Introduction
The management of castration-resistant prostate
cancer (CRPC) currently remains a challenge (1). Docetaxel (DTX) induces apoptotic cell
death by stabilizing β-tubulin, thereby blocking mitotic cell
division and inhibiting androgen receptor nuclear translocation
(2). DTX is the standard first-line
drug for the treatment of CRPC, however, only ~48% of patients
respond to DTX, and acquired drug resistance rapidly develops in
DXT chemotherapy (3). Patients with
DTX-resistant or -progressive CRPC previously treated with DTX,
cabazitaxel (4), abiraterone acetate
(5) enzalutamide (6), sipuleucel-T (7) and radium 223-dichloride (8) have shown an improvement in overall
survival time over the placebo group in phase III clinical trials.
However, recent cost-effectiveness analyses for patients with CRPC
have shown unfavorable results for these new drugs, since these
drugs are not affordable to numerous patients, particularly in less
developed countries (9). Thus,
cost-effective therapeutic strategies are urgently required for the
management of CRPC.
Multiple mechanisms are involved in the development
of DTX resistance in CRPC. Firstly, multi-drug resistance 1 (MDR1)
promotes drug efflux through a P-glycoprotein pump, and prostate
cancer cells with increased MDR1 expression demonstrate markedly
reduced intracellular concentrations of DTX (10,11).
Secondly, the modulation and phenotype changes of microtubules (the
primary target of DTX) are associated with reduced response rates
in patients receiving DTX-based chemotherapy (12). The accumulation of certain β-tubulin
isotypes (βIII-tubulin and βIV-tubulin) increases the dynamic
activity of microtubules, thereby decreasing the efficacy of DTX
(13). In addition, β-tubulin
mutations (T26A, A595G and F270I) lead to alterations in the
microtubule-binding site of DTX and reduce the efficacy of DTX
(14). Furthermore, the
overexpression of B-cell lymphoma-2 and the loss of p53 function
have been shown to contribute to the resistance of CRPC to DTX
(15). Finally, the high activity of
drug-detoxifying proteins, including glutathione-S-transferase and
cytochrome P450 3A, increases DTX metabolism (16,17).
Accordingly, several inhibitors that target these mechanisms have
been developed; however, clinical trials of these agents as single
or combination therapies have exhibited only limited clinical
efficacy (3,18). Thus, the identification of new
molecular mechanisms of DTX resistance and the development of novel
targeted treatment strategies are urgently required for CRPC.
Multi-drug resistance protein 4 (MRP4) is a member
of the MRP family, and transports a variety of molecules that are
principally organic anions, including the cyclic nucleotide cyclic
adenosine monophosphate, prostaglandins, dehydroepiandrosterone,
bile acids and numerous drugs such as the camptothecin-11
metabolite SN-38, topotecan, methotrexate and anti-retrovirals
(19). In recent years, accumulating
evidence has indicated that the overexpression of MRP4 is a
possible molecular mechanism for unexplained failures of
chemotherapy in certain tumors (20–22). In
gastric cancer, cisplatin (DDP) resistance was revealed to be
associated with MRP4 overexpression, and the decrease in MRP4
expression by RNA interference reversed DDP resistance in
vitro (23). Similarly, acquired
resistance to methotrexate was associated with the overexpression
of MRP4 in LNCaP cells, while disruption of MRP4 expression
sensitized LNCaP cells to methotrexate (24). In addition, it was reported that MRP4
expression levels increased with androgen treatment, but decreased
with anti-androgen treatment, in LNCaP cells (25). Furthermore, our previous study
demonstrated that DTX-resistance time in patients with low
testosterone levels was significantly delayed compared with that in
patients with high testosterone levels (26). These data indicated that androgen
levels may be associated with DTX resistance. However, the
association between MRP4 and DTX resistance, and the effect of
androgen on MRP4 expression in DTX-resistant prostate cancer cells
remain unknown.
In the present study, DTX-resistant prostate cancer
cell lines were established from prostate cancer
androgen-independent C4-2 cell lines to define the association
between MRP4 and DTX resistance, and to explore the effect of
androgen signaling on MRP4 expression and its efflux activity.
Materials and methods
Cell lines and reagents
C4-2 cells obtained from the American Type Culture
Collection (Manassas, VA, USA) were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% complete fetal bovine serum (Sigma-Aldrich; Merck KGaA), 100
U/ml penicillin and 100 µg/ml streptomycin, and were maintained at
37°C in a humidified incubator with 5% CO2. DTX (cat
no., 125354-16-7) was purchased from Shanghai Sanwei Pharmaceutical
Co., Ltd. (Shanghai, China). Bicalutamide (BKL; cat no.,
144701-48-4) and dihydrotestosterone (DHT; cat no., 521-18-6) were
purchased from Sigma-Aldrich (Merck KGaA). Antibodies against MRP4
(cat no., #ab15598) and GAPDH (cat no., #ab181603) were obtained
from Abcam (Cambridge, MA, USA). Plasmid pDONR223-based full-length
androgen receptor constructs [human androgen receptor (AR)
complementary DNA (cDNA) clone, AR (NM_000044)] and lentiviral
vector-based MRP4 short hairpin RNA (shRNA) constructs were
purchased from Chongqing Youbao Biotechnology Co., Ltd. (Chongqing,
China).
DTX-resistant culture
Cells were seeded onto 6-well plates at
2×105 cells/ml and maintained at 37°C for 2 weeks.
DTX-resistant cells were generated from parental C4-2 cells by
gradually increasing DTX concentrations (0, 5, 10, 20 or 40 nM) in
the culture medium. Cells that survived the maximum concentration
of DTX (40 nM) were selected for subsequent analysis and were
referred to as C4-2/D cells. Parental cells were passaged alongside
DTX-treated cells and were referred to as C4-2/S cells; these cells
were used as an appropriate control. DTX-resistant and -sensitive
cell lines were seeded onto 6-well plates at a density of
1×103 cells/well, then maintained at 37°C for 48 h.
Following treatment with or without 40 nM DTX for 21 days, total
cell numbers were counted using a ViCell Coulter counter (Beckman
Coulter, Inc., Brea, CA, USA).
Experiment grouping design and
treatment
C4-2/S and C4-2/D cells were divided into three
groups according to the culture medium added with different drugs:
Group A [PBS (+), DHT (−), BKL (−)]; group B [PBS (−), DHT (+), BKL
(−)]; and group C [PBS (−), DHT (+), BKL (+)]. Cells in group A
were cultured with PBS for 5 days at room temperature as a control.
Group B cells were cultured at 37°C with 20 nM DHT for 5 days,
while group C cells were treated with 20 nM DHT plus 50 nM BKL for
5 days. In addition, in order to observe the effect of different
levels of androgen on the expression of MRP4, cells were maintained
for 5 days under five different concentrations of DHT (0, 5, 10, 20
and 40 nM). Medium plus drugs were replenished each day. On the
sixth day, the plates were placed on a bed of ice and cells were
harvested by scraping.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
RNA isolation was performed using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
was subjected to RT using M-MLV reverse transcriptase (Fermentas;
Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) following the
manufacturer's protocol. The cDNA product was used for PCR (5 µl),
using the PCR Master Mix (Fermentas; Thermo Fisher Scientific,
Inc.) and the following thermocycler settings: Initially
pre-denatured at 94°C for 6 min, then denatured at 40 cycles of
95°C for 20 sec and annealed at 44°C for 30 sec. PCR products were
separated by 1.5% agarose gel electrophoresis and stained with
ethidium bromide. The respective forward and reverse primers used
for each gene were: MRP4 forward, 5′-CAGAGTCTTCGGTTTGGT-3′ and
reverse, 5′-GCTTCTCGGTTACATTTC-3′; AR forward,
5′-CCTACGGCTACACTCGG-3′ and reverse, 5′-CTGGCAGTCTCCAAACG-3′; and
GAPDH forward, 5′-AATCCCATCACCATCTTCC-3′ and reverse,
5′-AGTCCTTCCACGATACCAA-3′. GAPDH was used as control.
Clonogenic assay
C4-2/S and C4-2/D cells were treated with 50 µl 0.2%
dimethyl sulfoxide (DMSO) or different doses of DTX (10, 20 and 40
nM) at 37°C for 8 h, and 1×103 cells were plated on a
100-mm dish at 37°C for 21 days. Cells were fixed with 4%
formaldehyde for 10 min and stained with 0.5% crystal violet for 30
min at room temperature. Colonies were counted using a ViCell
Coulter counter (Beckman Coulter, Inc.).
Cell apoptosis assay
Cell apoptosis was assayed by flow cytometry
(Beckman Coulter, Inc.). Cells were harvested and re-suspended in
PBS containing 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA).
Following centrifugation at 620 × g at 4°C for 5 min, cells were
re-suspended with 500 µl binding buffer [including 50 mmol/l sodium
phosphate buffer, 0.3 mol/l NaCl, 10 mmol/l imidazole (pH 8.0);
Chongqing Youbao Biotechnology Co., Ltd., Chongqing, China] and
mixed with 5 µl of 0.5 mg/ml Annexin V-fluorescein isothiocyanate
(FITC; Oncogene Research Products, La Jolla, CA, USA). Cells were
then incubated with 5 µl of 0.6 mg/ml propidium iodide (Oncogene
Science; Nuclea Diagnostics Laboratories, LLC, Cambridge, MA, USA)
in the dark at room temperature for 5–15 min. Apoptotic cells with
positive Annexin V-FITC staining were detected by flow cytometry.
All assays were performed in triplicate.
MTT assay
C4-2/S and C4-2/D cells were seeded onto 96-well
plates at a density of 1×105 cells/well in
quadruplicate, treated with DTX (40 nM) and incubated at 37°C for
24 h. Cell viability was evaluated with MTT (Promega Corporation,
Madison, WI, USA). Briefly, 20 µl MTT was added to the cells, which
were incubated in the dark for an additional 4 h at 37°C. The
supernatant was then discarded and 100 µl DMSO (Sigma-Aldrich;
Merck KGaA) was added to each well to dissolve the formazan
product. The absorbance of the solution was measured at a 490-nm
excitation and a 520-nm emission wavelength using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Plasmids and cell transfection
C4-2/D cells were transiently transfected with
shRNAs specific against MRP4 or green fluorescent protein (as a
vector control) using Attractene Transfection Reagent (Qiagen GmbH,
Hilden, Germany). C4-2/D shMRP4- and empty vector-trnsfected stable
clones were selected with 2 µg/ml puromycin within 14 days after
transfection, and then maintained in culture medium containing 2
µg/ml puromycin.
Western blot analysis
Cells were washed twice with PBS buffer and lysed in
radioimunnoprecipitation assay lysis buffer (50 mM Tris-Cl pH 7.4,
150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS, 1 mM
EDTA, 100 mM NaF, 1 mM Na3VO4, 1 mM
phenylmethylsulfonyl fluoride and 2 µg/ml aprotinin) on ice. Total
protein (50 µg) was separated by 8% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
nonfat milk in TBS-Tween-20 (TBST; 10 mM Tris pH 7.4, 150 mM NaCl
and 0.1% Tween-20) at room temperature for 2 h, and blots were
probed with an anti-MRP4 antibody (dilution, 1:200; cat no.
ab15598) or anti-GAPDH antibody (dilution, 1:200; cat no. ab181603;
Abcam, Cambridge, MA, USA,) as a control for protein loading.
Subsequent to washing with TBST, the membrane was incubated with a
horseradish peroxidase-conjugated secondary antibody (dilution,
1:1,000; cat. no ab2116; Sigma-Aldrich; Merck KGaA,) for 2 h at
room temperature. Following extensive washing with TBST, the
presence of proteins was visualized by an enhanced
chemiluminescence detection kit according to the manufacturer's
protocol (GE Healthcare Life Sciences, Chalfont, UK).
Determination of DTX concentration
inside cells and in the cell culture supernatant
C4-2/D and C4-2/S cells were incubated with 250 µl
medium containing 40 nM DTX at 37°C for 0.5, 1, 2, 3, 4, 5, 6, 7,
8, 9 or 10 h. Following incubation, the medium at different time
points was transferred into a fresh tube, diluted in 500 µl PBS and
subjected to high-performance liquid chromatography (HPLC). In
order to determine intracellular DTX concentrations, cells were
treated with 0.25% trypsin (Invitrogen; Thermo Fisher Scientific,
Inc.), washed six times with PBS and resuspended in 500 µl PBS.
Cell samples were subsequently frozen in a refrigerator at −60°C
for 1 h and then thawed in a water bath at 37°C for 5 min to lyze
the cells. Following five repetitions of the frozen-and-thaw cycle,
cell lysates were collected and subjected to HPLC. In addition, in
order to observe the effect of androgen on intracellular DTX
concentrations in CRPC cells, cells treated with 40 nM DTX were
maintained at the aforementioned time points under one of the three
following conditions: Medium with PBS; medium with 20 nM DHT; or
medium with 20 nM DHT plus 50 nM BKL. Cell lysates were prepared as
aforementioned.
HPLC was performed on a Waters SunFire C18 column
(250×4.6 mm2; 5 µm; Waters Corporation, Milford, MA,
USA). The mobile phase consisted of water: 0.043 mol/l ammonium
acetate and acetonitrile (53:47). Flow rate was maintained at 1
ml/min with an injection volume of 20 µl. Effluents were monitored
at a detection wavelength of 232 nm.
Statistical analysis
All data are presented as the mean ± standard
deviation. Differences among multiple groups were determined using
one-way analysis of variance followed by the least significant
difference procedure for comparison of mean values. Student's
t-tests were used to assess differences in DTX concentrations
between drug-resistant and parental cells. P<0.05 was considered
to indicate a statistically significant difference. All reported
P-values were two-sided. Statistical analyses were performed using
SPSS 19.0 software (IBM SPSS, Armonk, NY, USA).
Results
Development and characterization of
DTX-resistant prostate cancer cells
In order to determine the association between MRP4
expression and acquired DTX resistance, DTX-resistant C4-2 cells
(C4-2/D) were first established from parental C4-2 (C4-2/S) cells
by culturing C4-2 cells with DTX in a dose-escalation manner (3–40
nM). Following 90 days of selection, cells that survived the
maximum concentration of DTX (40 nM) were used for subsequent
analysis and referred to as C4-2/D cells. In order to test the
effect of DTX on the proliferation of DTX-resistant and -sensitive
cells, a clonogenic ability assay was performed for C4-2/D and
C4-2/S cells following treatment with increasing concentrations of
DTX for 8 h. The clonogenic ability of resistant cells was
significantly increased compared with that of sensitive cells in
response to DTX treatment (P<0.05). When DTX concentration was
increased to 40 nM, there were almost no colonies formed by C4-2/S
cells, while the number of colonies formed by C4-2/D cells was
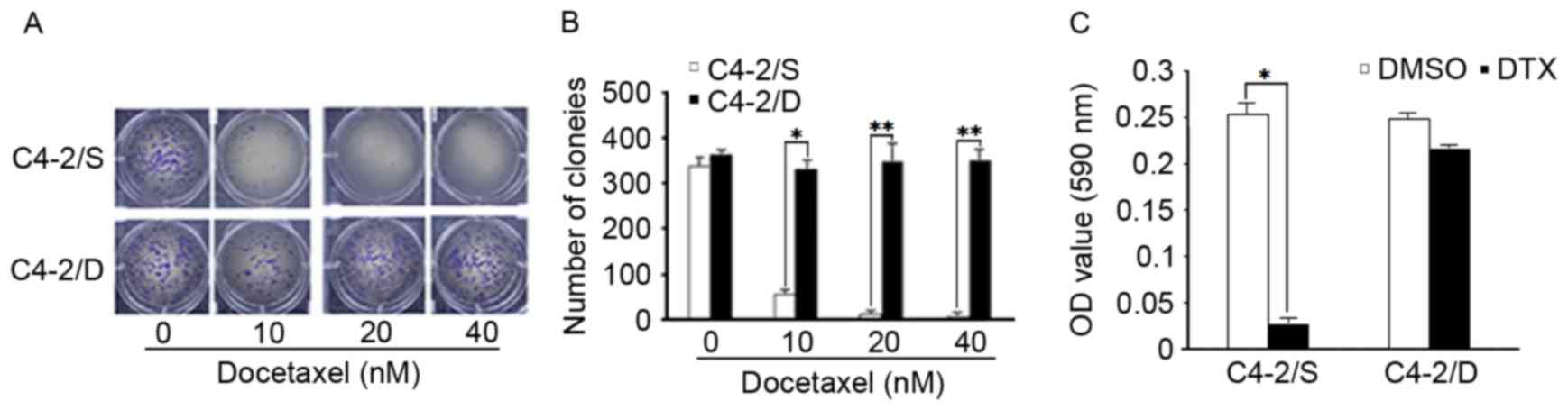
similar to that of the untreated cells (Fig. 1A and B). In order to test the toxicity
of DTX in DTX-resistant and -sensitive cells, C4-2/D and C4-2/S
cells were exposed to 40 nM DTX for 24 h, and cell apoptosis was
analyzed by MTT assay. DTX at a concentration of 40 nM reduced cell
viability in C4-2/S cells (P<0.05), but had little effect on
C4-2/D cells (Fig. 1C).
MRP4 is overexpressed in DTX-resistant
prostate cancer cells and inhibition of MRP4 expression reverses
DTX resistance
MRP4 is a membrane pump involved in the efflux of
chemotherapy drugs (22). In order to
define the expression pattern of MRP4 in DTX-resistant and
-sensitive prostate cancer cells, total RNA was isolated from
C4-2/D and C4-2/S cells, and MRP4 mRNA levels were analyzed by
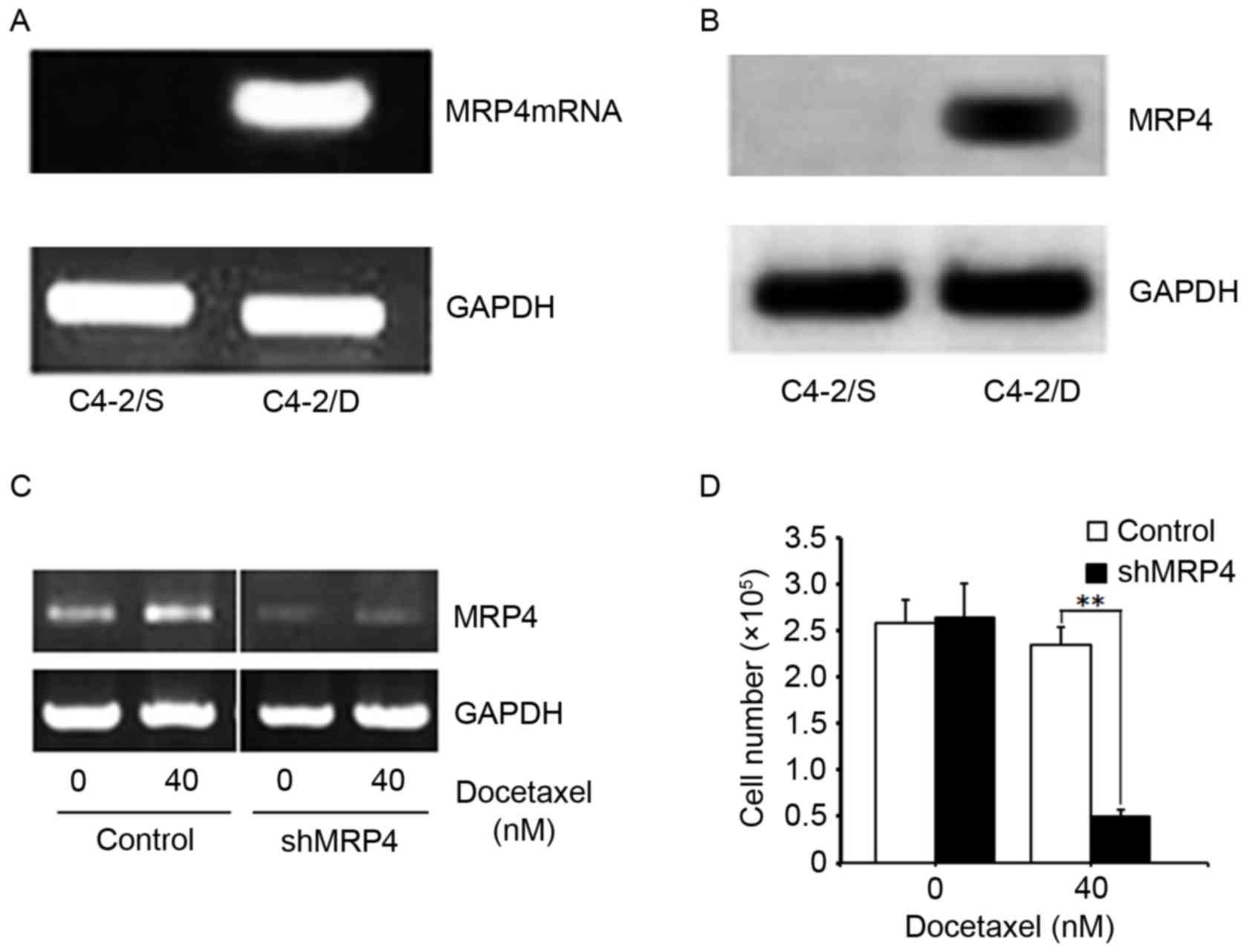
semi-quantitative RT-PCR. As shown in Fig. 2A, MRP4 mRNA was highly expressed in
C4-2/D cells, but was not detectable in C4-2/S cells. MRP4 protein
expression was analyzed by western blotting in whole cell extracts
of C4-2/D and C4-2/S cells. Similar to its mRNA expression, MRP4
protein was strongly expressed in C4-2/D cells (Fig. 2B). These results demonstrated that
MRP4 was overexpressed in DTX-resistant cells at the mRNA and
protein levels.
It was next tested whether overexpression of MRP4
leads to DTX resistance in C4-2/D cells. MRP4 protein expression
was knocked down by MRP4 shRNA in C4-2/D cells (Fig. 2C), followed by treatment with DTX. As
shown in Fig. 2D, inhibition of MRP4
expression by MRP4 shRNA re-sensitized C4-2/D cells to DTX
treatment. These data indicated that MRP4 may be an important
determinant of DTX resistance, and that inhibition of MRP4
expression reverses DTX resistance.
Androgen upregulates MRP4
expression
In our previous study, it was identified that the
DTX-resistance time in CRPC patients with low testosterone levels
was significantly delayed (19). In
the present study, a test was performed to determine whether
androgen leads to MRP4 overexpression in DTX-resistant cells.
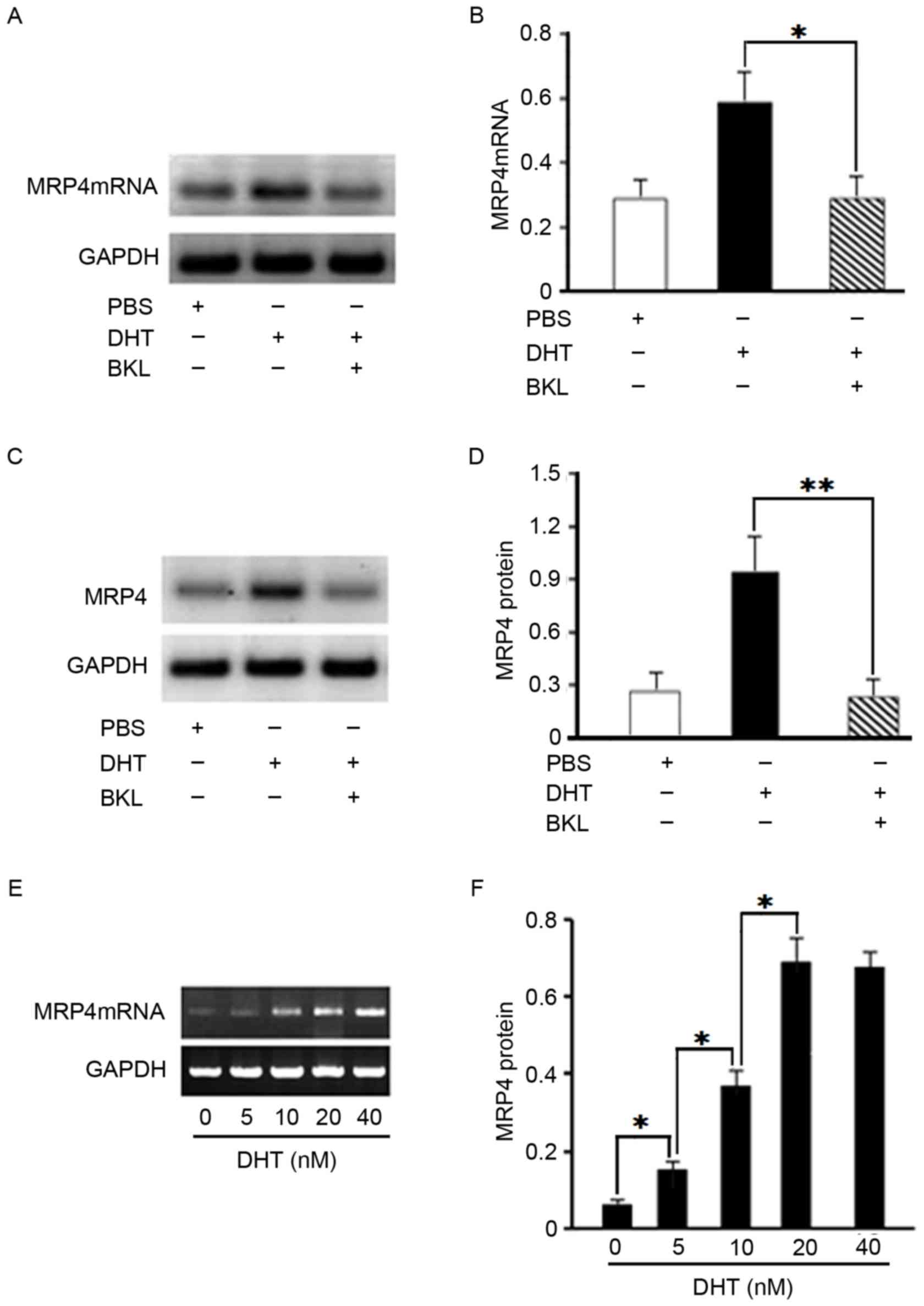
C4-2/D cells were treated with BKL or DHT, and the mRNA and protein
levels of MRP4 were determined by semi-quantitative RT-PCR and
immunoblotting, respectively. MRP4 mRNA levels were upregulated by
DHT treatment, which was significantly prevented by the
anti-androgen BKL in C4-2/D cells (P<0.05; Fig. 3A and B). Similarly, MRP4 protein
levels were increased by DHT treatment, which was significantly
abolished by BKL in C4-2/D cells (P<0.01; Fig. 3C and D). C4-2/D cells were then
treated with different concentrations of DHT (0, 5, 10, 20 or 40
nM), and MRP4 mRNA expression was determined by semi-quantitative
RT-PCR. The results revealed that DHT upregulated the mRNA
expression of MRP4 in C4-2/D cells in a dose-dependent manner
(P<0.05; Fig. 3E and F). The
alteration to MRP4 mRNA expression reached a peak at 20 nM DHT; a
dose of 40 nM DHT was not indicated as statistically more effective
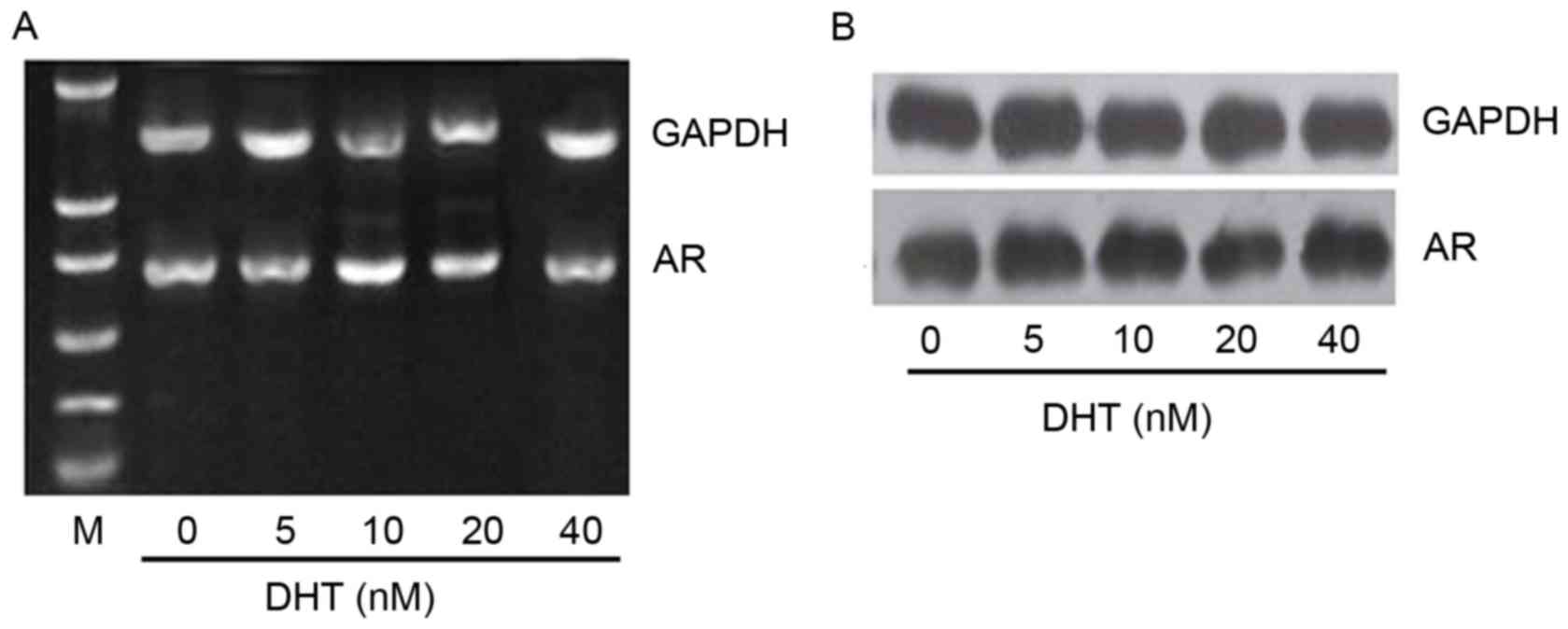
than 20 nM. To assess whether DHT-induced overexpression of MRP4
mRNA results from increased AR levels, the mRNA and protein levels
of AR were examined in C4-2/D cells treated with the different DHT
concentrations as mentioned previously. The results revealed that
neither the mRNA (Fig. 4A) or protein
(Fig. 4B) levels of AR were altered
following treatment of C4-2/D cells with different DHT
concentrations. These results demonstrated that MRP4 was
upregulated by androgen and downregulated by anti-androgen
treatment.
BKL reverses DTX resistance in
DTX-resistant prostate cancer cells
In order to examine whether BKL restores DTX
sensitivity in C4-2/D cells, C4-2/D cells were treated with 40 nM
DTX and 50 nM BKL in the presence or absence of 20 nM DHT. As shown
in Fig. 5A, MRP4 protein level was
significantly upregulated upon treatment of DTX alone or in
combination with DHT, which was abolished by BKL. In addition, MTT
assay revealed that BKL alone had little effect on the viability of
C4-2/D cells in the absence of DHT and DTX, while the combination
DTX and BKL reduced the viability of C4-2/D cells by ~60% in the
presence of DHT (Fig. 5B). Similarly,
the combination of BKL and DTX significantly induced apoptotic cell
death in C4-2/D cells in the presence of DHT (Fig. 5C). Taken together, these results
revealed that BKL restored DTX sensitivity in C4-2/D cells.
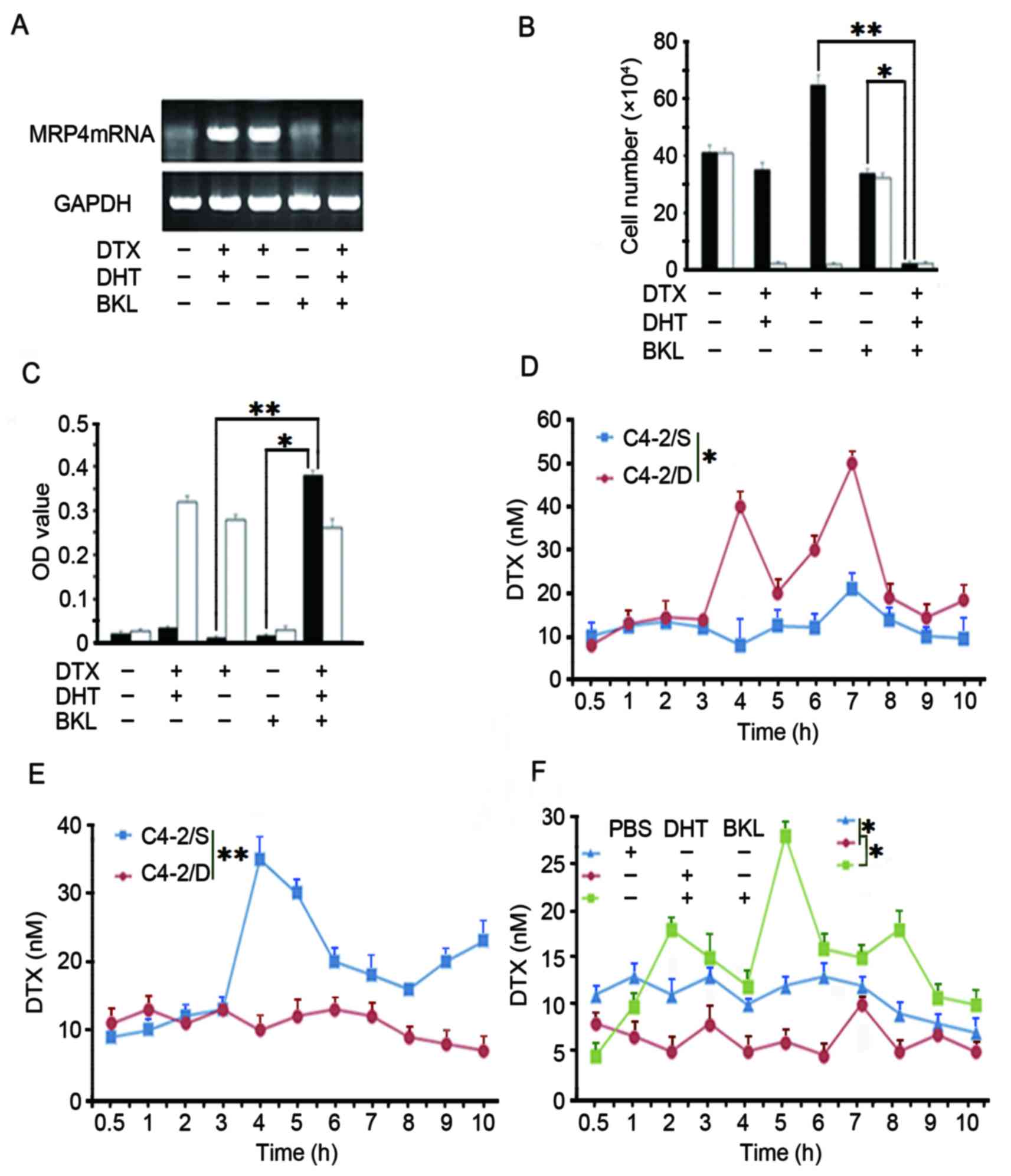 | Figure 5.Downregulation of MRP4 expression
sensitizes DTX-resistant C4-2/D cells to DTX treatment. (A)
DTX-resistant C4-2/D cells were subjected to different treatments
as indicated (40 nM DTX, 20 nM DHT or 50 nM BKL) for 5 days. MRP4
protein levels were determined by western blotting with GAPDH as
loading a control. (B) The extent of apoptotic cell death was
determined using Annexin V-fluorescein isothiocyanate staining
coupled with flow cytometry, following treatment of C4-2/D cells
with different drugs, as indicated. (C) Cell viability was assessed
by an MTT assay, following the treatment of C4-2/D cells with
different drugs, as indicated. (D) C4-2/D and DTX-sensitive C4-2/S
cells were treated with 40 nM DTX for the indicated times. The
concentration of DTX in extracellular fluids was detected at
different time points by HPLC. (E) Cell lysates from C4-2/D or
C4-2/S cells treated with 40 nM DTX were extracted and DTX
concentrations were tested by HPLC. (F) C4-2/D cells were treated
with different drugs as indicated (5 µl PBS, 20 nM DHT or 50 nM
BKL) and the intracellular concentration of DTX was determined by
HPLC. *P<0.05; **P<0.001. DHT, dihydrotestosterone; BKL,
bicalutamide; DTX, docetaxel; HPLC, high-performance liquid
chromatography; MRP4, multidrug resistance protein 4; OD, optical
density. |
In order to clarify whether the BKL reversal of DTX
resistance was caused by a decrease in MRP4 expression,
intracellular and extracellular concentrations of DTX in C4-2/D
cells following treatment with DTX in the presence or absence of
DHT or in combination with BKL were determined. As shown in
Fig. 5D, the cell culture supernatant
concentration of DTX in C4-2/D cells was increased compared with
that in C4-2/S cells. By contrast, the intracellular DTX
concentration in C4-2/D cells was lower compared with that in
C4-2/S cells (Fig. 5E). Notably, the
intracellular DTX concentration in C4-2/D cells treated with DHT
was lower than that in PBS-treated control cells. Furthermore, the
intracellular DTX concentration in C4-2/D cells treated with DHT
plus BKL increased compared with that in cells treated with only
DHT or PBS (Fig. 5F). These data
demonstrated that BKL increased the intracellular DTX concentration
of DTX-resistant prostate cancer cells, indicating that BKL
restores DTX sensitivity by reducing the expression of MRP4.
Discussion
DTX-based therapy is the standard first-line
chemotherapy in patients with metastatic CRPC. However, tumor
progression eventually occurs due to the development of resistance
to DTX treatment (27). In the
present study, a DTX-resistant prostate cancer C4-2/D cell line was
developed from parental androgen-independent C4-2 cells, and the
expression level of MRP4, as well as the association between its
expression and androgen, was investigated. It was revealed that
MRP4 was highly expressed and regulated by androgen in C4-2/D
cells. Notably, the downregulation of MRP4 by the androgen
antagonist BKL re-sensitized C4-2/D cells to DTX treatment. The
present findings indicated that overexpression of MRP4 contributes
to acquired DTX resistance, and indicated that targeting MRP4
expression by anti-androgen treatment is a potential approach to
reverse the sensitivity of DTX-resistant prostate cancer cells to
DTX chemotherapy.
The present study indicated that overexpression of
MRP4 is a novel mechanism of DTX resistance in CRPC. MRP4 mRNA and
protein levels were significantly increased in C4-2/D cells
compared with those in C4-2/S cells, and MRP4 expression levels in
C4-2/D cells were positively associated with cell growth and
negatively associated with cell apoptosis. Furthermore, following
DTX treatment, the DTX concentration was lower in C4-2/D cells than
in C4-2/S cells. By contrast, the extracellular DTX concentration
increased in C4-2/D cells compared with that in C4-2/S cells. This
indicated there is higher MRP4 expression in C4-2/D cells. Finally,
the present results revealed that MRP4 expression levels were
decreased by BKL, indicating that MRP4 is an androgen
signaling-regulated gene. Accordingly, the decrease in MRP4
expression by BKL reversed the intracellular DTX concentration in
C4-2/D cells and sensitized C4-2/D cells to DTX.
Another important question that arose from the
present findings is how AR signaling regulates MRP4 expression. It
has been reported that the binding of androgen to AR results in AR
translocation into the nucleus, whereby AR indirectly binds the
MRP4 promoter and promotes the transcription of the MRP4 gene
(24). In addition, the non-AR
signaling pathway also performs an important role in the regulation
of MRP4 expression. Androgens may increase the levels of cyclic
guanosine monophosphate, which has been implicated in inducing MRP4
expression (28). In addition,
another study demonstrated that androgen induction of E-twenty six
(ETS) translocation variant 1, which is a member of the ETS family
of transcription factors, is associated with enhanced MRP4 gene
expression in liver cancer cells (29). Similarly, in the present study, it was
observed that androgen induced the expression of MRP4 in
DTX-resistant prostate cancer cells. However, in contrast to
previous studies (24,29), the present study observed that MRP4
expression exhibited a certain dose of DHT dependence under
castration levels of testosterone (equivalent to ≤50 ng/dl of serum
testosterone) (30). The effect of
low levels of DHT on MRP4 expression indicates that androgen levels
should be reduced as much as possible during DTX chemotherapy in
order to reduce or delay the resistance of prostate cancer cells to
DTX.
Importantly, the present observation of the
re-sensitization of DTX-resistant cells to DTX treatment by
anti-androgen treatment may have potential clinical implications.
DTX chemotherapy in combination with anti-androgen therapy may
achieve beneficial outcomes for patients with CRPC. It was
considered that such increased activity of this combined treatment
may result from several mechanisms. First, DTX resistance was
associated with MRP4 overexpression, the mechanism of which is
regulated by androgen signaling. Effective reduction of MRP4
expression and restored sensitivity towards DTX by anti-androgen
therapy ultimately achieves the purpose of delaying the resistance
to DTX. In addition, one of the important functions of microtubules
is the transport of ARs. DTX can impair microtubule function and
lead to decreased AR nuclear translocation (31). However, AR nuclear translocation is
significantly enhanced following long-term DTX chemotherapy or the
occurrence of resistance to DTX in prostate cancer (3,32).
Therefore, DTX-induced AR signaling changes may be treated with
anti-androgen therapy, and DTX combined with anti-androgen therapy
may have a synergistic effect. Finally, prostaglandin E2 (PGE2)
performs an important role in the angiogenesis, invasion and
metastasis of tumor cells (33).
However, PGE2 secretion by prostate cancer cells requires the
involvement of MRP4 (34,35). Thus, the suppression of MRP4
expression by blocking androgens may reduce PGE2 secretion.
In summary, DTX may induce MRP4 expression, and DTX
resistance may be associated with the abnormal expression of MRP4
in prostate cancer cells. MRP4 is an androgen responsive- protein
whose expression is upregulated by androgen stimulation, but
downregulated by anti-androgen treatment. To the best of our
knowledge, the present study is the first to propose that DTX
chemotherapy may be combined with targeting MRP4 expression to
reduce androgen levels, or combined with an anti-androgen approach,
which may help delaying or reversing DTX-resistant prostate cancer
cells.
Acknowledgements
The present study was supported by the Hubei
Province Health and Family Planning Scientific Research Project of
China (grant no., WJ2015MB220). The authors would like to thank Dr
Xi Zhou for his comments on the manuscript (Hubei University of
Medicine, Shiyan, Hubei, China).
References
|
1
|
Caffo O, De Giorgi U, Fratino L, Alesini
D, Zagonel V, Facchini G, Gasparro D, Ortega C, Tucci M, Verderame
F, et al: Clinical outcomes of castration-resistant prostate cancer
treatments administered as third or fourth line following failure
of docetaxel and other second-line treatment: Results of an italian
multicentre study. Eur Urol. 68:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramaswamy B and Puhalla S: Docetaxel: A
tubulin-stabilizing agent approved for the management of several
solid tumors. Drugs Today (Barc). 42:265–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madan RA, Pal SK, Sartor O and Dahut WL:
Overcoming chemotherapy resistance in prostate cancer. Clin Cancer
Res. 17:3892–3902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan CJ, Smith MR, Fizazi K, Saad F,
Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small
EJ, et al: Abiraterone acetate plus prednisone versus placebo plus
prednisone in chemotherapy-naive men with metastatic
castration-resistant prostate cancer (COU-AA-302): Final overall
survival analysis of a randomised, double-blind, placebo-controlled
phase 3 study. Lancet Oncol. 16:152–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loriot Y, Miller K, Sternberg CN, Fizazi
K, De Bono JS, Chowdhury S, Higano CS, Noonberg S, Holmstrom S,
Mansbach H, et al: Effect of enzalutamide on health-related quality
of life, pain, and skeletal-related events in asymptomatic and
minimally symptomatic, chemotherapy-naive patients with metastatic
castration-resistant prostate cancer (PREVAIL): Results from a
randomised, phase 3 trial. Lancet Oncol. 16:509–521. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunisawa S, Tange C and Shimozuma K:
Realities in cost-effectiveness analyses: A study of
castration-resistant prostate cancer patients using a medical
claims database. Springerplus. 4:6242015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Liu C, Armstrong C, Lou W, Sandher
A and Gao AC: Antiandrogens Inhibit ABCB1 Efflux and ATPase
activity and reverse docetaxel resistance in advanced prostate
cancer. Clin Cancer Res. 21:4133–4142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hour TC, Chung SD, Kang WY, Lin YC, Chuang
SJ, Huang AM, Wu WJ, Huang SP, Huang CY and Pu YS: EGFR mediates
docetaxel resistance in human castration-resistant prostate cancer
through the Akt-dependent expression of ABCB1 (MDR1). Arch Toxicol.
89:591–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng WE, Chen H, Yuan SF, Wu LL, Zhang W,
Sun HY and Chen WJ: Overexpression of βIII-tubulin and survivin
associated with drug resistance to docetaxel-based chemotherapy in
advanced gastric cancer. J BUON. 17:284–290. 2012.PubMed/NCBI
|
|
13
|
Li W, Zhai B, Zhi H, Li Y, Jia L, Ding C,
Zhang B and You W: Association of ABCB1, β tubulin I, and III with
multidrug resistance of MCF7/DOC subline from breast cancer cell
line MCF7. Tumour Biol. 35:8883–8891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hara T, Ushio K, Nishiwaki M, Kouno J,
Araki H, Hikichi Y, Hattori M, Imai Y and Yamaoka M: A mutation in
beta-tubulin and a sustained dependence on androgen receptor
signalling in a newly established docetaxel-resistant prostate
cancer cell line. Cell Biol Int. 34:177–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamaki H, Harashima N, Hiraki M, Arichi N,
Nishimura N, Shiina H, Naora K and Harada M: Bcl-2 family
inhibition sensitizes human prostate cancer cells to docetaxel and
promotes unexpected apoptosis under caspase-9 inhibition.
Oncotarget. 5:11399–11412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arai T, Miyoshi Y, Kim SJ, Akazawa K,
Maruyama N, Taguchi T, Tamaki Y and Noguchi S: Association of GSTP1
expression with resistance to docetaxel and paclitaxel in human
breast cancers. Eur J Surg Oncol. 34:734–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hendrikx JJ, Lagas JS, Song JY, Rosing H,
Schellens JH, Beijnen JH, Rottenberg S and Schinkel AH: Ritonavir
inhibits intratumoral docetaxel metabolism and enhances docetaxel
antitumor activity in an immunocompetent mouse breast cancer model.
Int J Cancer. 138:758–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelly WK, Halabi S, Carducci M, George D,
Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, et
al: Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keppler D: Multidrug resistance proteins
(MRPs, ABCCs): Importance for pathophysiology and drug therapy.
Handb Exp Pharmacol. 1–323. 2011.
|
|
20
|
Oprea-Lager DE, Bijnsdorp IV, VAN
Moorselaar RJ, VAN DEN Eertwegh AJ, Hoekstra OS and Geldof AA:
ABCC4 Decreases docetaxel and not cabazitaxel efficacy in prostate
cancer cells in vitro. Anticancer Res. 33:387–391. 2013.PubMed/NCBI
|
|
21
|
Savaraj N, Wu C, Wangpaichitr M, Kuo MT,
Lampidis T, Robles C, Furst AJ and Feun L: Overexpression of
mutated MRP4 in cisplatin resistant small cell lung cancer cell
line: Collateral sensitivity to azidothymidine. Int J Oncol.
23:173–179. 2003.PubMed/NCBI
|
|
22
|
Russel FG, Koenderink JB and Masereeuw R:
Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux
transporter for drugs and signalling molecules. Trends Pharmacol
Sci. 29:200–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YH, Wu Q, Xiao XY, Li DW and Wang
XP: Silencing MRP4 by small interfering RNA reverses acquired DDP
resistance of gastric cancer cell. Cancer Lett. 291:76–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai C, Omwancha J, Hsieh CL and
Shemshedini L: Androgen induces expression of the multidrug
resistance protein gene MRP4 in prostate cancer cells. Prostate
Cancer Prostatic Dis. 10:39–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho LL, Kench JG, Handelsman DJ, Scheffer
GL, Stricker PD, Grygiel JG, Sutherland RL, Henshall SM, Allen JD
and Horvath LG: Androgen regulation of multidrug
resistance-associated protein 4 (MRP4/ABCC4) in prostate cancer.
Prostate. 68:1421–1429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YF, Zhang SF, Zhang TT, Li L, Gan W,
Jia HT, Xie S, Ji HH and He DL: Intermittent tri-weekly docetaxel
plus bicalutamide in patients with castration-resistant prostate
cancer: A single-arm prospective study using a historical control
for comparison. Asian J Androl. 15:773–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lattanzio L, Tonissi F, Monteverde M,
Milano G, Merlano MC and Lo Nigro C: Differential molecular
mechanism of docetaxel-octreotide combined treatment according to
the docetaxel-resistance status in PC3 prostate cancer cells.
Anticancer Drugs. 24:120–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sampath J, Adachi M, Hatse S, Naesens L,
Balzarini J, Flatley RM, Matherly LH and Schuetz JD: Role of MRP4
and MRP5 in biology and chemotherapy. AAPS PharmSci. 4:E142002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lasagna N, Fantappiè O, Solazzo M,
Morbidelli L, Marchetti S, Cipriani G, Ziche M and Mazzanti R:
Hepatocyte growth factor and inducible nitric oxide synthase are
involved in multidrug resistance-induced angiogenesis in
hepatocellular carcinoma cell lines. Cancer Res. 66:2673–2682.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moul JW and Dreicer R: Focusing on
testosterone. Urology. 78 5 Suppl:S476–S477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang G, Liu X, Li J, Ledet E, Alvarez X,
Qi Y, Fu X, Sartor O, Dong Y and Zhang H: Androgen receptor splice
variants circumvent AR blockade by microtubule-targeting agents.
Oncotarget. 6:23358–23371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thadani-Mulero M, Portella L, Sun S, Sung
M, Matov A, Vessella RL, Corey E, Nanus DM, Plymate SR and
Giannakakou P: Androgen receptor splice variants determine taxane
sensitivity in prostate cancer. Cancer Res. 74:2270–2282. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jain S, Chakraborty G, Raja R, Kale S and
Kundu GC: Prostaglandin E2 regulates tumor angiogenesis in prostate
cancer. Cancer Res. 68:7750–7759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madrigal-Martinez A, Cazaña FJ and
Fernández-Martínez yA: Role of intracellular prostaglandin E2 in
cancer-related phenotypes in PC3 cells. Int J Biochem Cell Biol.
59:52–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Terada N, Inoue T, Kamba T and Ogawa O:
Novel treatment for prostate cancer targeting prostaglandins. Nihon
Rinsho. 72:2141–2146. 2014.(In Japanese). PubMed/NCBI
|