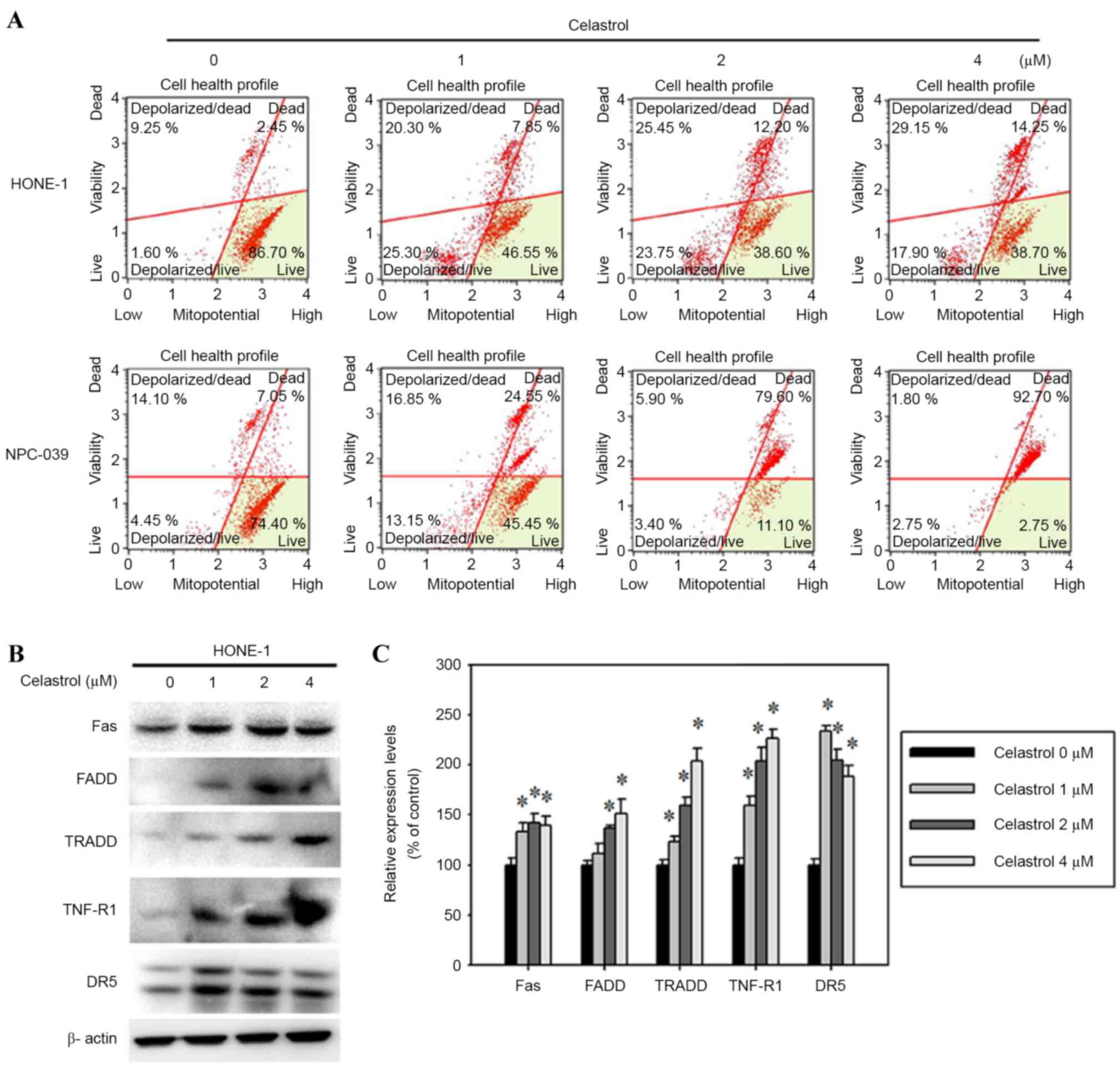 | Figure 6.Celastrol induces cell apoptosis by
the death receptor and mitochondrial pathways. Cells were treated
with celastrol, at doses of 1, 2 or 4 µM, for 24 h. (A) The
mitochondrial membrane potential was analyzed with flow cytometry.
(B) Western blot analysis detected the change in the expression
level of Fas, FADD, TRADD, TNF-R1 and DR5. (C) The western blot was
quantified, with detected protein levels normalized to β-actin
using a densitometer. *P<0.05 vs. 0 µM control. FADD, Fas
associated via death domain; TRADD, TNFRSF1A associated via death
domain; TNF-R1, TNF receptor superfamily member 1A; DR5, TNF
receptor superfamily member 10B. |
Celastrol increases JNK1/2 activation
and decreases ERK1/2 and p38 activation
The mitogen-activated protein kinase (MAPK)
signaling pathway is involved in the induction of apoptosis by
chemotherapeutic drugs (14). Western
blot analysis was used to assess whether MAPKs were activated
following celastrol treatment of HONE-1 cells. The relative
phosphorylation level of JNK1/2 was increased and the
phosphorylation of p38 and ERK1/2 was decreased, but AKT
phosphorylation was not significantly altered (Fig. 7A). Celastrol treatment significantly
increased the extent of JNK1/2 phosphorylation; phosphorylation was
increased by 34% compared with the control (Fig. 7B). In addition, celastrol treatment
significantly decreased the levels of ERK1/2 and p38
phosphorylation by 52 and 82%, respectively, compared with the
control (Fig. 7B). Celastrol combined
with an ERK1/2 inhibitor (U0126) or p38 inhibitor (SB203580)
increased the rate of apoptosis in HONE-1 cells, compared with
celastrol alone (Fig. 7C).
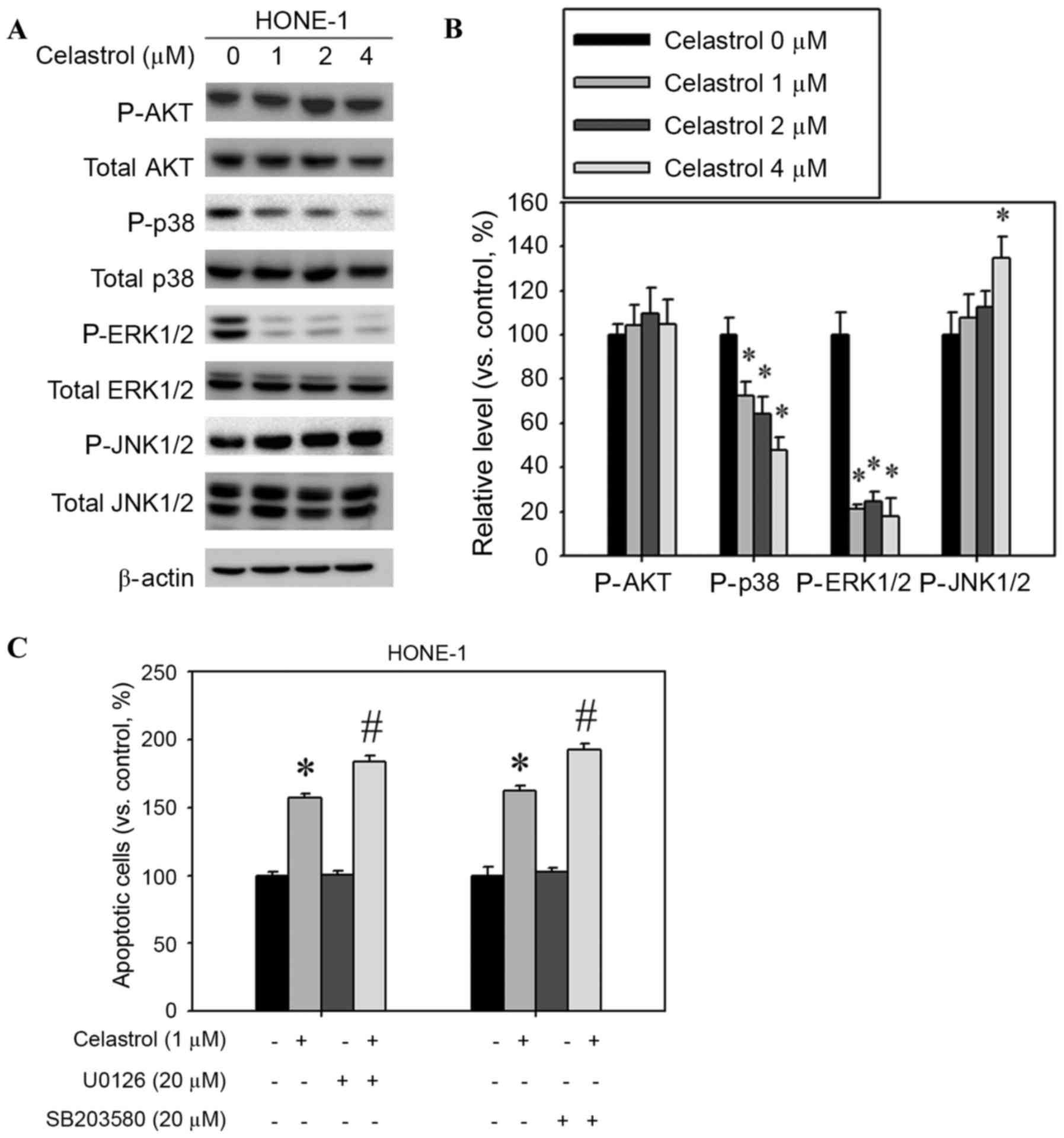 | Figure 7.Effect of celastrol on the expression
of mitogen-activated protein kinase pathway components. Cells were
treated with celastrol, at doses of 1, 2 or 4 µM, for 24 h. (A)
Western blot analysis detected the change in AKT, p38, ERK1/2 and
JNK1/2 phosphorylation. (B) The western blot was quantified, with
detected protein levels normalized to β-actin with a densitometer.
(C) Cells were treated with a combination of celastrol (1 µM) and
U0126 (20 µM) or SB203580 (20 µM) for 24 h. The number of apoptotic
cells was detected with flow cytometry. *P<0.05 vs. 0 µM
control. #P<0.05 vs. celastrol-only group. AKT,
protein kinase B; p38, mitogen-activated protein kinase 14; ERK1/2,
extracellular signal-regulated kinase 1/2; JNK1/2, c-Jun N-terminal
kinase 1/2. |
Discussion
Celastrol is a bioactive isolate from T.
wilfordii. Multiple in vitro studies have demonstrated
that celastrol exhibits promising results against several types of
cancer, including lung cancer, esophageal cancer, osteosarcoma,
prostate cancer, breast cancer, hepatocellular carcinoma, and
gastric cancer (7–9). However, the effect of celastrol on NPC
cells remains uncertain. The purpose of the present study was to
investigate the effect of celastrol on human NPC cells, and to
identify the mechanisms underlying the effect. The findings
revealed that celastrol may inhibit cancer cell viability, delay
cell cycle progression and induce apoptosis. The viability of NPC
cells was decreased in a dose-dependent manner. Apoptosis was
induced in NPC-039 and HONE-1 cells following 24 h of celastrol
treatment, and the extent of apoptosis was dose-dependent.
Cell cycle arrest, which is induced by a number of
novel chemotherapeutic medications, is a potential strategy to
hinder the proliferation of cancer cells (13). During cell cycle arrest, cells may
initiate self-repairing mechanisms or enter the apoptosis cascade.
A number of studies have previously identified that celastrol may
induce cell cycle arrest. A study by Peng et al (15) demonstrated that celastrol arrested the
human monocytic leukemia cell line U937 in G0/G1. Another study, by
Rajendran et al (16),
revealed that celastrol causes the accumulation of human
hepatocellular carcinoma cells in the sub-G1 phase of the cell
cycle. Furthermore, G2/M arrest has been identified in human
cervical carcinoma and prostate cancer cells (15,17). The
present study demonstrated that celastrol treatment caused the
accumulation of HONE-1 and NPC-039 cells in the sub-G1 and G2/M
phases. Therefore, celastrol may cause cell cycle arrest and prompt
apoptosis in NPC cells.
Celastrol treatment (4 µM) significantly decreased
the expression levels of Bcl-2 and Bcl-xL compared with the control
(Fig. 5B). Members of the Bcl-2
family regulate the intrinsic mitochondrial mediated apoptotic
pathway (18). The Bcl-2 family
includes pro-apoptotic and anti-apoptotic members. Pro-apoptotic
Bcl-2-like proteins, including Bax, promote apoptosis by opening
the mitochondrial voltage-dependent anion channel to cause
permeabilization of the mitochondrial outer membrane and the
release of other pro-apoptotic factors (19). In the present study, celastrol
treatment increased Fas, FADD, TRADD, TNF-R1 and DR5 expression and
reduced the mitochondrial membrane potential (Fig. 6).
Caspases are also involved in mediating apoptotic
processes. Western blot analysis revealed that the administration
of celastrol led to increased expression levels of cleaved
caspase-9, −8 and −3 and the cleavage of PARP in HONE-1 cells. A
previous study has identified that celastrol treatment resulted in
a significant activation of p38 and ERK and a marginal activation
of stress-activated protein kinases/JNK in a dose- and
time-dependent manner (17). Zhu
et al (20) hypothesized that
DR4 and DR5 are involved in the sensitization of celastrol-treated
cells to TRAIL/Apo-2L-induced apoptosis, in a p38-independent
manner. In addition, Choi et al (21) observed that celastrol reduced the
extent of rotenone-induced generation of reactive oxygen species
and mitochondrial membrane potential loss by inhibiting p38
activation in the SH-SY5Y human neuroblastoma cell line. In the
present study, celastrol treatment significantly decreased the
phosphorylation of p38 and ERK1/2, however, the level of JNK1/2
phosphorylation was increased (Fig.
7).
In conclusion, the results of the present study
demonstrated that celastrol inhibited the viability of NPC cells,
caused G2/M cycle arrest and the accumulation of cells in the
sub-G1 phase, and induced apoptosis. Therefore, it is possible to
hypothesize that celastrol may be a promising candidate in the
development of drugs against NPC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: Phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bensouda Y, Kaikani W, Ahbeddou N, Rahhali
R, Jabri M, Mrabti H, Boussen H and Errihani H: Treatment for
metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head
Neck Dis. 128:79–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji N, Li J, Wei Z, Kong F, Jin H, Chen X,
Li Y and Deng Y: Effect of celastrol on growth inhibition of
prostate cancer cells through the regulation of hERG channel in
vitro. Biomed Res Int. 2015:3084752015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH
and Chun KH: Celastrol inhibits gastric cancer growth by induction
of apoptosis and autophagy. BMB Rep. 47:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li PP, He W, Yuan PF, Song SS, Lu JT and
Wei W: Celastrol induces mitochondria-mediated apoptosis in
hepatocellular carcinoma Bel-7402 cells. Am J Chin Med. 43:137–148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shrivastava S, Jeengar MK, Reddy VS, Reddy
GB and Naidu VG: Anticancer effect of celastrol on human triple
negative breast cancer: Possible involvement of oxidative stress,
mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol
Pathol. 98:313–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J and Wu CL: Anti-metastasis of
celastrol on esophageal cancer cells and its mechanism. Sheng Li
Xue Bao. 67:341–347. 2015.(In Chinese). PubMed/NCBI
|
|
12
|
Xu J, Wu CL and Huang J: Effect of
celastrol in inhibiting metastasis of lung cancer cells by
influencing Akt signaling pathway and expressing integrins.
Zhongguo Zhong Yao Za Zhi. 40:1129–1133. 2015.(In Chinese).
PubMed/NCBI
|
|
13
|
Yu X, Zhou X, Fu C, Wang Q, Nie T, Zou F,
Guo R, Liu H, Zhang B and Dai M: Celastrol induces apoptosis of
human osteosarcoma cells via the mitochondrial apoptotic pathway.
Oncol Rep. 34:1129–1136. 2015.PubMed/NCBI
|
|
14
|
Chen T and Wong YS: Selenocystine induces
S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7
cells by modulating ERK and Akt phosphorylation. J Agric Food Chem.
56:10574–10581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng B, Xu L, Cao F, Wei T, Yang C, Uzan G
and Zhang D: HSP90 inhibitor, celastrol, arrests human monocytic
leukemia cell U937 at G0/G1 in thiol-containing agents reversible
way. Mol Cancer. 9:792010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajendran P, Li F, Shanmugam MK, Kannaiyan
R, Goh JN, Wong KF, Wang W, Khin E, Tergaonkar V, Kumar AP, et al:
Celastrol suppresses growth and induces apoptosis of human
hepatocellular carcinoma through the modulation of STAT3/JAK2
signaling cascade in vitro and in vivo. Cancer Prev Res (Phila).
5:631–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang WB, Feng LX, Yue QX, Wu WY, Guan SH,
Jiang BH, Yang M, Liu X and Guo DA: Paraptosis accompanied by
autophagy and apoptosis was induced by celastrol, a natural
compound with influence on proteasome, ER stress and Hsp90. J Cell
Physiol. 227:2196–2206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anilkumar U and Prehn JH: Anti-apoptotic
BCL-2 family proteins in acute neural injury. Front Cell Neurosci.
8:2812014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Primikyri A, Chatziathanasiadou MV, Karali
E, Kostaras E, Mantzaris MD, Hatzimichael E, Shin JS, Chi SW,
Briasoulis E, Kolettas E, et al: Direct binding of Bcl-2 family
proteins by quercetin triggers its pro-apoptotic activity. ACS Chem
Biol. 9:2737–2741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H, Liu XW, Ding WJ, Xu DQ, Zhao YC, Lu
W, He QJ and Yang B: Up-regulation of death receptor 4 and 5 by
celastrol enhances the anti-cancer activity of TRAIL/Apo-2L. Cancer
Lett. 297:155–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi BS, Kim H, Lee HJ, Sapkota K, Park
SE, Kim S and Kim SJ: Celastrol from ‘Thunder God Vine’ protects
SH-SY5Y cells through the preservation of mitochondrial function
and inhibition of p38 MAPK in a rotenone model of Parkinson's
disease. Neurochem Res. 39:84–96. 2014. View Article : Google Scholar : PubMed/NCBI
|