Introduction
The incidence of brain metastases may be increasing,
due to both improved detection of small metastases by magnetic
resonance imaging (MRI) and improved control of extracerebral
disease as a result of improved systematic therapy (1,2). Whole
brain radiotherapy (WBRT) and Gamma Knife radiosurgery (GKR) are
standard modalities of treatment for brain metastases. WBRT has
been a classical treatment for almost all cases of brain
metastases. GKR has been performed for a limited number and small
sizes of brain metastases (≤4 and <3 cm in diameter) (3,4), but
recent multiple randomized trials support the use of GKR in the
initial management of patients with <5 brain metastases
(5). GKR provides a high control rate
due to spatially accurate and high-conformal isodose curve to the
target. However, the recurrence of brain metastases has been
identified to occur in 10–16% of lesions following treatment with
GKR (5–9). Tumor recurrence usually presents with a
progressive increase in lesion size on gadolinium-enhanced MRI
(Gd-MRI) (10–13). Certain enlargements are caused by
tumor progression, whereas others are caused by radiation effects
without tumor progression. It is often difficult to distinguish
radiation effects from tumor progression. Pathological examination,
including surgical resection or biopsy, is performed to predict the
cause of enlarged lesion size on MRI scans. Although there are
numerous studies (14–17) comparing the pathological findings from
surgical resection or biopsy with MRI findings, this method may not
always be accurate because of the uncertain location information.
Investigations comparing pathological findings from autopsy with
MRI findings is required to acquire accurate interpretations of
MRI.
The aim of the present study was to investigate the
usefulness of MRI for the detection of residual tumors following
GKR for brain metastases. Two hypotheses were investigated: i)
Whether a single MRI may detect tumor existence; and ii) whether a
series of MRIs may detect tumor existence. Follow-up MRI and
pathological results in brain metastases were compared using
autopsy cases to answer these questions.
Materials and methods
Study design
This study was a retrospective case series performed
in a single institution; The University of Tokyo Hospital (Tokyo,
Japan).
Ethics statement
The study protocol was approved by the ethical
review board of the University of Tokyo Hospital (authorization no.
10857). Written informed consent was acquired from patients in
advance, or from surrogates following mortality.
Patients
Patients with metastatic tumors in the brain, who
were treated with GKR and received autopsy with craniotomy between
August 2003 and April 2011 at The University of Tokyo Hospital were
included. Patients with brain metastases without follow-up MRIs
were excluded. The characteristics of the included patients are
summarized in Table I.
 | Table I.Summary of clinical data and
pathological outcomes of 11 lesions. |
Table I.
Summary of clinical data and
pathological outcomes of 11 lesions.
| Lesion no. | Patient age/sex | Primary site | ExCr
lesiona | BT location | Size (mm) | Volume (cc) | Margin dose (Gy) | Isodose curve
(%) | WBRT | Total BED
(3Gy)b (Gy) | CTxc | Intervald (months) | Acute
MRIe | Late MRIf | Pathology |
|---|
| 1a | 71/M | Colon (AC) | Bone | R. frontal | 30 | 9.3 | 20 | 50 | − | 153 | − | 10 | SD | SD | Failure |
| 2a | 64/M | Unknown (AC) | Unknown | L. thalamus | 30 | 8.8 | 18 | 50 | 30 Gy/10 fr
(pre-GKR) | 186 | − | 1.6 | SD | − | Failure |
| 3a | 77/M | Kidney (ccRCC) | Kidney | R. parietal | 20 | 3.01 | 20 | 60 | − | 153 | − | 19 | PD | SD | Remission |
| 4a | 35/F | Breast (AC) | Bone | L. frontal | 15 | 1.88 | 18 | 40 | 40 Gy/20 fr
(pre-GKR) | 193 | + | 19 | SD | PD | Failure |
| 4bg |
|
| Bone | R. temporal | 1st 18; 2nd 32 | 1st 1.76; 2nd
9.85 | 1st 18; 2nd 18 | 1st 40; 2nd 40 | 40 Gy/20 fr
(pre-GKR) | 319 | + | 1st 19; 2nd 7 | 1st SD | 1st PD | Failure |
| 5a | 63/M | Lung (NSCLC) | Lung (primary) | L. parietal | 29 | 9.01 | 20 | 40 | 40 Gy/20 fr
(post-GKR) | 220 | + | 7 | SD | SD | Remission |
| 5b |
|
| Lung (primary) | R. temporal | 13 | 0.934 | 20 | 40 | 40 Gy/20 fr
(post-GKR) | 220 |
| 7 | PR | CR | Remission |
| 6a | 59/M | Kidney (ccRCC) | − | R. frontal | 25 | 6.6 | 20 | 40 | − | 153 | + | 20 | SD | PR | Remission |
| 6b |
|
| − | R. frontal | 8 | 0.27 | 20 | 60 | − | 153 | + | 18 | PD | CR | Remission |
| 6c |
|
| − | R. frontal | 8 | 0.27 | 20 | 50 | − | 153 | + | 10 | PR | CR | Remission |
| 6d |
|
| − | R. occipital | 11 | 0.63 | 20 | 75 | − | 153 | + | 2 | PD | − | Remission |
| Median (range) | − | − | − | − | 18 (8–30) | − | 20 (18–20) | 50 (40–75) | − | − | − | 10 (1.6–20) | − | − | − |
Radiation techniques
All lesions were treated with GKR. A Leksell frame
(Elekta Instruments AB, Stockholm, Sweden) was attached to secure
the head of the patient in place. Subsequently, contrast-enhanced
stereotactic MRI was performed to obtain precise data of the 3D
coordinates of the tumors. The Leksell Gamma Plan (Elekta
Instruments AB) was used to plan the treatment for tumors by
stereotactic Gd-MRI and thin-slice diagnostic Gd-MRI. Treatments
were delivered using the Leksell Gamma Knife B or 4C (Elekta
Instruments AB). A dose of 20 Gy was prescribed for patients in
whom GKR was the initial radiotherapy, and 18 Gy for patients who
had undergone WBRT prior to GKR. The appropriate isodose curves
were calculated considering the shape of the target tumor. Target
volumes were defined based on the area of contrast enhancement plus
a margin of 1 mm. For each patient, the recommendation for WBRT was
based on the attending physician's discretion, using a linear
accelerator with a median prescribed dose of 30 Gy in 3-Gy
fractions or 40 Gy in 2-Gy fractions.
Follow-up MRI and other
modalities
Patients received follow-up MRIs every 1–3 months
following GKR to detect recurrence and/or newly occurring tumors.
Routine MRI sequences included axial T1-weighted imaging (T1WI),
axial T2-weighted imaging (T2WI), coronal fluid-attenuated
inversion recovery (FLAIR), coronal and axial Gd-MRI, axial
diffusion-weighted imaging (DWI), and axial apparent diffusion
coefficient. Fluorodeoxyglucose-positron emission tomography
(FDG-PET) was performed to distinguish tumor recurrence from
radiation effects in an enlarged lesion, or to perform systemic
investigation for the patient with the adenocarcinoma of unknown
origin.
Autopsy and histological
examination
Autopsy was performed within 24 h after mortality.
Brain sections were fixed in 20% neutral-buffered formaldehyde for
2–4 weeks. The cerebrum was cut into coronal slices 7–10-mm thick.
The specimens were cut into small quadrangular pieces and embedded
in paraffin, then cut again to produce 4–6-µm-thick
paraffin-embedded tissue sections. The specimens were mounted on
glass microscope slides coated with 0.01% poly-L-lysine, and
maintained at 37°C in an incubator overnight. Subsequent to
dehydration with an alcohol and xylene series, hematoxylin staining
(2 min 30 sec ×3) and eosin staining (2 min ×1) at room temperature
was performed for primary examination. Sections from formalin-fixed
paraffin-embedded tissue blocks were subjected immunohistochemical
analysis using a Ventana BenchMark XT automated immunostainer
(Roche Diagnostics, Basel, Switzerland). Staining conditions were
as follows; samples were incubated with primary antibody incubation
for 30 min at room temperature, following addition of CC1 standard
(CC1-buffer, 60 min, 95°C). The applied primary antibody was Ki-67
(cat. no. M7240; Clone MIB-1; dilution, 1:200; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA). Immunohistochemical
results of Ki-67 were evaluated for identifying tumor
proliferation. Pathological failure was defined as the detection of
Ki-67-positive tumor cells by histological examination in treated
brain metastases of autopsy specimens.
Analysis
First, the most recent MRI scans and the
pathological results from autopsy were compared. The MRI scans were
reconstructed using a free 3D imaging program, OsiriX (Pixmeo,
Geneva, Switzerland), to adapt the slices of the scans to the cut
slices of brain at autopsy. The last single MRI was compared with
pathological specimen and pathological results in the
gadolinium-enhanced area were obtained. Subsequently, the maximum
diameters of the lesions were measured on a series of Gd-MRI scans,
the percentage of the tumor diameter was calculated, referring to
the diameter of the lesion at the time of GKR as baseline, and a
time-volume curve was produced. The treatment responses of the
tumors were classified as follows: Complete response (CR),
disappearance of target lesion; partial response (PR), ≥30%
decrease in the diameter of the target lesion, taking as reference
the baseline diameter; progressive disease (PD), ≥20% increase in
the diameter of the target lesion, taking as reference the baseline
diameter; stable disease (SD), neither sufficient shrinkage to
qualify for PR nor sufficient increase to qualify for PD, taking as
reference the baseline diameter. The follow-up period following GKR
was divided into three phases: Acute (0–3 months); sub-acute (3–6
months); and late (>6 months). Treatment responses were
categorized by the size changes (CR, PR, SD or PD) and the
follow-up period (acute or late phases). In the present study,
‘temporary enlargement’ was defined an initial growth of >20%,
and reduction to less than the baseline size within 3 months. To
assess the total radiation effect of GKR and WBRT, the total
biological effective dose (BED; 3 Gy) of the tumor margins was
calculated. The present study presumed that BED (3 Gy) reflected
late biological effect from radiation therapy.
Results
Patient characteristics and
treatments
A total of 9 patients with 14 metastatic lesions in
the brain were treated with GKR and received autopsy with
craniotomy between June 1995 and June 2013 at The University of
Tokyo Hospital. Brain metastases without follow-up MRIs were
excluded. As a result, 6 patients with a total of 11 brain
metastases were eligible for the present study. These lesions were
treated with GKR between October 2002 and February 2011.
The median age at diagnosis was 63.5 years (range,
35–77 years). The male:female ratio was 5:1. Sites of primary
tumors were the kidneys in 2 patients, lung in 1 patient, breast in
1 patient, colon in 1 patient and an adenocarcinoma of unknown
origin in 1 patient. The numbers of brain metastases at diagnosis
were 1 tumor in 3 patients, 2 tumors in 2 patients, and 4 tumors in
1 patient, respectively. WBRT was performed in 3 patients prior or
subsequent to GKR. Surgery was performed in 1 patient prior to GKR.
The median follow-up time was 15 months (range, 1.6–20 months).
None of the patients succumbed with any clear evidence of
neurological symptoms. Table I
summarizes the clinical data and pathological outcomes. A total of
11 brain metastases in 6 patients were treated with GKR. The median
prescribed dose was 20 Gy (range, 18–20 Gy) at the tumor margin,
with a median maximal dose of 40 Gy (range, 27–50 Gy). GKR was
performed twice in the same lesion in 1 brain metastasis from
breast cancer (lesion no. 4b).
The pathological outcomes were 7 remissions and 4
failures. The pathological outcomes of the all lesions were the
same in every patient: Either all remissions or all failures.
Although 1 lesion (no. 4b) received the highest total BED (3 Gy) of
319 Gy due to repeated GKR, it did not exhibit radiation
necrosis.
Comparison of the last MRI results
with pathological results
Table II summarizes
the associations between the details of the last MRI and
pathological results. A case of pathological failure is presented
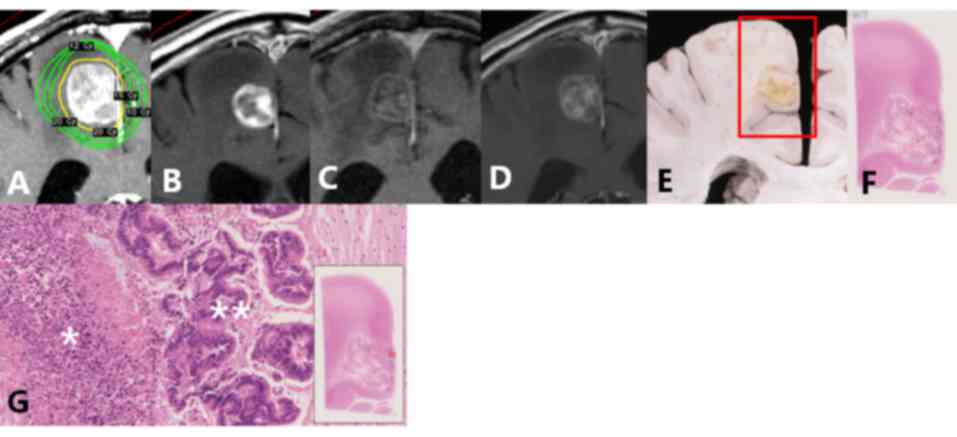
in Fig. 1 (lesion no. 1a). The
contrast-enhanced areas on Gd-MRI contained various pathological
components, including viable tumor cells, tumor necrosis,
hemorrhage, inflammation and vessels. The degree of contrast on
Gd-MRI was similar in all components. All viable tumor tissue and
hemorrhages demonstrated contrast enhancement. Some areas of tumor
necrosis, inflammation and vessels exhibited contrast enhancement,
while others did not.
 | Table II.Comparison of the last MRI results
with pathological results. |
Table II.
Comparison of the last MRI results
with pathological results.
| Lesion no. | Primary site | Size of lesion
(mm) | Pathology | Final MRI
classification | Final MRI
results | Pathological
results |
|---|
| 1a | Colon | 30 | Failure | SD | CE+
(heterogeneity) | Degenerated tumor
(center)+viable tumor (periphery)+TN+ICs |
| 2a | Unknown | 30 | Failure | SD | CE+ (solid) | Viable tumor
(periphery)+TN+ICs |
| 3a | Kidney | 20 | Remission | SD |
CE+/hetero-nodule/CE- |
TN/hemorrhage/fibrosis+TN+ICs |
| 4a | Breast | 15 | Failure | PD | CE+/CE- | Viable tumor
(periphery)+TN+ICs/fibrosis |
| 4ba |
| 1st 18 | Failure | 1st PD | CE+/CE- | Viable tumor
(periphery)+TN+ICs/fibrosis |
| 5a | Lung | 29 | Remission | SD | CE+/CE- | ICs/lack of
tissue |
| 5b |
| 13 | Remission | CR | CE- | TN+ICs |
| 6a | Kidney | 25 | Remission | PR | CE+ | Scar+ICs |
| 6b |
| 8 | Remission | CR | CE- |
Fibrosis+ICs+hemosiderin-phagocytes |
| 6c |
| 8 | Remission | CR | CE- | Fibrosis+TN |
| 6d |
| 11 | Remission | PD | CE+
(heterogeneity) | Hemorrhage+TN |
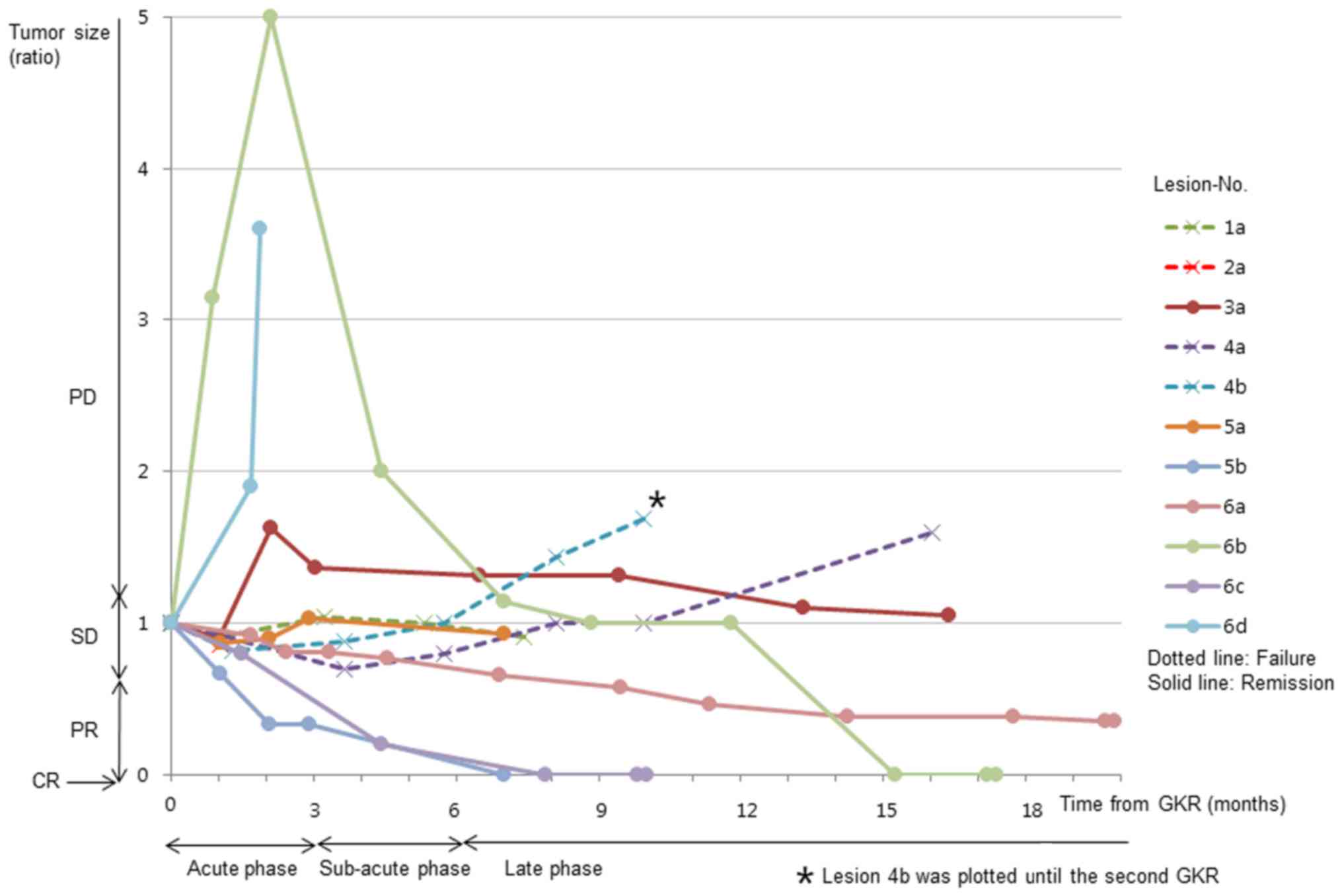
Time-volume curves from a series of
MRI scans
Time-volume curves determined by Gd-MRI are shown in
Fig. 2, a summary of which is listed
in Table III. PD indicated
pathological failures in the late phase. Treatment response,
remission or failure and on time-volume curves of MRI scans were in
agreement with pathological findings, excluding acute PD lesions
(Table II; lesion nos. 3a, 6b and
6d). A total of 2 acute PD lesions (nos. 6b and 6d) demonstrated
high intensity on non-enhanced T1WI, indicating hemorrhage.
Histopathological examination revealed hemosiderin-phagocytes
(lesion no. 6b) and hemorrhages (lesion no. 6d) without viable
tumor tissue. A partially enlarged, high-intensity area on Gd-MRI
was observed in 1 acute PD lesion (no. 3a). The enlarged area on
Gd-MRI was iso-intense on T2WI, indicating an expansion of contrast
enhancement, and not hemorrhage. As the enlarged area on Gd-MRI
reduced in size within 3 months, enlargement was judged to be
temporary. Histopathological examination indicated only edema in
the temporarily enlarged area. Certain SD lesions (nos. 1a and 2a)
demonstrated remnant viable tumor, whereas others (nos. 3a and 5a)
did not in either the acute or the late phase. Late PD lesions
(nos. 4a and 4b) exhibited tumor recurrences (no. 4b).
 | Table III.Association between MRI
classification and pathological outcomes. |
Table III.
Association between MRI
classification and pathological outcomes.
| MRI
classification | Pathological
remission | Pathological
failure | Total |
|---|
| Acute phase |
|
|
|
| CR | 0 | 0 | 0 |
| PR | 2 | 0 | 2 |
| SD | 2 | 4 | 6 |
| PD | 3 | 0 | 3 |
| Late phase |
|
|
|
| CR | 3 | 0 | 3 |
| PR | 1 | 0 | 1 |
| SD | 2 | 1 | 3 |
| PD | 0 | 2 | 2 |
| No
follow-up until late phase | 1 | 1 | 2 |
| Total | 7 | 4 | 11 |
Other sequences and modalities
Regarding DWI, FLAIR and FDG-PET scans, the
examination outcomes are summarized in Table IV. From the results of the DWI scans,
only 1 lesion (no. 2a) exhibited abnormal intensity among the 4
pathological failures (nos. 1a, 2a, 4a and 4b). As for FLAIR,
enlarged peritumoral high intensity was observed in 2 lesions (nos.
4a and 4b) among the 4 pathological failures. Concerning FDG-PET,
increased uptake was observed in 2 lesions (nos. 2a and 4b) but not
in 1 lesion (no. 4a) among the 4 pathological failures.
 | Table IV.Results of DWI, FLAIR, and FDG-PET
scans. |
Table IV.
Results of DWI, FLAIR, and FDG-PET
scans.
| Scan findings | Pathological
remission | Pathological
failure | Total |
|---|
| DWI |
|
|
|
| Normal
intensity | 7 | 3 | 10 |
|
Abnormal intensity | 0 | 1 | 1 |
| FLAIR |
|
|
|
|
Stable | 7 | 2 | 9 |
|
Enlarged peritumoral high
intensity | 0 | 2 | 2 |
| FDG-PET |
|
|
|
| Normal
uptake | 0 | 1 | 1 |
|
Increased uptake | 0 | 2 | 2 |
| No
image | 7 | 1 | 8 |
Discussion
To the best of our knowledge, the present study is
the first to report a precise correlation between MRI and
pathological results in autopsy cases of brain metastases treated
with GKR. In previous studies, correlations between MRI and
pathology using surgical or biopsy specimens were studied while
relying on insufficient orientation; the locations of the MRI
features were not adapted to the location of pathological features
directly (15,18). To resolve this problem, the slices of
the reconstructed MRIs were adapted to the macroscopically viewed
slices of the brain in autopsy cases. This enabled the correct
study of the correlations between MRI and pathological findings.
The treatment method of GKR was standardized, making it easy to
compare its effects. At various timings from acute to late phase,
the histopathological outcomes were evaluated.
Although pathological failure was evident in 4/11
lesions (36%), no patient succumbed to brain metastases during the
course of the present study. It is often hypothesized that
pathological confirmation of remnant viable tumors by surgical
specimen may be an overdiagnosis in view of the clinical course
(15,19).
To investigate the usefulness of MRI for the
detection of residual tumor following GKR for brain metastases, two
hypotheses were investigated: i) Whether a single MRI scan may
detect tumor existence; and ii) whether a series of MRI scans may
detect tumor existence.
According to certain studies (20,21),
contrast enhancement in the central nervous system is a combination
of two primary processes: Vascular (intravascular) enhancement and
interstitial (extravascular) enhancement. New blood vessels
(angiogenesis), active inflammation (infectious and noninfectious),
cerebral ischemia and pressure overload (eclampsia and
hypertension) are known as extravascular enhancement. All of these
changes are associated with alterations in permeability of the
blood-brain barrier (BBB). In the present study, five pathological
components were observed: Viable tumor cells, tumor necrosis,
hemorrhage, inflammation and vessels in the area of contrast
enhancement on Gd-MRI. There were abundant tumor vessels adjacent
to viable tumor tissue and tumor necrosis. BBB of tumor vessels
were vulnerable. Hemorrhage indicated injury of the vessels, and
inflammation occasionally indicated active inflammation. As all of
these components were associated with alterations in BBB
permeability, contrast enhancement itself was not useful for
distinguishing viable tumor tissues from the other components.
Other MRI sequences, such as DWI and FLAIR, were not always useful.
Thus, regarding the first hypothesis, a single MRI was not able to
assess the existence of viable tumor tissue.
Of 3 acute PD lesions, 2 (lesion nos. 6b and 6d)
were confirmed as hemorrhage by histopathology. These two lesions
were metastases from renal cell carcinoma. Brain metastases,
particularly from renal cell carcinoma, tend to exhibit bleeding as
part of the natural course of the disease (22,23), and
bleeding was identified in 9% of patients following GKR in a
previous study (24). Bleeding at the
tumor site is not a rare event following stereotactic irradiation
in renal cell carcinoma.
The third acute PD lesion (no. 3a) exhibited
temporary high-intensity enlargement on Gd-MRI, which demonstrated
iso-intensity on T1WI/T2WI. The histopathology indicated that the
temporarily enlarged area consisted only of edema. Several previous
studies (25,26) demonstrated temporary enlargement of
brain metastases following stereotactic radiosurgery: One study
reported a rate of 12% (11/87 cases) and a median duration of 3
months (range, 2–6 months) (25);
while the other study reported temporary enlargement in 7% (5/73
cases) of all lesions following GKR (26). However, the detailed mechanism of
temporary enlargement is not clear. It was hypothesized that
extravascular enhancement by vessel changes and consequential
increased BBB permeability caused temporary, enhancing enlargement
on MRI. Park et al (27)
suggested that endothelial cells were injured and the permeability
of the vessels rose to a peak within 24 h after irradiation when
normal vessels were irradiated. This increased permeability then
remained for ~1 month. The magnitude of these changes depended on
the radiation dose. An increase in the permeability of vessels led
to invasion of inflammatory cells and fibroblasts, facilitating
fibrosis (22). Similar changes may
have occurred in 1 patient of the present study (no. 3a). As
chronic changes of normal vessels, such as increased wall
thickness, were observed in the area of temporary enlargement,
these normal vessels were assumed to have presented increased
permeability as a result of radiation injury in the acute phase.
Following these acute changes, permeability of these normal vessels
would decrease with increasing wall thickness.
In the present study, 2 acute PR lesions (nos. 5b
and 6c) continued to shrink, becoming CR on the last MRI in the
late phase, and these lesions were confirmed as pathological
remission. Da Silva et al (28) identified that tumors with a histology
associated with high radiosensitivity, such as breast cancer and
non-small-cell lung cancer (NSCLC), tended to shrink in the acute
phase. In the present study, acute shrinkage was observed in the
metastases that arose from NSCLC (lesion no. 5b) and renal cell
carcinoma (lesion no. 6c). Lesions from breast cancer were reduced
by a small amount, but were diagnosed as SD. Tumor necrosis,
inflammation and hemorrhages occurred in the acute or late phases,
and exhibited contrast enhancement in the absence of viable tumor
tissues.
The time-volume curves demonstrated a good
association with pathological outcomes, excluding acute PD. The
determination of acute PD was due to hemorrhage or temporary
enlargement on Gd-MRI. Acute PD lesions decreased in size during
the late phase, indicating that they should be observed for an
extended period. In addition, tumor necrosis should be monitored in
the late phase. There was no case of radiation necrosis in the
present study, but generally it is difficult to distinguish
radiation necrosis from tumor recurrence by MRI results in late PD
lesions (15). Thus, as regards the
second hypothesis, a series of MRI scans may detect the presence of
residual tumor. For acute PD, subsequent time-volume curves were
useful to distinguish pathological remission from failure.
High intensity on DWI, enlarged size of high
intensity on FLAIR, and increased uptake on FDG-PET were
occasionally useful for detecting tumor recurrence, but the absence
of these signs did not always rule out tumor existence.
The present study was subject to certain
limitations. First, it comprised a small number of patients, and
more patients are required to confirm the findings. In addition,
there was a selection bias due to the use of only autopsy cases.
Furthermore, there was a delay between the last MRI and autopsy,
and the MRI results may change within that interval. Additionally,
there was no patient with radiation necrosis included in the
present study.
It may be concluded that time-volume curves from a
series of MRI scans are useful for predicting pathological tumor
responses. Particularly for acute PD lesions exhibiting hemorrhage
or temporary enlargement, subsequent time-volume curves were useful
to distinguish pathological remission from failure. It is
impossible to distinguish pathological failure from remission with
a single MRI. This is because contrast enhancement on Gd-MRI
contained viable tumor tissues, tumor necrosis, hemorrhage,
inflammation and vessels.
Acknowledgements
The authors gratefully acknowledge the work of
members of Department of Pathology and Radiology of The University
of Tokyo Hospital. The authors would also like to thank Mr. Gerz
for careful English proofreading.
Glossary
Abbreviations
Abbreviations:
|
GKR
|
Gamma Knife radiosurgery
|
|
Gd-MRI
|
gadolinium-enhanced magnetic resonance
imaging
|
|
WBRT
|
whole brain radiotherapy
|
|
T1WI
|
T1-weighted imaging
|
|
T2WI
|
T2-weighted imaging
|
|
FLAIR
|
fluid-attenuated inversion
recovery
|
|
DWI
|
diffusion-weighted imaging
|
|
FDG-PET
|
fluorodeoxyglucose-positron emission
tomography
|
|
PR
|
partial response
|
|
PD
|
progressive disease
|
|
SD
|
stable disease
|
|
BBB
|
blood-brain barrier
|
|
NSCLC
|
non-small-cell lung cancer
|
|
BED
|
biological effective dose
|
References
|
1
|
Wen PY and Loeffler JS: Management of
brain metastases. Oncology (Williston Park). 13:941–954, 957-962,
969. 1999.PubMed/NCBI
|
|
2
|
Bouffet E, Doumi N, Thiesse P, Mottolese
C, Jouvet A, Lacroze M, Carrie C, Frappaz D and Brunat-Mentigny M:
Brain metastases in children with solid tumors. Cancer. 79:403–410.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrews DW, Scott CB, Sperduto PW,
Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J,
Bahary JP, et al: Whole brain radiation therapy with or without
stereotactic radiosurgery boost for patients with one to three
brain metastases: Phase III results of the RTOG 9508 randomised
trial. Lancet. 363:1665–1672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kocher M, Soffietti R, Abacioglu U, Villà
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: Adjuvant whole-brain radiotherapy versus
observation after radiosurgery or surgical resection of one to
three cerebral metastases: Results of the EORTC 22952-26001 study.
J Clin Oncol. 29:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto M, Serizawa T, Shuto T, Akabane
A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et
al: Stereotactic radiosurgery for patients with multiple brain
metastases (JLGK0901): A multi-institutional prospective
observational study. Lancet Oncol. 15:387–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Young RF: Radiosurgery for the treatment
of brain metastases. Semin Surg Oncol. 14:70–78. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mori Y, Kondziolka D, Flickinger JC, Logan
T and Lunsford LD: Stereotactic radiosurgery for brain metastasis
from renal cell carcinoma. Cancer. 83:344–353. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mori Y, Kondziolka D, Flickinger JC,
Kirkwood JM, Agarwala S and Lunsford LD: Stereotactic radiosurgery
for cerebral metastatic melanoma: Factors affecting local disease
control and survival. Int J Radiat Oncol Biol Phys. 42:581–589.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirzkall A, Debus J, Lohr F, Fuss M, Rhein
B, Engenhart-Cabillic R and Wannenmacher M: Radiosurgery alone or
in combination with whole-brain radiotherapy for brain metastases.
J Clin Oncol. 16:3563–3569. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuruda JS, Kortman KE, Bradley WG,
Wheeler DC, Van Dalsem W and Bradley TP: Radiation effects on
cerebral white matter: MR evaluation. AJR Am J Roentgenol.
149:165–171. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dooms GC, Hecht S, Brant-Zawadzki M,
Berthiaume Y, Norman D and Newton TH: Brain radiation lesions: MR
imaging. Radiology. 158:149–155. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain R, Narang J, Sundgren PM, Hearshen D,
Saksena S, Rock JP, Gutierrez J and Mikkelsen T: Treatment induced
necrosis versus recurrent/progressing brain tumor: Going beyond the
boundaries of conventional morphologic imaging. J Neurooncol.
100:17–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar AJ, Leeds NE, Fuller GN, Van Tassel
P, Maor MH, Sawaya RE and Levin VA: Malignant gliomas: MR imaging
spectrum of radiation therapy- and chemotherapy-induced necrosis of
the brain after treatment. Radiology. 217:377–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smirniotopoulos JG, Murphy FM, Rushing EJ,
Rees JH and Schroeder JW: Patterns of contrast enhancement in the
brain and meninges. Radiographics. 27:525–551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alomari A, Rauch PJ, Orsaria M, Minja FJ,
Chiang VL and Vortmeyer AO: Radiologic and histologic consequences
of radiosurgery for brain tumors. J Neurooncol. 117:33–42. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamada K, Mastuo T, Tani M, Izumo T,
Suzuki Y, Okimoto T, Hayashi N, Hyashi K and Shibata S: Effects of
stereotactic radiosurgery on metastatic brain tumors of various
histopathologies. Neuropathology. 21:307–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirato M, Hirato J, Zama A, Inoue H, Ohye
C, Shibazaki T and Andou Y: Radiobiological effects of gamma knife
radiosurgery on brain tumors studied in autopsy and surgical
specimens. Stereotact Funct Neurosurg. 66 Suppl 1:S4–S16. 1996.
View Article : Google Scholar
|
|
18
|
Truong MT, St Clair EG, Donahue BR, Rush
SC, Miller DC, Formenti SC, Knopp EA, Han K and Golfinos JG:
Results of surgical resection for progression of brain metastases
previously treated by gamma knife radiosurgery. Neurosurgery.
59:86–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsao MN, Rades D, Wirth A, Lo SS,
Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD,
Wang JZ, et al: Radiotherapeutic and surgical management for newly
diagnosed brain metastasis(es): An American Society for Radiation
Oncology evidence-based guideline. Pract Radiat Oncol. 2:210–225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sage MR, Wilson AJ and Scroop R: Contrast
media and the brain. The basis of CT and MR imaging enhancement.
Neuroimaging Clin N Am. 8:695–707. 1998.PubMed/NCBI
|
|
21
|
Provenzale JM, Mukundan S and Dewhirst M:
The role of blood-brain barrier permeability in brain tumor imaging
and therapeutics. AJR Am J Roentgenol. 185:763–767. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bucci L, Giannini A, Bellotti R,
Castellano AE and Carillo C: Role of hypernephroma in a case of
intracerebral hemorrhage as a 1st sign of metastasis. Case report.
Riv Neurol. 56:325–335. 1986.PubMed/NCBI
|
|
23
|
Bitoh S, Hasegawa H, Ohtsuki H, Obashi J,
Fujiwara M and Sakurai M: Cerebral neoplasms initially presenting
with massive intracerebral hemorrhage. Surg Neurol. 22:57–62. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hernandez L, Zamorano L, Sloan A,
Fontanesi J, Lo S, Levin K, Li Q and Diaz F: Gamma knife
radiosurgery for renal cell carcinoma brain metastases. J
Neurosurg. 97 5 Suppl:S489–S493. 2002.
|
|
25
|
Huber PE, Hawighorst H, Fuss M, van Kaick
G, Wannenmacher MF and Debus J: Transient enlargement of contrast
uptake on MRI after linear accelerator (linac) stereotactic
radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys.
49:1339–1349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peterson AM, Meltzer CC, Evanson EJ,
Flickinger JC and Kondziolka D: MR imaging response of brain
metastases after gamma knife stereotactic radiosurgery. Radiology.
211:807–814. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park KR, Monsky WL, Lee CG, Song CH, Kim
DH, Jain RK and Fukumura D: Mast cells contribute to
radiation-induced vascular hyperpermeability. Radiat Res.
185:182–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Da Silva AN, Nagayama K, Schlesinger D and
Sheehan JP: Early brain tumor metastasis reduction following Gamma
Knife surgery. J Neurosurg. 110:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|