Introduction
Metastatic deposits from carcinomas are by far the
most common types of malignant tumor affecting the skeleton
(1). The majority of metastases
originate from common types of cancer such as breast, lung,
prostate, kidney and thyroid gland carcinomas, which account for
93% of all deposits (2). The most
prominent symptoms are pain, with or without swelling, and symptoms
associated with pathological fracture. Patients with bone
metastasis typically exhibit poor general medical condition and
bone metastasis usually heralds incurability, therefore treatment
is usually palliative. We have previously performed curettage and
packing with bone cement without resection for metastatic bone
tumors to maintain physical activity in patients (3,4). However,
complications of local recurrence and subsequent osteolysis
persisted. Tumor-associated osteolysis precipitates the development
of pathological fractures and causes loosening of previously placed
devices for internal fixation of pathological fractures (5–7).
Previously, the prognosis of patients with bone metastases has
improved through the development of effective adjuvant or
neoadjuvant chemotherapy regimens. With improved prognosis, local
control of metastasis has become increasingly important for the
quality of life of the patient.
Bisphosphonates (BPs) are effective inhibitors of
bone resorption and have been used in the treatment of metabolic
bone diseases and metastatic bone tumors (8). Nitrogen-containing BPs (N-BPs), also
termed the second- and third-generation BPs, induce apoptosis in
osteoclasts by inhibiting protein prenylation of small G proteins
through the inhibition of farnesyl pyrophosphate synthase in the
mevalonate pathway (9). It has been
suggested that third-generation BPs such as zoledronic acid (ZOL),
the most potent N-BP clinically available, may not only reduce bone
loss but may also exert direct antitumor effects against a number
of malignant cells (10,11). We previously identified the effects of
ZOL against osteosarcoma and fibrosarcoma cells (12–15). ZOL
is rapidly cleared from the circulation within 1–2 h (10). Additionally, following an infusion of
a standard dose of ZOL, peak plasma levels were only 1–2 µM
(16). It is therefore likely that
peripheral tumors are exposed to a low concentration of ZOL for
only a few h, and that the effects of ZOL alone may be insufficient
in vivo. Therefore, for clinical applications, a novel
approach is needed to maintain a high concentration of BP for
prolonged tumor exposure.
For the treatment of osteomyelitis, in order to
maintain a high local concentration of antibiotics, sustained drug
release systems using hydroxyapatite (HA) or polymethyl
methacrylate (PMMA) bone cement have been used widely. HA is a
naturally occurring mineral form of calcium apatite with the
formula Ca5(PO4)3(OH). Up to 50%
by volume and 7% by weight of human bone is a modified form of HA,
also termed bone mineral (17). As HA
exhibits a high affinity to bone with a moderate strength, it is
commonly used as a filler to replace bone defects subsequent to
tumor resection or as a coating to promote bone ingrowth into
prosthetic implants. PMMA bone cement is also clinically used in
orthopedic surgery as a bone graft substitute (18,19), for
implant arthroplasty, and to strengthen bone. These materials may
also be applied in orthopedic surgery as sustained-release systems
for certain drugs, including antibiotics and antitumor agents
(20–22).
At present, the in vivo antitumor effects of
BP-loaded HA or bone cement have not been examined. ZOL released
from HA or bone cement represents an attractive potential treatment
strategy for osteosarcoma and metastatic bone tumors. Therefore,
the present study examined the antitumor effects of ZOL-loaded HA
and ZOL-loaded bone cement in metastatic sites of malignant tumor
cells in vitro and in vivo. Additionally, to
investigate the effects of polymerization heat on sustained release
of ZOL two types of bone cement were used; these were produced at
different polymerization temperatures.
Materials and methods
Reagents
ZOL was purchased from Novartis Pharma AG (Basel,
Switzerland). To study the effects of polymerization heat, two
types of bone cement were prepared. Simplex P® bone cement,
composed of 40 g PMMA powder (75% methyl methacrylate-styrene
copolymer, 15% PMMA and 10% barium sulfate) and 20 ml solvent (2.6%
polymerization accelerator N,N'-dimethyl-p-toluidine and
97.4% methylmethacrylate monomer), was purchased from Stryker
Corporation (Kalamazoo, MI, USA). The temperature produced during
polymerization reached 70–110°C. Cemex RX® bone cement, composed of
40 g of powder (88.27% PMMA, 9% barium sulfate and 2.73% benzoyl
peroxide) and 13.30 g solvent (99.10% methylmethacrylate, 0.90%
N,N-dimethyl-p-toluidine and 75 ppm hydroquinone), was
purchased from Tecres S.P.A. (Sommacampagna, Italy). The
temperature used for polymerization was lower compared with that
for Simplex P®, at 40–55°C. HA (Primafix®), was purchased from NGK
Spark Plug Co. Ltd. (Nagoya, Japan). Primafix® consisted of a
mixture of powder and liquid. The powder contained tetracalcium
phosphate and dibasic calcium phosphate anhydrous, and the liquid
contained sulfate sodium sulfur 5 and water for injection.
Cell lines and cell culture
For the in vivo studies, the mouse
osteosarcoma LM8 (23), human
osteosarcoma SaOS2 (24), human
fibrosarcoma HT1080 (25), human
synovial sarcoma Syo-1 (26), human
renal cancer 786-O (27), human
prostate cancer PC-3 (28) and human
lung cancer A549 (29) cell lines
were used. LM8, SaOS2, Syo-1, 786-O, and A549 cells were maintained
in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Inc.,
Kyoto, Japan) containing 10% fetal calf serum (FCS) (Nacalai
Tesque, Inc.) and 100 U/ml penicillin G and 100 mg/ml streptomycin
antibiotics (Nacalai Tesque, Inc.). HT1080 and PC-3 cells were
maintained in RPMI-1640 medium (Nacalai Tesque, Inc.) containing
FCS and antibiotics. All cell lines were maintained at 37°C in a
humidified atmosphere of 5% CO2 and 95% air.
Preparation of bone cement and HA
cylinders for in vitro use
Bone cement and HA cylinders were manually prepared
under sterile conditions, with or without ZOL, using 10 ml syringes
(Terumo Corporation, Japan; cat. no., ss-10ESzp) as templates. The
two types of bone cement, Simplex P® and Cemex RX®, and Primafix®
were mixed with or without 2.0 mg ZOL and the appropriate solvent
in the template for 1 h. Each cylinder was 15.9 mm in diameter and
7.5 mm in length, with a volume of ~1.5 ml, and was incubated in 10
ml of medium for 24 h at 37°C. The medium was collected, cylinders
were washed with 10 ml PBS, and fresh medium was added every 24 h
for 14 days. The collected medium was preserved in a humidified
atmosphere of 5% CO2 and 95% air at 37°C for the
cytotoxicity assay.
In vitro cytotoxicity assay
The viability of cell lines was determined using the
MTT assay, as previously described (30). Briefly, LM8, SaOS-2, HT1080, Syo-1,
786O, PC-3 and A549 cells were cultivated in flat-bottomed 96-well
plates (Greiner Bio-One, Frickenhausen, Germany) at 2,500, 3,000,
2,500, 1×104, 3,000, 1×104, and
1×104 cells per well, respectively, in 100 µl of medium.
Following 24 h of incubation, these media were changed to the 100
µl of medium collected from Simplex P®, Cemex RX® and Primafix®
cylinders from day 0–14, and cells were incubated for an additional
72 h. Namely, experiments were performed with six types of obtained
culture medium for one cell line group. The means of 6 replicates
for each treatment were calculated. For all cell lines the linear
association between the degree of proliferation and cell numbers
within the range of the experiment were evaluated.
Preparation of bone cement cylinders
for in vivo use
Bone cement cylinders were manually prepared under
sterile conditions, with and without ZOL. For xenograft assays in
C3H/He mice, a 96-well microplate (Nunc; Nalge Nunc International;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used as the
template and Cemex RX® was mixed with or without 0.265 mg ZOL and
solvent in the template for 1 h. The base area of resulting
cylinders was 0.33 mm2, with a thickness of 6.1 mm and a
volume of ~200 µl. For the assays used to determine the effects of
ZOL-loaded cylinders in rabbits, 10 ml syringes were used as
templates, as aforementioned, and 300 mg Cemex RX® was mixed with
or without 1.0 mg ZOL and solvent in the template for 3 min, at
which point the bone cement was not completely set.
In vivo xenograft assay
A total of 10 5-week-old male C3H/He mice (purchased
from SLC, Inc., Japan) weighing 20.0–23.0 g were housed at the
Animal Center of Kyoto Prefectural University of Medicine (Kyoto,
Japan), fed nutritionally adequate food daily, and had free access
to clean drinking water. The committee for Animal Research of Kyoto
Prefectural University of Medicine authorized all animal
experimental procedures, and the study conformed to international
guidelines on the ethical use of animals. Quantities of
1×107 LM8 cells suspended in 0.1 ml DMEM (Nacalai
Tesque, Inc., Kyoto, Japan) were injected into the subcutaneous
soft tissue of the lateral lumbar region, and mice were randomized
into two groups (n=5 per group). At 3 weeks subsequent to the
injection of cells, mice were anesthetized using 2.0% isoflurane
(Abbott Japan, Co., Ltd., Tokyo, Japan), the lateral lumbar region
was shaved and a skin incision was made at the tumor site. A
longitudinal incision was then made on the dorsal side of the
tumor, which was curettaged for bone cement implantation.
Subsequent to implantation of ZOL-loaded Cemex RX® cylinders in the
tumor cavity, the tumor capsule was sutured. Control mice received
Cemex RX® cylinders without ZOL. At five weeks subsequent to tumor
cell transplantation, control and experimental mice were sacrificed
by cervical dislocation and their primary tumors and lungs were
harvested and fixed for analysis. Subsequent to the removal of the
Cemex RX® cylinders from tumors, tumor length and width were
measured to determine tumor volume. The greatest longitudinal
diameter (length) and the greatest transverse diameter (width) were
measured using calipers. Tumor volume based on these measurements
was calculated using the modified ellipsoidal formula (31–33):
Tumorvolume=1/2(lengthxwidth2)
Histological examination
The samples of tumors were fixed in 10% buffered
formaldehyde for 96 h at 25°C and then embedded in paraffin.
Sections, 4 µm thick, were mounted onto glass slides and stained
with hematoxylin for 5 min at 25°C, washed in running tap water,
then stained with hematoxylin and eosin for 2 min at 25°C. These
samples of tumors were visually assessed using a fluorescence
microscope. The lungs were blindly assessed by 3 orthopedic
surgeons using a stereomicroscope, and visual comparisons were made
between the two groups.
In vivo histotoxic assay
All animal experimentation was performed under the
review and approval of the Animal Experimentation Ethics Committee
of Kyoto Prefectural University of Medicine. The animals were
housed at the Animal Center of Kyoto Prefectural University of
Medicine, fed nutritionally adequate food daily, and had free
access to clean drinking water. A total of 12 3-month-old male
Japanese white rabbits, purchased from Shimizu Laboratory Supplies
Co., Ltd (Kyoto, Japan) weighing 2.0–2.5 kg were anesthetized by
intravenous injection of pentobarbital (Abbott Laboratories, Abbott
Park, IL, USA) at a dose of 30 mg/animal and local anesthesia with
lidocaine at 5 mg/animal.
The front foot of each rabbit was shaved and,
subsequent to sterilization, covered with a drape in order to
perform aseptic surgery. A 3 cm incision was made on the proximal
tibia of the foreleg, and the soft tissue overlaying the proximal
tibia was dissected. A 1.5 cm cortical window was prepared in the
tibia using a 1.2 mm diameter Kirschner wire, a surgical
oscillating saw and a chisel. Through the cortical window, the bone
marrow cavity was enlarged using a bone curette, manually packed
with ZOL-loaded Cemex RX® which had not completely set, and covered
with the cortical window. The wound was closed using interrupted
4–0 Vicryl sutures (Ethicon, Bridgewater, NJ, USA). A total of 6
control rabbits received Cemex RX® without ZOL.
Radiographic examination
To determine if ZOL-loaded bone cement inhibited
osteogenesis, radiographic examinations were performed weekly for 4
weeks subsequent to surgery. Images were captured with TECHNOMOBILE
II (Hitachi, Ltd., Tokyo, Japan) at a voltage of 50 kV and a
current of 100 mA. The focal distance was maintained at 100 cm with
an exposure time of 0.04 sec. The radiolucent region and callus
formation around the bone cement were blindly assessed by 3
orthopedic surgeons and compared between the 2 groups.
Histological examination
A total of 8 weeks subsequent to Cemex RX®
implantation, control and experimental rabbits were sacrificed.
Cemex RX® implants were removed from the tibia and bone tissue
sections were prepared for histological examination. Excised tibia
bone were fixed using 4% paraformaldehyde in 0.1 M phosphate buffer
at pH 7.4 with 0.2% picric acid for 7 days at 4°C, and then
decalcified in 0.5 M EDTA at pH 7.5 for 8 weeks. Tissue sections
from a 30 µm longitudinal axis view were prepared and stained with
H&E as aforementioned.
Blood test
To identify the toxicities caused by Cemex RX®
implants, 2 ml blood samples were collected at 1-week intervals
from the ear marginal vein (needle gauge 26) of all rabbits in each
group for 4 weeks. Whole blood was centrifuged using the KN-70
Tabletop Centrifuge (Kubota, Tokyo, Japan) at 1,378 × g for 10 min
at 25°C and the resulting serum was collected for biochemical
examination.
Statistical analysis
Data are expressed as means ± standard deviation of
experiments. Statistical evaluation of data was performed using
Student's t-test for simple comparisons between groups and
treatments. Microsoft Excel for MAC 2011 version 14.7.2 was used to
perform statistical analysis (Microsoft Corporation, Redmund, WA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
In vitro growth inhibitory effects of
ZOL-loaded HA and bone cement in tumor cells
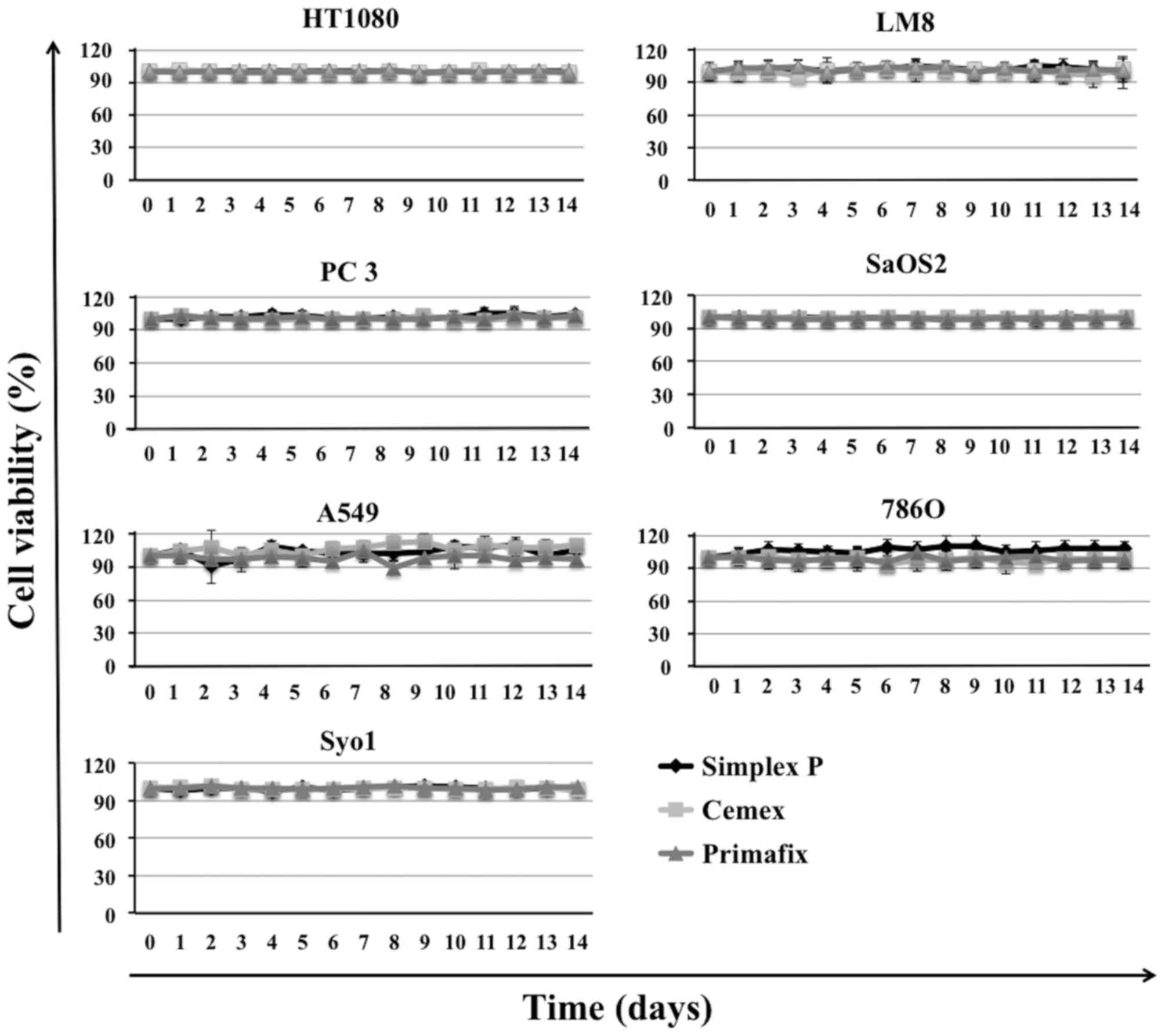
HA and bone cement without ZOL did not affect the
growth of cell lines (Fig. 1).
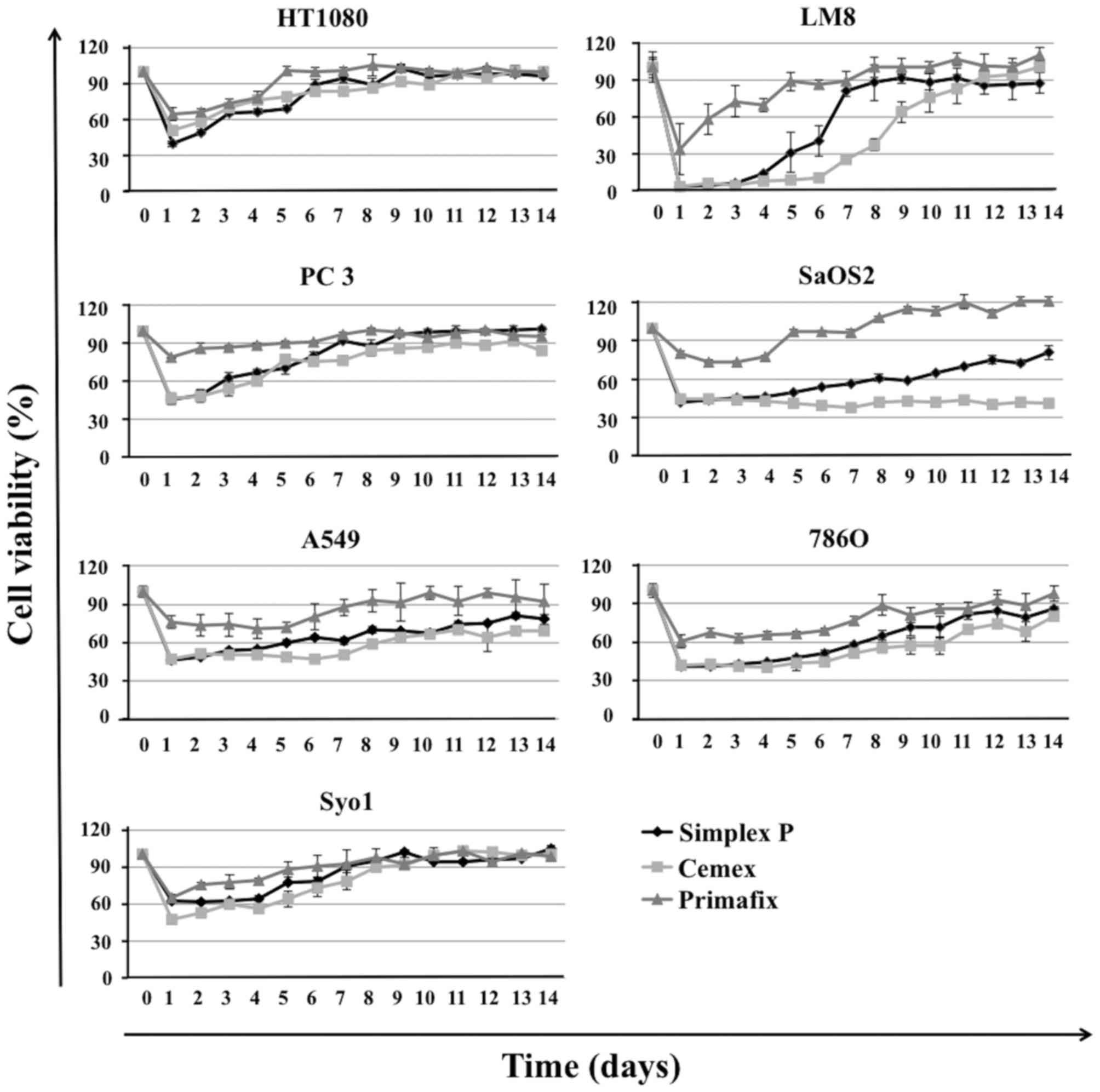
However, ZOL-loaded Simplex P®, Cemex RX®, and Primafix® inhibited
the growth of all cell lines. Although ZOL-loaded Primafix®
exhibited inhibitory effects in HT1080, LM8, PC-3, SaOS2, A549,
786-O and Syo-1 cells, the growth inhibitory effects were weak and
short-acting compared with those treated by ZOL-loaded Simplex P®
and Cemex RX®. No significant differences in antitumor effects and
duration of drug activity were observed between the two types of
ZOL-loaded bone cement evaluated. Growth inhibitory effects of
these materials gradually decreased over the 14 day treatment
period. ZOL-loaded bone cement and Primafix® demonstrated
particularly strong inhibitory effects in LM8 cells (Fig. 2).
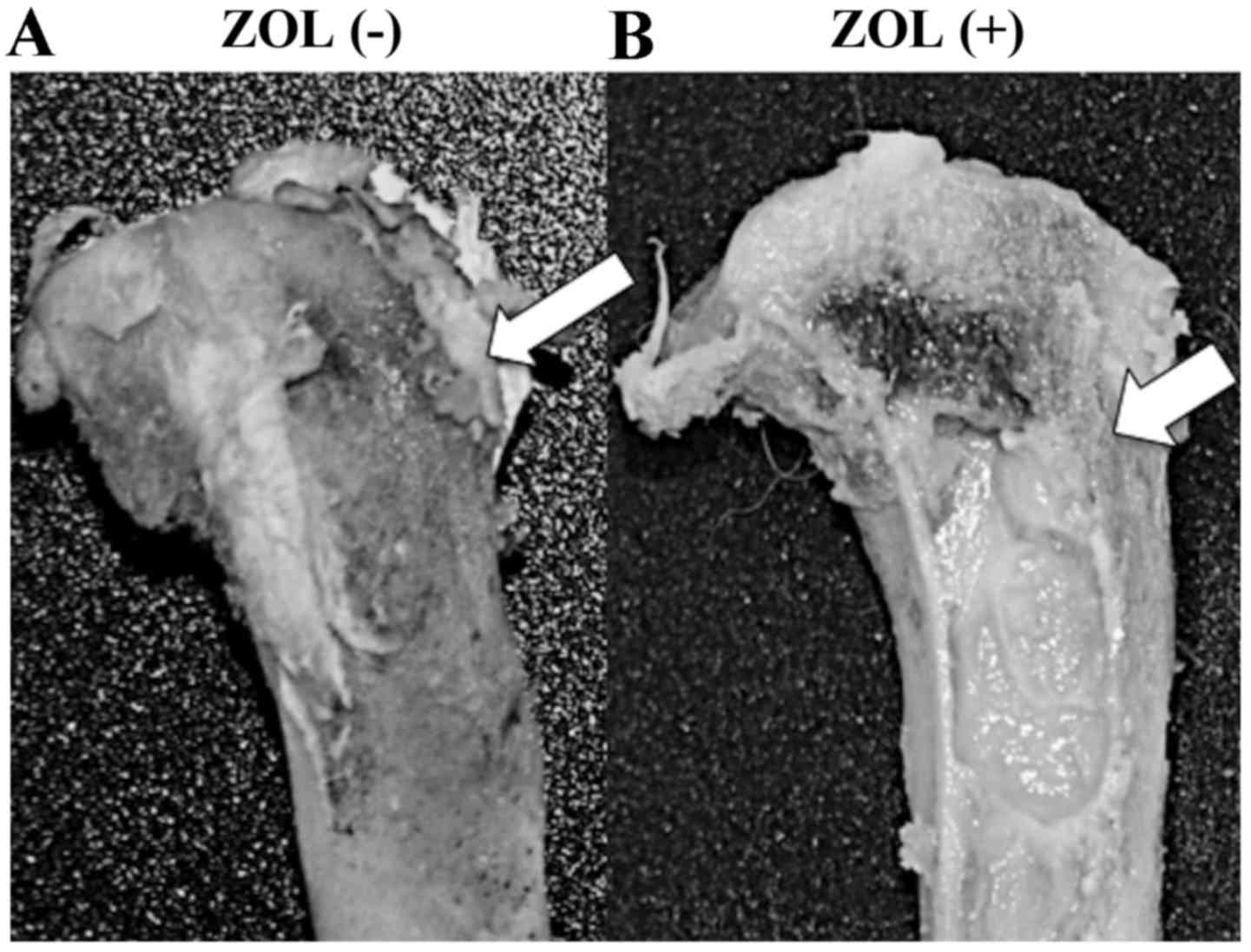
In vivo antitumor effects of
ZOL-loaded bone cement
ZOL-loaded Cemex RX® inhibited the growth of LM8
cells in vivo. Prior to initiating clinical trials, it is
important that the in vivo efficacy of potential anticancer
agents is determined in an animal model. Therefore, an in
vivo study to determine whether ZOL-loaded bone cement inhibits
the growth of LM8 cell xenografts in C3H/He mice was performed.
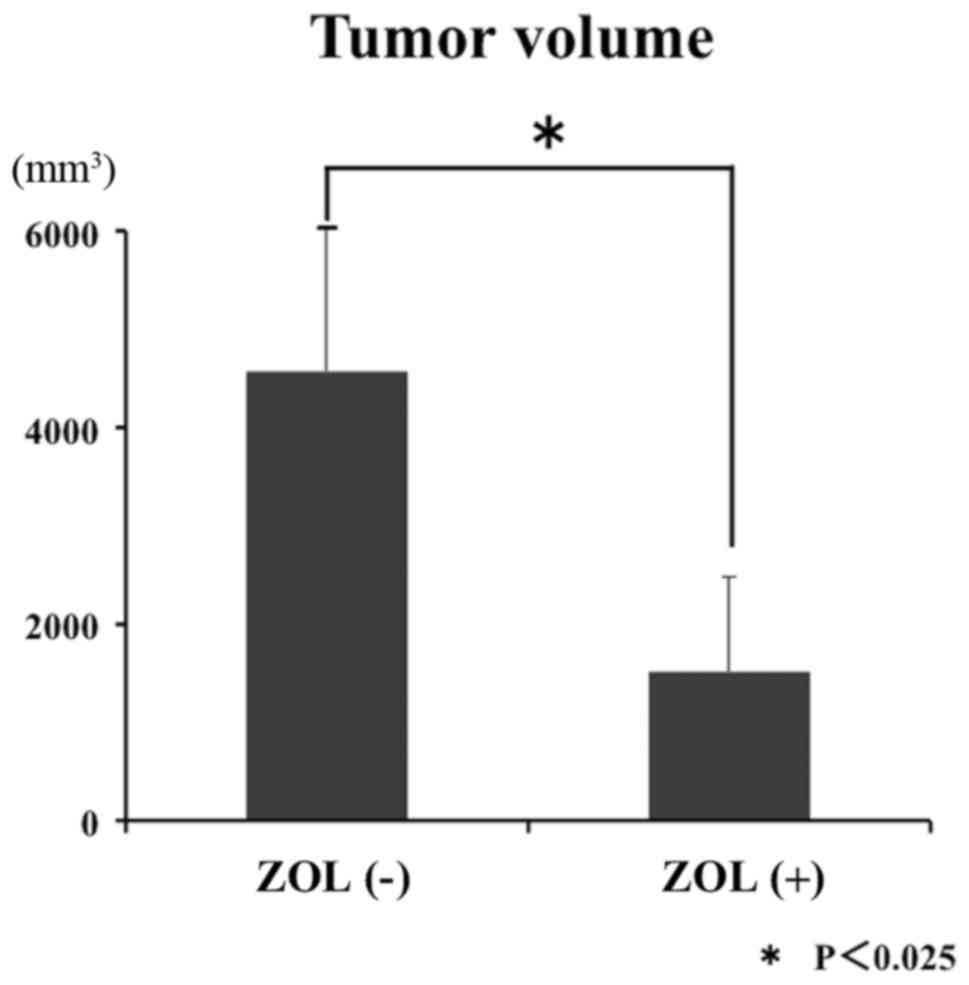
ZOL-loaded Cemex RX® caused significant inhibition of LM8 tumor
growth. At 5 weeks subsequent to bone cement implantation, the
average tumor volume in C3H/He mice treated with ZOL-loaded Cemex
RX® was less compared with that in mice treated with Cemex RX®
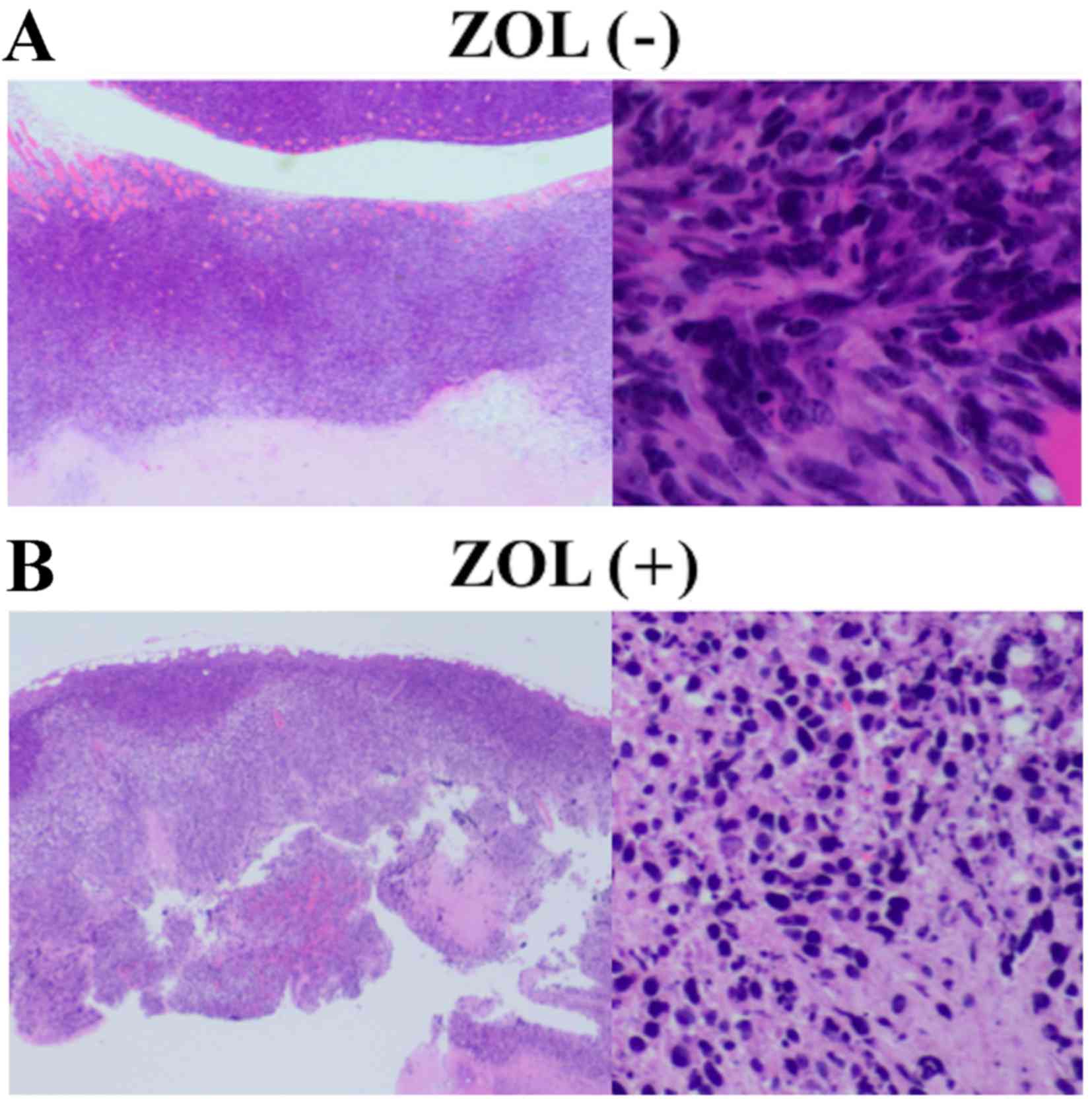
control (Fig. 3). Tumor necrosis was
also observed around the implanted ZOL-loaded Cemex RX® (Fig. 4).
Effects of ZOL-loaded bone cement on
lung metastasis
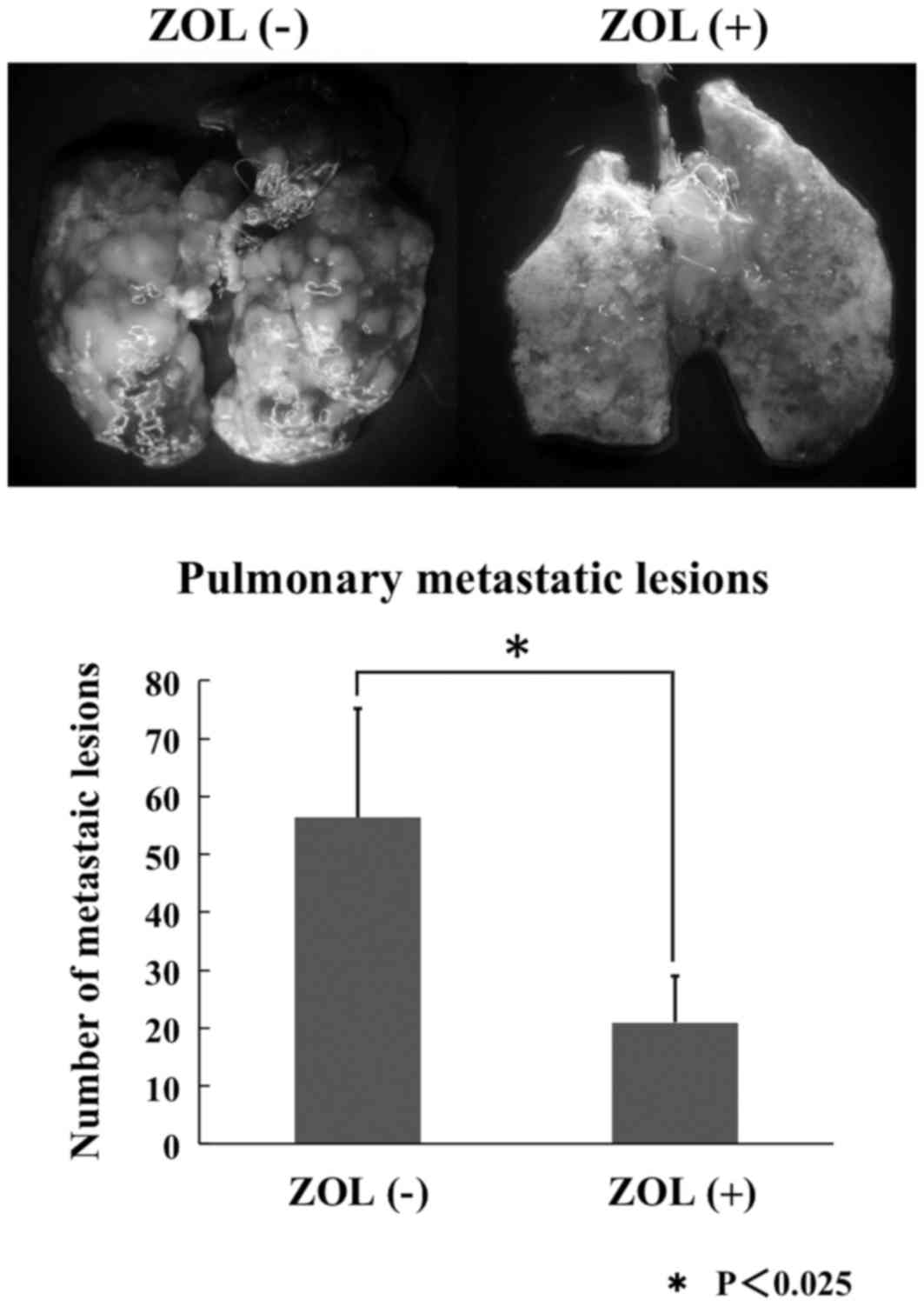
Spontaneous lung metastases were observed in all
groups subsequent to LM8 cell inoculation. The numbers of pulmonary
metastatic lesions were counted in each group. The growth of these
metastases subsequent to 3 weeks of ZOL-loaded Cemex RX® treatment
was significantly reduced compared with controls (Fig. 5).
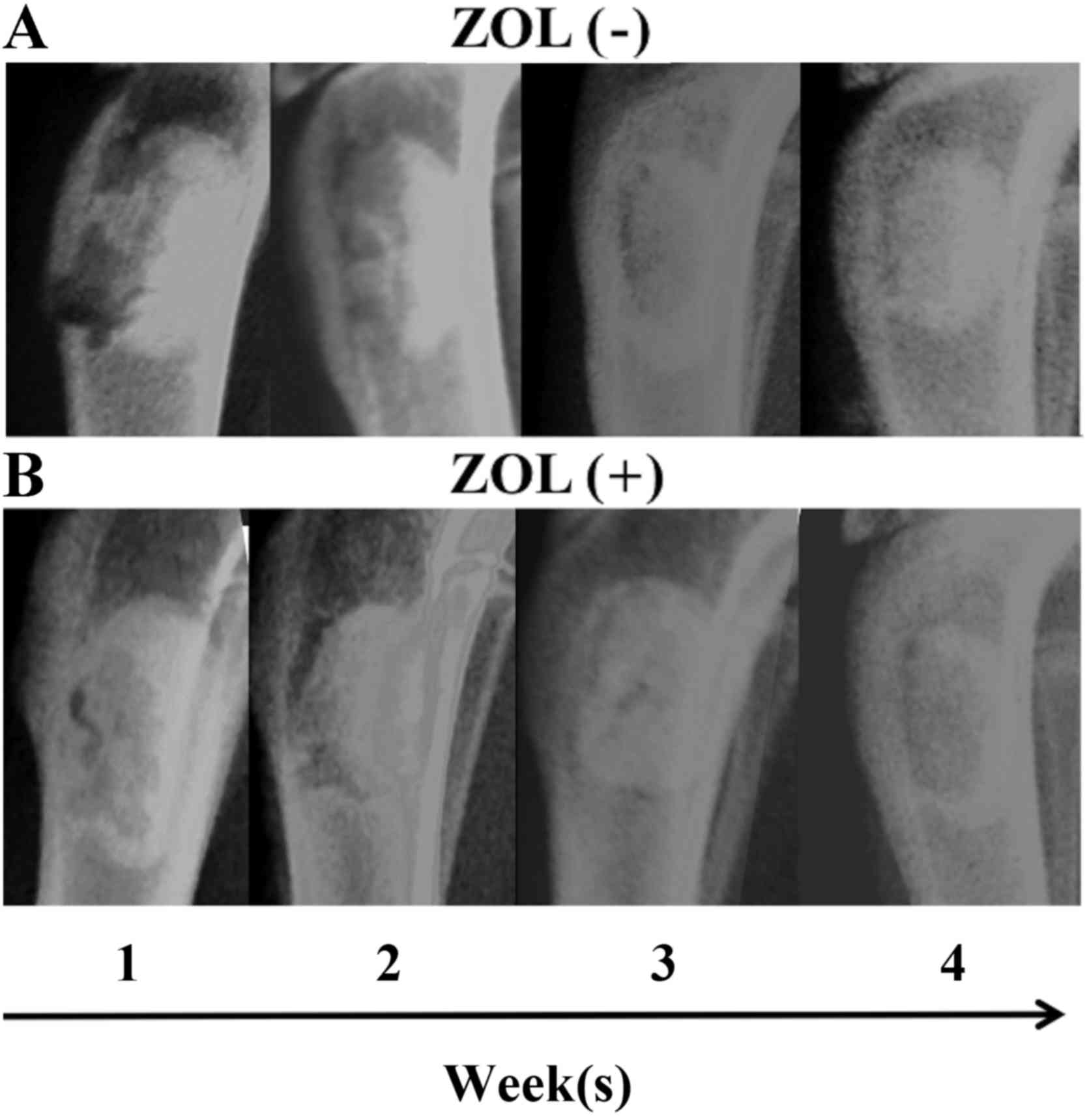
Radiological and histological
results
X-ray imaging did not reveal increased lucent and
sclerotic regions that represent osteonecrosis and inhibition of
bone formation. The lucent zone around the bone cement became less
clear over time in each group (Fig.
6). Novel bone formation was observed in the bone tissue
specimens in both groups (Fig.
7).
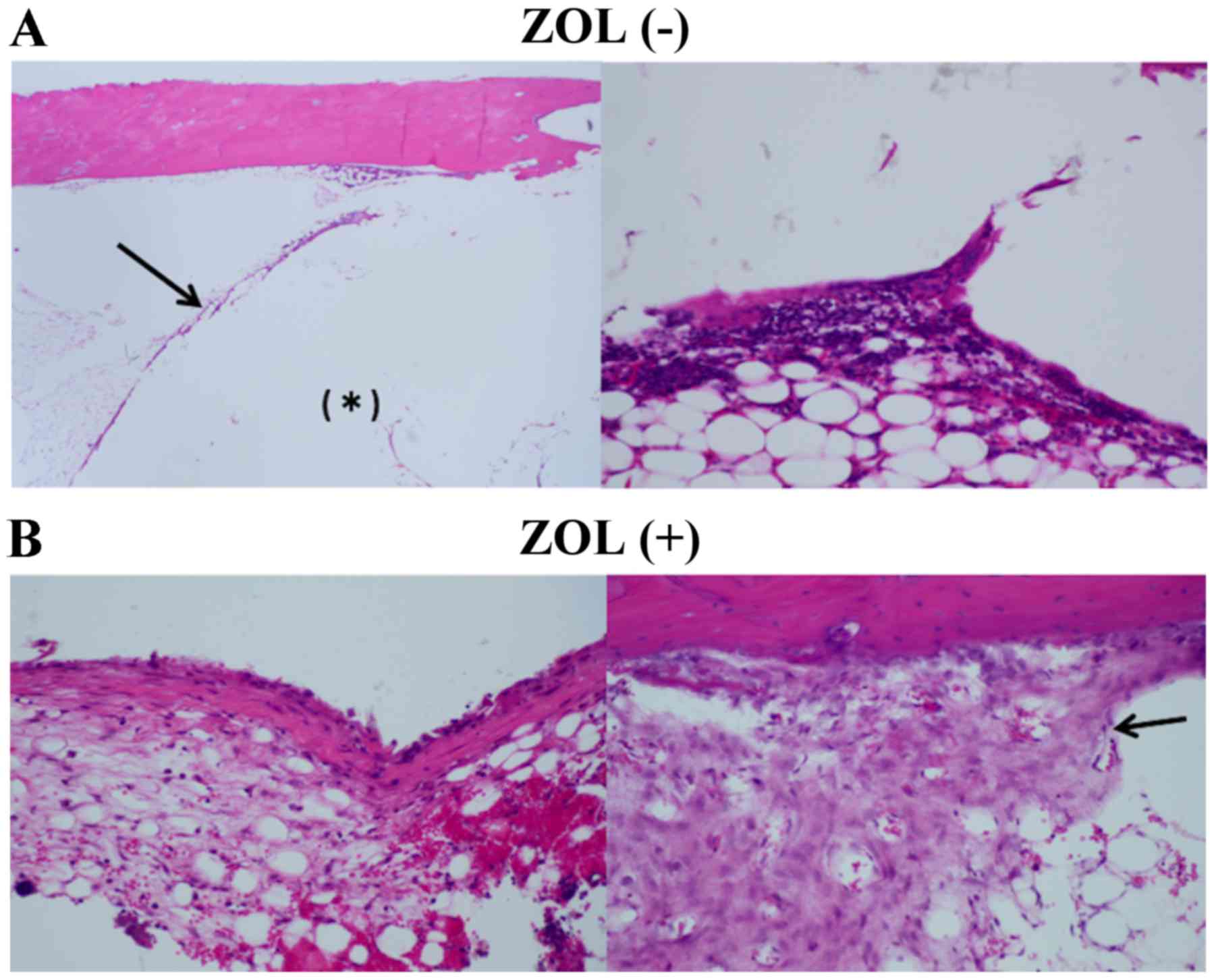
Histologically, certain fibrous tissue and
multinucleated giant cells were observed around the bone cement in
each group. Certain necrotic tissue was also observed in the bone
marrow in both groups, but significant differences were not
observed between the two groups (Fig.
8).
Laboratory data
Laboratory blood tests demonstrated no remarkable
signs of toxicity. Blood urea nitrogen, creatinine, aspartate
transaminase, alanine transaminase, calcium, potassium, phosphorus,
sodium and chloride were all within normal limits. No significant
differences between the two groups were observed (Fig. 9).
Discussion
In the present study, it has been demonstrated that
ZOL is released from bone cement and HA, and that its cytotoxic
effects were demonstrated by MTT assay in 7 cultured cell lines:
Murine osteosarcoma, human osteosarcoma, human fibrosarcoma, human
synovial sarcoma, human prostate cancer, human lung cancer, and
human renal cancer. In addition, ZOL-loaded bone cement inhibited
both malignant bone tumor growth at the primary site and metastases
from the primary site in vivo. Antitumor effects were
similar between ZOL-loaded Simplex-P® and Cemex RX®. The
temperature produced during polymerization of Simplex-P® and Cemex
RX® is 70–110°C and 40–55°C, respectively. Similar to previous
studies (34), these results
suggested that the heat produced during polymerization of bone
cement did not affect the biological activity of ZOL. Additionally,
no toxicity associated to the bone cement used in the present study
on all cell lines was observed. By contrast, certain antitumor
drugs and antibiotics may lose their activity during the
polymerization of bone cement. Thus, the negative effects of
polymerization and temperature produced during the solidification
of the bone cement should be considered when selecting other
cytotoxic agents for local control of malignant bone tumors,
including metastatic bone tumors. To address these concerns, the
effects of ZOL-loaded HA were also evaluated. Although ZOL-loaded
HA exerted antitumor effects, these effects were weak compared with
ZOL-loaded bone cement. BPs exhibit a high affinity to bone and HA
(35), so this difference may be
attributed to the weaker releasing ability of ZOL-loaded HA.
The contents of ZOL in HA and bone cement
preparations was determined by the following process: When 2.5 mg
of ZOL was mixed with 2.0 g Primafix® and its relevant solvent,
solidification capacity was lost. However, 2.0 mg ZOL did not
affect the solidification of the two types of bone cement evaluated
(data not shown). These results may be explained by the affinity
between ZOL and HA. Although 2.0 mg ZOL was selected for these
reasons as the dose for the present study, additional investigation
is needed to determine the optimum ratio of ZOL to HA/bone
cement.
ZOL exerts strong antitumor effects and may also
induce apoptosis in osteoclasts. Bone cement has compressive and
tensile strength, and may be used as a drug delivery system for the
local and slow release of drugs (20). These characteristics enable ZOL-loaded
bone cement to maintain a high concentration of ZOL for a prolonged
period of time. Therefore, ZOL-loaded bone cement exhibits a
significantly smaller risk of systemic side effects such as renal
disturbance (36) and inhibition of
osteogenesis compared with drugs administered by intravenous
injection. From the data suggesting that ZOL-loaded bone cement
exerted antitumor effects against lung, prostate and renal cancer,
all of which have high potential for bone metastasis, the clinical
application of this preparation as a filling material subsequent to
the curettage of bone metastasis possesses strong potential. A
number of studies have described the combined effects of
third-generation BPs with antitumor agents and radiation in various
malignant tumor cell lines (14,37,38), and
we have also identified that ZOL synergistically augments the
effects of antitumor agents and ionizing radiation in fibrosarcoma
cell lines (12). These results
indicate that ZOL-loaded bone cement used alongside other
anticancer agents or ionizing radiation is likely to exert additive
or synergistic therapeutic effects against several types of
malignant tumors. Additionally, from these data that demonstrate
the strong antitumor effects of these drug delivery systems in
primary malignant bone tumor, the potential for clinical use to
pack the cortical bone window during bone tumor biopsy and to fix
the implant prosthesis during surgery for primary malignant bone
tumor is clear.
To the best of our knowledge, this is the first
in vivo study demonstrating release of a third-generation BP
from bone cement. In addition, the antitumor effects of ZOL-loaded
HA in several malignant tumor cell lines were demonstrated for the
first time. The decreased viability of all tumor cell lines exposed
to ZOL eluted from bone cement indicates that ZOL is not
inactivated by polymerization heat. The antitumor effects of
ZOL-loaded HA were weak compared with ZOL-loaded bone cement,
possibly as BPs exhibit a high affinity to bone and HA. Although
additional studies to determine the optimum content of ZOL in
HA/bone cement are needed, the packing of an osseous defect with
ZOL-loaded bone cement/HA subsequent to tumor curettage may have
beneficial effects. These include beneficial effects at the
surgical site and against pulmonary metastases, through antitumor
activity and reduction of osteolysis without systemic toxicity.
Finally, these medical materials are already in clinical use, so
these antitumor strategies may be readily applied in clinical
settings.
Acknowledgements
This work was supported by a grant-in-aid from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (grant no. 26462274, to HM).
Glossary
Abbreviations
Abbreviations:
|
ZOL
|
zoledronic acid
|
|
BPs
|
bisphosphonates
|
|
PMMA
|
polymethyl methacrylate
|
|
HA
|
hydroxyapatite
|
References
|
1
|
Unni KK and Inwards CY: Dahlin's bone
tumors, General Aspects and Data on 10,165 Cases. Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 3052010
|
|
2
|
Desai S and Jambhekar N:
Clinicopathological evaluation of metastatic carcinomas of bone: A
retrospective analysis of 114 cases over 10 years. Indian J Pathol
Microbiol. 38:49–54. 1995.PubMed/NCBI
|
|
3
|
Doung YC, Kenan S and Rapp T: Metastatic
lesions of the proximal femur. Bull NYU Hosp Jt Dis. 69:81–86.
2011.PubMed/NCBI
|
|
4
|
Weiss RJ, Forsberg JA and Wedin R: Surgery
of skeletal metastases in 306 patients with prostate cancer. Acta
Orthop. 83:74–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wedin R, Bauer HC and Wersäll P: Failures
after operation for skeletal metastatic lesions of long bones. Clin
Orthop Relat Res. 1–139. 1999.PubMed/NCBI
|
|
6
|
Wedin R: Surgical treatment for pathologic
fracture. Acta Orthop Scand Suppl. 72:2pp1–29. 2001. View Article : Google Scholar
|
|
7
|
Chien SH, Hung SH, Cheng YM, Lin GT, Lin
SY, Ko CY, Chen LH and Chiang HC: Surgical treatment of pathologic
fracture of the femur. Kaohsiung J Med Sci. 13:556–561.
1997.PubMed/NCBI
|
|
8
|
Russell RG and Rogers MJ: Bisphosphonates:
From the laboratory to the clinic and back again. Bone. 25:97–106.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Green JR: Antitumor effects of
bisphosphonates. Cancer. 97 3 Suppl:S840–S847. 2003. View Article : Google Scholar
|
|
10
|
Lee MV, Fong EM, Singer FR and Guenette
RS: Bisphosphonate treatment inhibits the growth of prostate cancer
cells. Cancer Res. 61:2602–2608. 2001.PubMed/NCBI
|
|
11
|
Kubo T, Shimose S, Matsuo T, Tanaka K,
Yasunaga Y, Sakai A and Ochi M: Inhibitory effects of a new
bisphosphonate, minodronate, on proliferation and invasion of a
variety of malignant bone tumor cells. J Orthop Res. 24:1138–1144.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koto K, Murata H, Kimura S, Horie N,
Matsui T, Nishigaki Y, Ryu K, Sakabe T, Itoi M, Ashihara E, et al:
Zoledronic acid inhibits proliferation of human fibrosarcoma cells
with induction of apoptosis, and shows combined effects with other
anticancer agents. Oncol Rep. 24:233–239. 2010.PubMed/NCBI
|
|
13
|
Horie N, Murata H, Nishigaki Y, Matsui T,
Segawa H, Nogawa M, Yuasa T, Kimura S, Maekawa T, Fushiki S and
Kubo T: The third-generation bisphosphonates inhibit proliferation
of murine osteosarcoma cells with induction of apoptosis. Cancer
Lett. 238:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horie N, Murata H, Kimura S, Takeshita H,
Sakabe T, Matsui T, Maekawa T, Kubo T and Fushiki S: Combined
effects of a third-generation bisphosphonate, zoledronic acid with
other anticancer agents against murine osteosarcoma. Br J Cancer.
96:255–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koto K, Horie N, Kimura S, Murata H,
Sakabe T, Matsui T, Watanabe M, Adachi S, Maekawa T, Fushiki S and
Kubo T: Clinically relevant dose of zoledronic acid inhibits
spontaneous lung metastasis in a murine osteosarcoma model. Cancer
Lett. 274:271–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jagdev SP, Coleman RE, Shipman CM, Rostami
HA and Croucher PI: The bisphosphonate, zoledronic acid, induces
apoptosis of breast cancer cells: Evidence for synergy with
paclitaxel. Br J Cancer. 84:1126–1134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Junqueira LC and Carneiro J: Basic
Histology, Text & Atlas. 10th. MacGrow-Hill Companies; New
York: pp. 1442003
|
|
18
|
Yamamoto T, Onga T, Marui T and Mizuno K:
Use of hydroxyapatite to fill cavities after excision of benign
bone tumours. J Bone Joint Surg Br. 82:1117–1120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida A, Araki N, Shinto Y, Yoshikawa H,
Kurisaki E and Ono K: The use of calcium hydroxyapatite ceramic in
bone tumour surgery. J Bone Joint Surg Br. 72:298–302.
1990.PubMed/NCBI
|
|
20
|
Shinto Y, Uchida A, Korkusuz F, Araki N
and Ono K: Calcium hydroxyapatite ceramic used as a delivery system
for antibiotics. J Bone Joint Surg Br. 74:600–604. 1992.PubMed/NCBI
|
|
21
|
Hirata M, Murata H, Takeshita H, Sakabe T,
Tsuji Y and Kubo T: Use of purified beta-tricalcium phosphate for
filling defects after curettage of benign bone tumours. Int Orthop.
30:510–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itokazu M, Kumazawa S, Wada E and Wenyi Y:
Sustained release of adriamycin from implanted hydroxyapatite
blocks for the treatment of experimental osteogenic sarcoma in
mice. Cancer Lett. 107:11–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asai T, Ueda T, Itoh K, Yoshioka K, Aoki
Y, Mori S and Yoshikawa H: Establishment and characterization of a
murine osteosarcoma cell line (LM8) with high metastatic potential
to the lung. Int J Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fogh J, Fogh JM and Orfeo T: One hundred
and twenty-seven cultured human tumor cell lines producing tumors
in nude mice. J Natl Cancer Inst. 59:221–226. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rasheed S, Nelson-Rees WA, Toth EM,
Arnstein P and Gardner MB: Characterization of a newly derived
human sarcoma cell line (HT-1080). Cancer. 33:1027–1033. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawai A, Naito N, Yoshida A, Morimoto Y,
Ouchida M, Shimizu K and Beppu Y: Establishment and
characterization of a biphasic synovial sarcoma cell line, SYO-1.
Cancer Lett. 204:105–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams RD, Elliott AY, Stein N and
Fraley EE: In vitro cultivation of human renal cell cancer. II.
Characterization of cell lines. In Vitro. 14:779–786. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohnuki Y, Marnell MM, Babcock MS, Lechner
JF and Kaighn ME: Chromosomal analysis of human prostatic
adenocarcinoma cell lines. Cancer Res. 40:524–534. 1980.PubMed/NCBI
|
|
29
|
Giard DJ, Aaronson SA, Todaro GJ, Arnstein
P, Kersey JH, Dosik H and Parks WP: In vitro cultivation of human
tumors: Establishment of cell lines derived from a series of solid
tumors. J Natl Cancer Inst. 51:1417–1423. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Euhus DM, Hudd C, LaRegina MC and Johnson
FE: Tumor measurement in the nude mouse. J Surg Oncol. 31:229–234.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jensen MM, Jorgensen JT, Binderup T and
Kjaer A: Tumor volume in subcutaneous mouse xenografts measured by
microCT is more accurate and reproducible than determined by
18F-FDG-microPET or external caliper. BMC Med Imaging. 8:162008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zwolak P, Manivel JC, Jasinski P, Kirstein
MN, Dudek AZ, Fisher J and Cheng EY: Cytotoxic effect of zoledronic
acid-loaded bone cement on giant cell tumor, multiple myeloma, and
renal cell carcinoma cell lines. J Bone Joint Surg Am. 92:162–168.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Gangal G and Uludağ H: ‘Magic
bullets’ for bone diseases: Progress in rational design of
bone-seeking medicinal agents. Chem Soc Rev. 36:507–531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamasaki M, Yuasa T, Uehara S, Fujii Y,
Yamamoto S, Masuda H, Fukui I and Yonese J: Improvement of renal
function by changing the bone-modifying agent from zoledronic acid
to denosumab. Int J Clin Oncol. 21:1191–1195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kubo T, Shimose S, Matsuo T, Sakai A and
Ochi M: Efficacy of a nitrogen-containing bisphosphonate,
minodronate, in conjunction with a p38 mitogen activated protein
kinase inhibitor or doxorubicin against malignant bone tumor cells.
Cancer Chemother Pharmacol. 62:111–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ottewell PD, Mönkkönen H, Jones M, Lefley
DV, Coleman RE and Holen I: Antitumor effects of doxorubicin
followed by zoledronic acid in a mouse model of breast cancer. J
Natl Cancer Inst. 100:1167–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|