Introduction
Transforming growth factor-β (TGF-β) has an
important role in essential cell phenotypes, particularly in cell
proliferation, differentiation and apoptosis. Paradoxically, TGF-β
can be either a tumor suppressor or a tumor promoter, depending on
the cell context and tissue (1). The
canonical signaling pathway activated by TGF-β is the SMAD pathway
(2). It is also involved in other
signaling pathways, including the mitogen-activated protein kinase,
protein kinase B, mammalian target of rapamycin and nuclear
factor-κB signaling pathways (3).
However, the downstream mechanisms of TGF-β that induce cell growth
suppression have not been verified yet.
The E2F family of transcription factors is a group
of DNA-binding proteins that are involved in cell-cycle
progression, and therefore play a key role in cell proliferation
(4). E2F1has a more pivotal role than
other E2Fs, due to its unique character of inducing both cell-cycle
progression and apoptosis (5). The
transcriptional activity of E2F1 is regulated through the
association with retinoblastoma tumor-suppressor protein (pRb)
(6). Certain studies have reported
that TGF-β regulates the transcription of a number of pro-apoptotic
genes in an E2F1-dependent manner in cancer cell lines from various
tissues (7). To investigate whether
E2F1 could also mediate the TGF-β-induced cytostatic response in
multiple myeloma cells, the MM.1S cell line was used in the present
study.
The present study found that cell proliferation was
markedly inhibited in TGF-β-treated MM.1S cells, and this process
was reversed subsequent to anti-E2F1siRNA treatment. The present
data support the conclusion that E2F1 is a central mediator of the
TGF-β induced growth arrest, at least in one of the multiple
myeloma cell lines.
Materials and methods
Cell lines, reagents, treatments and
transfection
Human myeloma MM.1S cells were obtained from
American Type Culture Collection (Manassas, VA, USA). The multiple
myeloma cells were maintained in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS) (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 50 U/ml penicillin and streptomycin, and cells
were grown at 37°C in a 5% CO2 atmosphere. All TGF-β
treatments were performed in serum-free medium with or without 5
ng/ml TGF-β1 (PeproTech, Rocky Hill, NJ, USA) to cells which were
serum-starved for 24 h. A total of 105 cells per well
were seeded into a 6-well cell culture plate in 2 ml of
antibiotic-free normal growth medium supplemented with FBS. Cells
were transiently transfected with siRNAs against E2F1 (cat. no.
sc-29297) or control siRNA (cat. no. sc-37007; both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), according to the
manufacturer's instructions.
Cell viability assays
The MM.1S cells were seeded in 96-well plates at a
density of 10,000 cells per well. After 24, 48 or 72 h, cell
viability was determined by assaying with MTS assay (Promega Corp.,
Madison, WI, USA). The MTS assay was performed according to the
manufacturer's instructions. Absorbance was measured at 490 nm with
a Chameleon plate reader (Bioscan, Inc., Poway, CA, USA).
Cell cycle analysis
In total, 104 cells from each group were
seeded into a 6-well culture plate. Cells were then harvested
subsequent to culture for 72 h and fixed in 80% ethanol. The fixed
cells were stained with propidium iodide (PI). The cell cycle
distribution analysis was performed by FACS can Flow Cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) and analyzed by the CellQuest
software package. Each procedure was repeated three times.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered
saline, and total proteins were isolated using
radioimmunoprecipitation assay lysis buffer, which included
protease inhibitors (leupeptin, antipain and aprotinin), 0.5 mM
PMSF and 0.2 mM sodium orthovanadate (Thermo Fisher Scientific,
Inc.). Protein amounts were quantified by the Bradford Protein
Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of proteins were loaded and separated on Criterion™
Tris-HCl Precast Gels (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), transferred onto polyvinylidene fluoride membranes, and
probed with the appropriate antibody, as follows: Rabbit polyclonal
anti-E2F1 (cat. no. 3742; dilution, 1:1,000) or anti-phospho-pRb
(cat. no. 9308; dilution, 1:1,000) (Cell Signaling Technology,
Danvers, MA, USA). The antibody was added for an overnight
incubation at 4°C. Membranes were then washed, incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin
(cat. no. P0448; dilution, 1:2,000; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA), and developed with SuperSignal
chemiluminescent substrate (Pierce; Thermo Fisher Scientific,
Inc.).
Statistical analysis
The results are expressed as the mean ± standard
deviation. Subsequent to identifying a normal distribution, data
were compared using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Bonferroni
correction was adopted as the post-hoc test used to control for
multiple comparisons with the same control.
Results
TGF-β-mediated growth suppression in
MM.1S cells
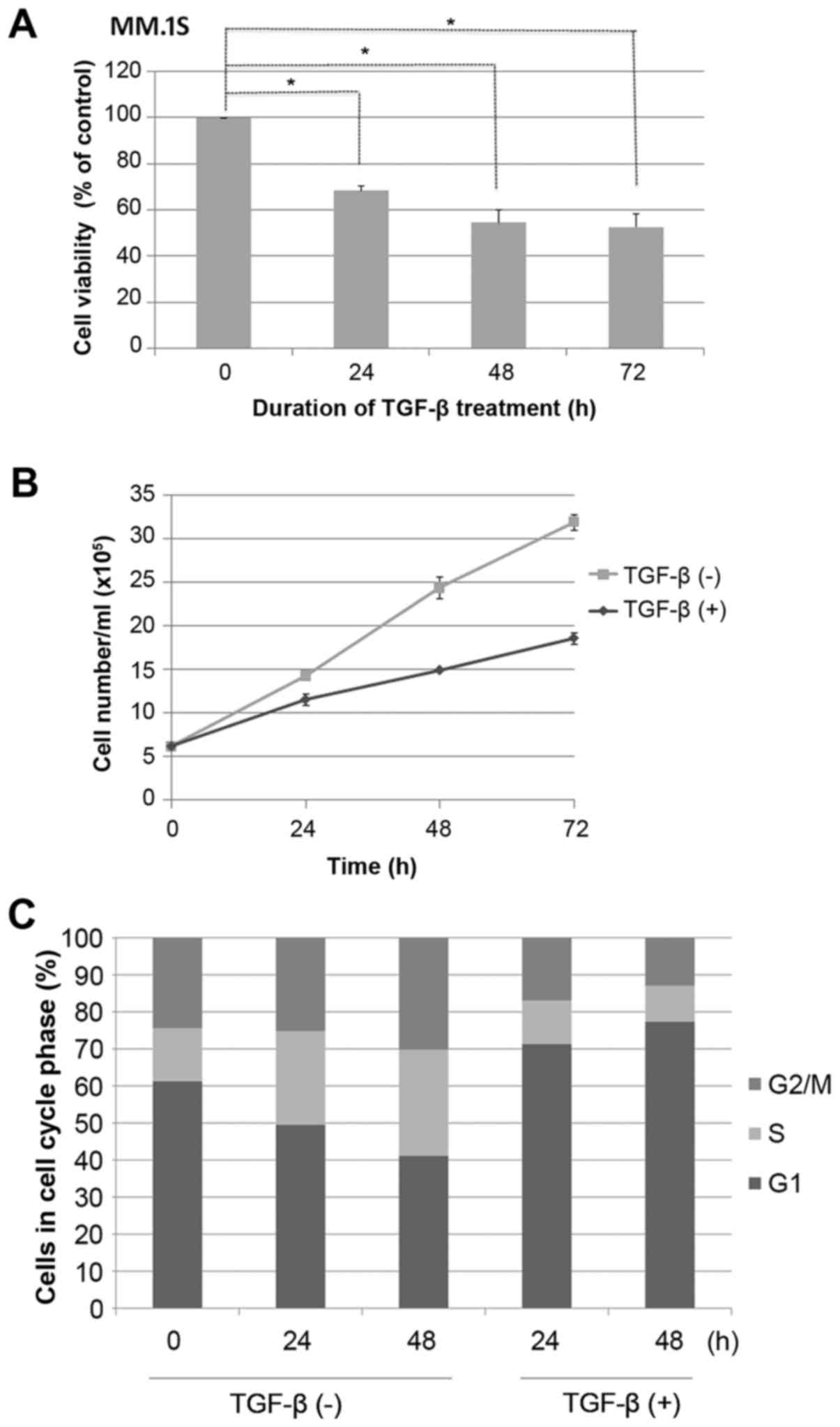
MM.1S cells were grown in the presence or absence of
TGF-β, as indicated, and cell viability was assessed using a MTS
assay. It was found that growth of MM.1S cells was inhibited by
TGF-β in a time-dependent manner (P<0.05; Fig. 1A). This was confirmed by a reduction
in cell count (P<0.05; Fig. 1B).
MM.1S cells were seeded overnight at 3×105/ml, during
which time almost 60% of cells were synchronized in the G1 phase of
the cell cycle, as measured by PI staining (Fig. 1C). Cells were then stimulated or not
with TGF-β for 48 h. Control cells progressed from G1 to S phase
through the cell cycle, whereas TGF-β-treated cells exhibited
efficient G1 cell cycle suppression (P<0.05; Fig. 1C).
E2F1 is required for TGF-β-mediated
growth suppression
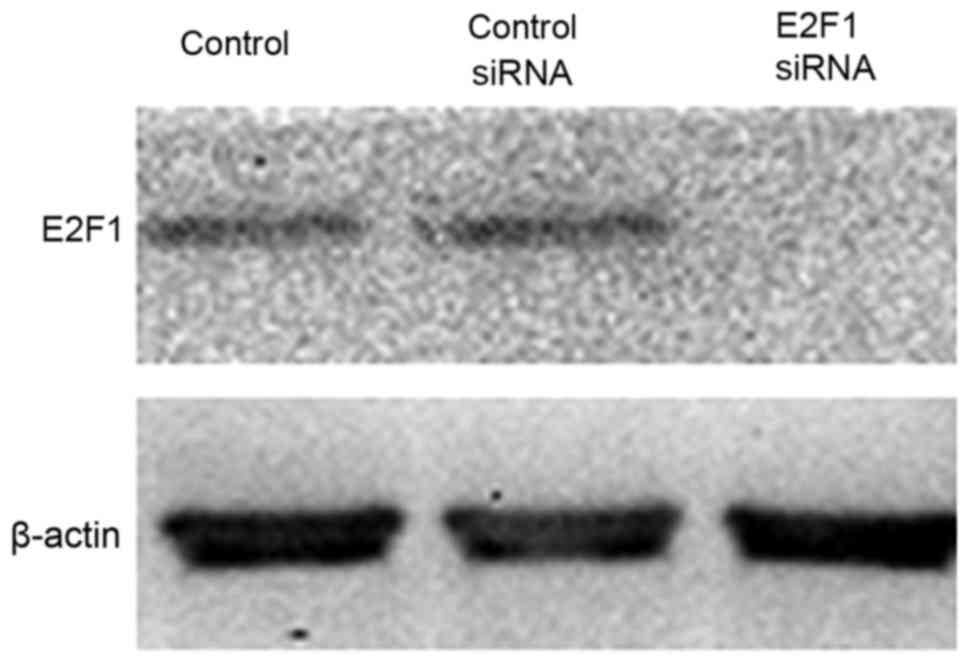
To study the contribution of E2F1 in mediating this
TGF-β response, RNA interference was used to reduce the expression
of endogenous E2F1 (Fig. 2). It was
found that the effect of TGF-β on cell viability in the MM.1S cell
lines tested was almost completely prevented when E2F1 expression
was silenced, indicating that E2F1 is required for mediating the
TGF-β-induced response in the multiple myeloma MM.1S cell line
(P<0.05; Fig. 3A). The cells
transfected with anti-E2F1 siRNA had reduced TGF-β-mediated growth
suppression in cell counting experiments (P<0.05; Fig. 3B and C). The effect of anti-E2F1 siRNA
on the MM.1S cell cycle was examined and each test was repeated
three times. Fig. 3D showed the mean
values of triplicate experiments. Compared with the control MM.1S
cells, the percentage of G1 stage cells was reduced following
transfection with siRNA E2F1 construct. Although the percentage of
S stage cells was significantly increased, no significant change
was identified in the proportion of G2/M stage cells.
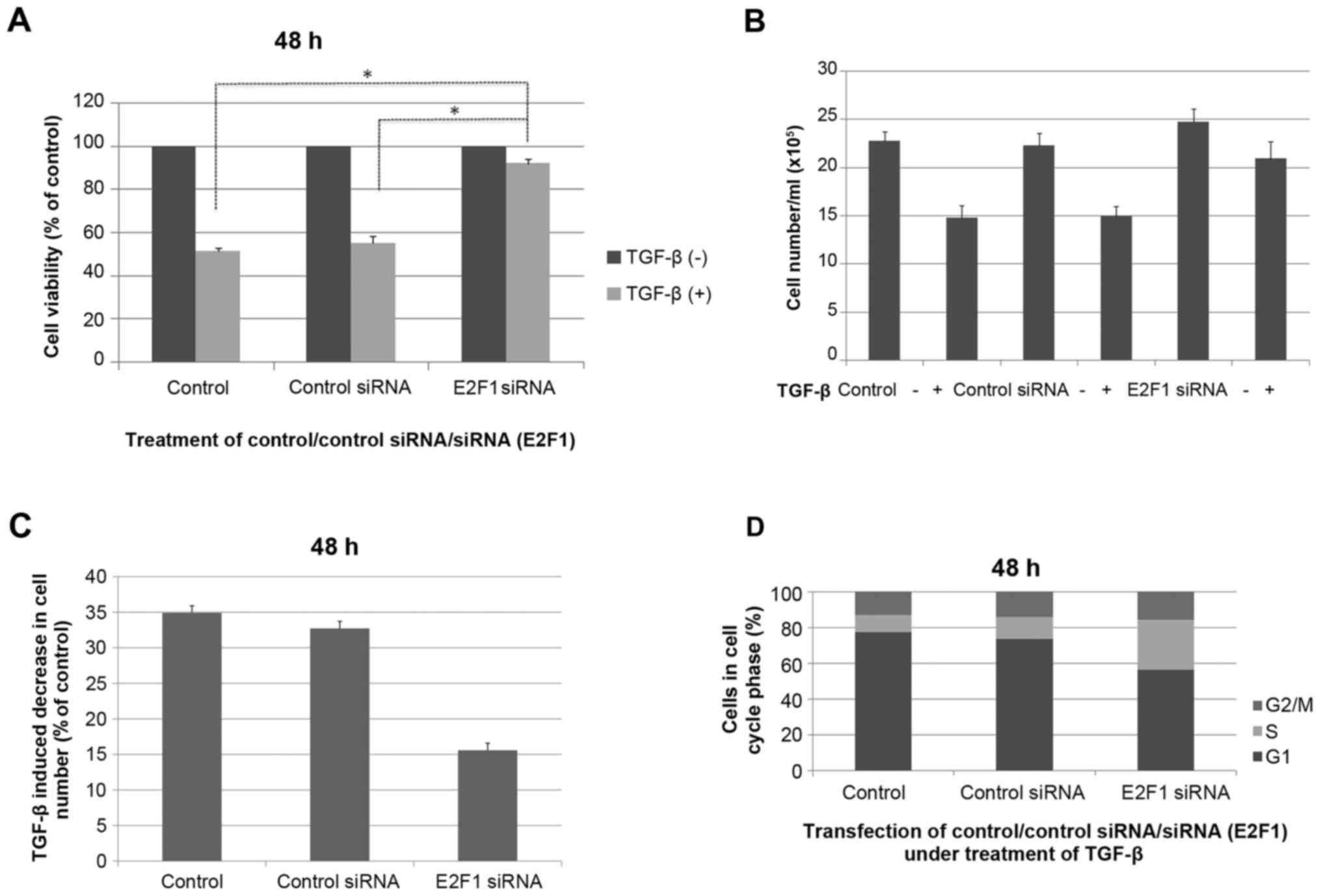 | Figure 3.TGF-β-mediated cell growth suppression
is impaired in E2F1-null MM.1S cells. (A) Cells without
transfection, cells transfected with unrelated siRNA controls and
cells transfected with anti-E2F1 siRNA were stimulated or not with
TGF-β for 48 h, and cell viability was assessed by MTS assay. (B)
Cell numbers following 48 h of TGF-β treatment. Cells were seeded
overnight at 3×105/ml and treated with or without 5 ng/ml TGF-β for
experiment and analyzed as the mean ± standard deviation. (C)
Reduction in cell number induced by TGF-β (expressed as a
percentage of untreated control cultures) determined from three
independent experiments (n=3). (D) MM.1S cells without
transfection, cells transfected with unrelated siRNA controls and
cells transfected with anti-E2F1 siRNA were stimulated with TGF-β
for 48 h, stained with propidium iodide, and analyzed by flow
cytometry. Bar graphs show the percentage of MM.1S cells in each
cell cycle phase for the average of three independent experiments.
Ctl, cells without transfection; siRNA, small interfering RNA; Ctl
siRNA, cells transfected with unrelated siRNA controls; E2F1 siRNA,
cells transfected with anti-E2F1 siRNA; TGF-β, transforming growth
factor-β. *P<0.05. |
TGF-β induces E2F1 protein expression
levels rapidly and transiently
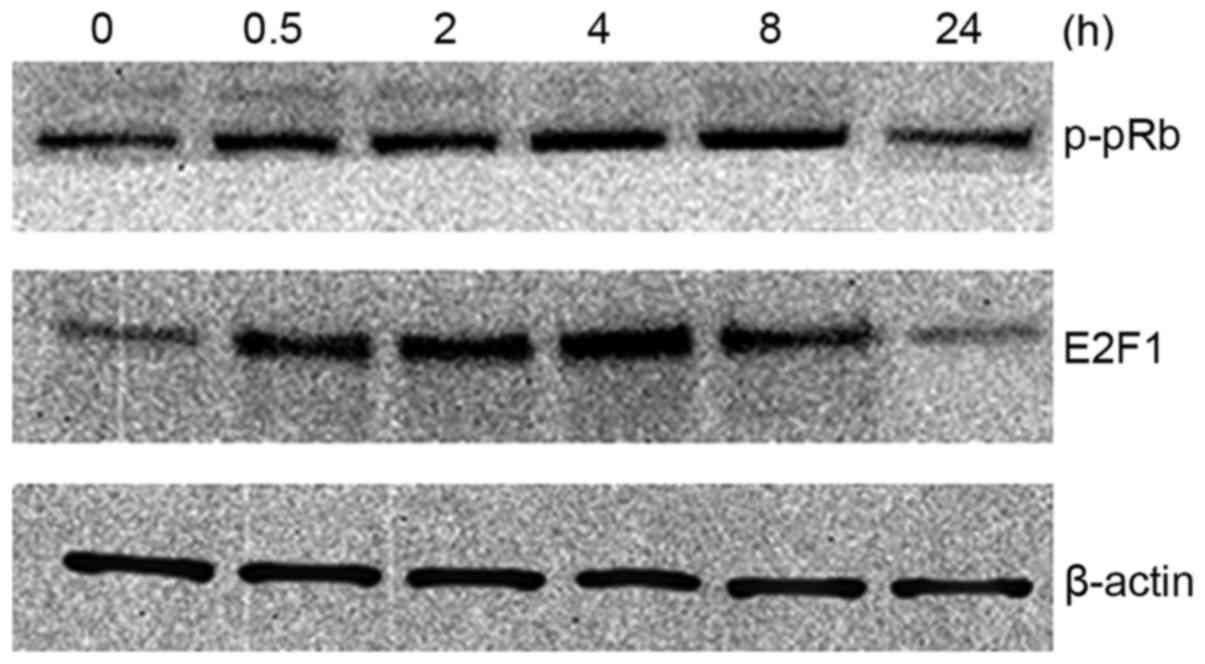
To determine whether E2F1 expression itself is
regulated by TGF-β, the TGF-β effect on E2F1 protein expression
levels was examined in MM.1S cells. As shown in Fig. 4, E2F1 protein levels are induced by
TGF-β. This effect was transient (within 4 h); however, longer
exposure to TGF-β resulted in a return to basal E2F1 protein levels
(within 24 h). It was also found that the phosphorylation state of
pRb showed a similar trend.
Discussion
Although numerous cell proliferation mediators and
signaling pathways have been implicated in TGF-β mediated growth
arrest, the majority of these mechanisms appear to be
tissue-specific and cell type-dependent (8). Previous studies have indicated that the
key event in TGF-β-mediated cytostatic responses in epithelial
cells is c-myc and its target genes. TGF-β-mediated cytostasis is
induced by Smad-dependent regulation of target genes involved in
cell cycle control (9–11). Progression between G1 and S phase is
dependent on cyclin-dependent kinases (CDKs), including CDK2, CDK4,
CDK6, cyclin D and cyclin E (9,10,12,13). The
downregulation of c-myc prevents TGF-β mediated cell cycle arrest
(11). At present, evidence whether
the mechanism is suitable for non-epithelial cells, such as
hematological cells, is lacking (14). The present study analyzed the common
multiple myeloma MM.1S cell line and defined a novel process of the
TGF-β-E2F1 signaling axis, and identified E2F1 as a critical
mediator of the TGF-β proliferation inhibition program in multiple
myeloma cells.
To examine the association between TGF-β, E2F1
expression and proliferation of MM.1S cells, the expression of E2F1
was inhibited by siRNA in MM.1S cells in the present study. The
current study showed that TGF-β-induced growth suppression was
reversed in MM.1S cells subsequent to transfection with anti-E2F1
siRNA. It demonstrated that E2F1 has an essential role in TGF-β
activity and cell proliferation in MM.1S cells.
The pRb-E2F1 complex has been shown to be a
suppressor of E2F target genes, with phosphorylation of pRb leading
to disruption of the pRb-E2F1 complex, which is required to release
free E2F1, in order to induce transcription of its target genes
(6,15,16). Loss
of pRb function induces resistance to TGF-β (17). It is also evident that E2F1 has an
important role in cell growth control and regulation of G1
progression. The control of E2F1 activity may be a downstream event
from the action of G1 CDKs. The growth-suppressing effects of TGF-β
coincide with the downregulation of G1 kinase activities and the
concomitant conversion of Rb to the phosphorylated form (17,18).
Therefore, the E2F1 activity may be an important target of the
TGF-β growth-inhibitory signaling pathway. The present results
support this model, since the TGF-β-mediated growth suppression can
be overcome by reduced expression of the E2F1 product. In the
present study, TGF-β signaling rapidly induced phosphorylation of
pRb, and E2F1 protein levels strongly increased accordingly.
However, the effect was transient, with both the p-pRb and E2F1
protein levels returning to the basal level within 24 h. This
supports the hypothesis that E2F1 activity is controlled through
association with pRb.
In multiple myeloma, TGF-β is secreted by myeloma
and bone marrow stromal cells (19,20). TGF-β
is an important factor during the disease development, which
involves a complex signaling pathway network. The canonical
signaling SMAD (21,22) pathway and the non-SMAD (23–25)
signaling pathways have been studied extensively. Numerous genes,
including c-myc, CDK, p53 and numerous other inhibitors of DNA
binding genes are described as key growth-promoting factors;
however, E2F1is not (26). Spender
and Inman (27) reported that
downregulation of E2F1 is the predominant mechanism by which TGF-β
could induce cell cycle arrest in Burkitt lymphoma cells. Korah
et al (28) reported that E2F1
is central to TGF-β-mediated apoptosis. In the present study, it
was found that the transcription factor E2F1 is involved, at least
partially, in the TGF-β-induced growth arrest. However, the
possibility that induction of other targets or pathways also
contributes to TGF-β-mediated cell growth arrest cannot be
excluded. The possible mechanisms are independent of other
well-established genes and are likely to have occurred downstream
of known signaling pathways. The present data support the notion
that E2F1 is a central mediator of TGF-β induced growth suppression
in MM.1S cells. An improved understanding of the mechanisms by
which both TGF-β and E2F1 exert their roles maybe useful for the
identification of therapeutic strategies in multiple myeloma.
Acknowledgements
The authors thank Dr Baimeng Zhang (Department of
Hepatobiliary Surgery, The Fifth Hospital of Sun Yat-Sen
University) for useful discussions. The present study received
financial support from The Fifth Affiliated Hospital of Sun Yat-sen
University Grants Commission (grant no. 2009058) and
infrastructural support from the Department of General Surgery, The
Fifth Affiliated Hospital of Sun Yat-sen University.
Glossary
Abbreviations
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
pRb
|
retinoblastoma tumor-suppressor
protein
|
|
CDK
|
cyclin-dependent kinase
|
References
|
1
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YG: Endocytic regulation of TGF-beta
signaling. Cell Res. 19:58–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bell LA and Ryan KM: Life and death
decisions by E2F-1. Cell Death Differ. 11:137–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Field SJ, Tsai FY, Kuo F, Zubiaga AM,
Kaelin WG Jr, Livingston DM, Orkin SH and Greenberg ME: E2F-1
functions in mice to promote apoptosis and suppress proliferation.
Cell. 85:549–561. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dyson N: The regulation of E2F by
pRB-family proteins. Genes Dev. 12:2245–2262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pardali K and Moustakas A: Actions of
TGF-beta as tumor suppressor and pro-metastatic factor in human
cancer. Biochim Biophys Acta. 1775:21–62. 2007.PubMed/NCBI
|
|
9
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CR, Kang Y and Massagué J: Defective
repression of c-myc in breast cancer cells: A loss at the core of
the transforming growth factor beta growth arrest program. Proc
Natl Acad Sci USA. 98:pp. 992–999. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warner BJ, Blain SW, Seoane J and Massagué
J: Myc downregulation by transforming growth factor beta required
for activation of the p15(Ink4b) G(1) arrest pathway. Mol Cell
Biol. 19:5913–5922. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iavarone A and Massagué J: Repression of
the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta
in cells lacking the CDK inhibitor p15. Nature. 387:417–422. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baughn LB, Di Liberto M, Niesvizky R, Cho
HJ, Jayabalan D, Lane J, Liu F and Chen-Kiang S: CDK2
phosphorylation of Smad2 disrupts TGF-beta transcriptional
regulation in resistant primary bone marrow myeloma cells. J
Immunol. 182:1810–1817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molina-Privado I, Rodríguez-Martínez M,
Rebollo P, Martín-Pérez D, Artiga MJ, Menárguez J, Flemington EK,
Piris MA and Campanero MR: E2F1 expression is deregulated and plays
an oncogenic role in sporadic Burkitt's lymphoma. Cancer Res.
69:4052–4058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hallstrom TC, Mori S and Nevins JR: An
E2F1-dependent gene expression program that determines the balance
between proliferation and cell death. Cancer Cell. 13:11–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun B, Wingate H, Swisher SG, Keyomarsi K
and Hunt KK: Absence of pRb facilitates E2F1-induced apoptosis in
breast cancer cells. Cell Cycle. 9:1122–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwarz JK, Bassing CH, Kovesdi I, Datto
MB, Blazing M, George S, Wang XF and Nevins JR: Expression of the
E2F1 transcription factor overcomes type beta transforming growth
factor-mediated growth suppression. Proc Natl Acad Sci USA. 92:pp.
483–487. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chellappan SP, Hiebert S, Mudryj M,
Horowitz JM and Nevins JR: The E2F transcription factor is a
cellular target for the RB protein. Cell. 65:1053–1061. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohammad KS, Chen CG, Balooch G, Stebbins
E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH,
Ionova-Martin SS, et al: Pharmacologic inhibition of the TGF-beta
type I receptor kinase has anabolic and anti-catabolic effects on
bone. PLoS One. 4:e52752009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto T and Abe M: TGF-β-related
mechanisms of bone destruction in multiple myeloma. Bone.
48:129–134. 2001. View Article : Google Scholar
|
|
21
|
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu
YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL
and Falb D: The MAD-related protein Smad7 associates with the
TGFbeta receptor and functions as an antagonist of TGFbeta
signaling. Cell. 89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hata A, Lagna G, Massagué J and
Hemmati-Brivanlou A: Smad6 inhibits BMP/Smad1 signaling by
specifically competing with the Smad4 tumor suppressor. Genes Dev.
12:186–197. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bakin AV, Rinehart C, Tomlinson AK and
Arteaga CL: p38 mitogen-activated protein kinase is required for
TGFbeta-mediated fibroblastic transdifferentiation and cell
migration. J Cell Sci. 115:3193–3206. 2002.PubMed/NCBI
|
|
24
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhowmick NA, Ghiassi M, Bakin A, Aakre M,
Lundquist CA, Engel ME, Arteaga CL and Moses HL: Transforming
growth factor-beta1 mediates epithelial to mesenchymal
transdifferentiationthrough a RhoA-dependent mechanism. Mol Biol
Cell. 12:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spender LC and Inman GJ: TGF-beta induces
growth arrest in Burkitt lymphoma cells via transcriptional
repression of E2F-1. J Biol Chem. 284:1435–1442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korah J, Falah N, Lacerte A and Lebrun JJ:
A transcriptionally active pRb-E2F1-P/CAF signaling pathway is
central to TGFβ-mediated apoptosis. Cell Death Dis. 3:e4072012.
View Article : Google Scholar : PubMed/NCBI
|