Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors, with a mortality rate of ~1 million people
per year worldwide (1–4). However, the precise molecular mechanism
of HCC tumorigenesis remains largely unknown. Potentially curative
therapies include surgical resection, transplantation and
percutaneous ablation, but are not suitable for all patients.
Surgery is effective for early-stage HCC and the postoperative
recurrence rate is low; however, in a large number of patients, HCC
is identified at an advanced disease stage, and thus are not
suitable for surgical intervention. Chemotherapy is an alternative
option for these patients, but development of resistance limits the
success of these approaches (5).
Thus, the development of potent and effective therapeutic
modalities for the treatment of HCC, particularly late-stage HCC,
is urgently required.
It has been well established that the aberrant
activation of signaling pathways controlling normal cellular
proliferation (including epidermal growth factor and
RAS/mitogen-activated protein kinase pathways), survival (including
the AKT/mechanistic target of rapamycin pathway), differentiation
(including the Wnt and hedgehog pathways) and angiogenesis
(including the vascular endothelial growth factor and
platelet-derived growth factor pathways) are heterogeneously
dysregulated in the majority of solid tumors (6). The dependence of cancer cells on
gain-of-function mutations to oncogenes and loss-of-function
mutations to tumor suppressor genes has led to the development of
successful targeted molecular therapies. In 2008, sorafenib, a
small molecule inhibitor of multiple tyrosine kinases, was approved
for the clinical treatment of several cancer types. Sorafenib
significantly prolongs median overall survival, resulting in a 44%
improvement in the survival of HCC patients compared with placebo
(7). Targeted molecular therapy is an
emerging field for the treatment of various types of cancer, with
numerous agents targeting different cancer-associated signaling
molecules currently undergoing preclinical and clinical trials.
The Wnt signaling pathway has been investigated
intensively due to its pivotal role in regulating the expression of
numerous genes that are critical for cell proliferation and
differentiation. The Wnt signaling pathway is perturbed in a number
of diseases, including cancer, and diseases of the bone and
cardiovascular system (8). The Wnt
pathway is upregulated in >30% of HCCs (9,10),
suggesting that targeting Wnt signaling is a promising strategy for
the treatment of HCC.
Qin Pi is a traditional Chinese medicine and a
common natural antioxidant. Qin Pi contains several
pharmacologically active ingredients, including esculetin, that
have in vivo anti-inflammatory and peripheral analgesic
activity (11). Esculetin has been
reported to suppress oxidative damage to cellular DNA induced by
lipid hydroperoxide (12). Notably,
esculetin has been demonstrated to suppress the proliferation of
human colon cancer cells by directly targeting β-catenin (13). Kim et al (14) reported that esculetin induced the
death of human colon cancer cells via the reactive oxygen
species-mediated mitochondrial apoptosis pathway. In addition, Wang
et al (15) reported that
esculetin induces apoptosis in HCC cells by initiating a
mitochondrial-dependent apoptosis pathway. However, whether
esculetin inhibits the growth and proliferation of HCC via the Wnt
signaling pathway remains to be determined.
In the present study, the effects of esculetin on
the growth and proliferation of human HCC SMMC-7721 cells were
investigated and the activity of the Wnt signaling pathway was
assessed following esculetin treatment.
Materials and methods
Chemicals and reagents
RPMI-1640, fetal bovine serum, and trypsin were
purchased from Hyclone (GE Healthcare, Logan, UT, USA). Penicillin,
streptomycin, and trypsin were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham MA, USA). Sodium dodecylsulfate, Ponceau
S, dithiothreitol, phenylmethylsulfonyl fluoride and bovine serum
albumin were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). The polymerase chain reaction (PCR) kit and the
SYBR-Green Quantitative RT-PCR kit were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Anti-β-catenin (cat. no.
8480; dilution 1:1,000), anti-cyclin D1 (cat. no. AB20509a;
dilution 1:1,000), anti-c-Myc (cat. no. BS2462; dilution 1:1,000),
anti-β-actin (cat. no. bs-0061R; dilution 1:1,000),
anti-phospho-β-catenin (Ser33/Ser37/Thr41) (cat. no. 9561; dilution
1:1,000) and horseradish peroxidase-conjugated goat anti-rabbit
(cat. no. BA1054; dilution 1:10,000) antibodies were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Esculetin was obtained from the Food and Drug
Verification Research Institute in China (batch number,
110741-200506; Beijing, China). Esculetin was dissolved in dimethyl
sulfoxide (DMSO) to obtain a stock solution with a concentration of
0.8 M, which was stored at 4°C. Cells were treated with esculetin
at a final concentration of 50, 100, 200, 300, 400 or 500
µmol/l.
Cell culture and treatment
Human HCC SMMC-7721 cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). Normal liver
HL-7702 cells were obtained from Obio Technology Co., Ltd.
(Shanghai, China). The cells were maintained in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum and 1%
penicillin-streptomycin at 37°C in a 5% CO2 incubator.
Cells were treated with vehicle (0.5% DMSO) alone, or 50, 100, 200,
300, 400 or 500 µmol/l esculetin for 24, 48 and 72 h.
Cell viability assay
Cell viability was determined using an MTT assay.
SMMC-7721 cells and HL-7702 cells were seeded at a density of
1×105 cells/well in 96-well plates. After incubation for
~24 h at 37°C in a 5% CO2 incubator, the cells were
treated with the aforementioned concentrations of esculetin. The
MTT assay was performed after 24, 48 and 72 h of treatment. The
culture medium was discarded, 30 µl 0.5% (w/v) MTT dissolved in 1X
PBS was added to each well and the plate was incubated for 3 h at
37°C. After incubation for 3 h, the culture medium was discarded
and 120 µl DMSO was added into each well. Following incubation for
30 min and slow shaking for 15 min at 37°C, the absorbance at 490
nm was measured with a microplate reader. The experiment was
repeated in triplicate. Cell viability was expressed as a
percentage of proliferation against the control (untreated cells),
which was set at 100%.
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR) analysis
SMMC-7721 cells were cultured in 6-well plates.
Following treatment with 100 or 300 µmol/l esculetin or DMSO for
24, 48 and 72 h, the total RNA was extracted using TRIzol (Gibco;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The RNA concentration was determined using a Nanodrop
8000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE,
USA), and the integrity of RNA was visualized on a 1% agarose gel
using a gel documentation system (Universal Hood II; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). qPCR was performed in a
20-µl reaction mixture using SYBR-Green Quantitative RT-PCR kit and
one-step PCR.
The sequences of the primers were designed using
Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA, USA) and are in
Table I. The PCR procedure was as
follows: Pre-denaturation at 95°C for 30 sec, denaturation at 95°C
for 5 sec, annealing at 60°C for 30 sec, and extension at 72°C for
15 sec. The PCR was performed for 40 cycles, followed by a final
extension at 72°C for 10 min. The threshold cycle (Cq)
correlates inversely with the target mRNA level, and the relative
quantitation of the relative gene expression levels was calculated
using the 2−ΔΔCq method (16).
 | Table I.Sequences of primers for reverse
transcription-polymerase chain reaction. |
Table I.
Sequences of primers for reverse
transcription-polymerase chain reaction.
| Gene | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| β-catenin |
CCAAGTGGGTGGTATAGAGG |
AGTCCATAGTGAAGGCGAAC |
| c-Myc |
TTGTTGCGGAAACGACG |
TCATAGGTGATTGCTCAGGAC |
| cyclin D1 |
GCATGTTCGTGGCCTCTAAG |
TTCAATGAAATCGTGCGGGG |
| GAPDH |
ACCAAATTGCCAGAGTGACC |
CAAAGCAGCATCTCATCCAA |
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer containing 1% protease
inhibitor cocktail (Roche Applied Science, Penzberg, Germany).
Equal amounts of protein extracts (50 µg) were separated using 10%
SDS-PAGE and subsequently transferred onto a polyvinylidene
difluoride membrane. Membranes were blocked with 5% (w/v) skimmed
milk dissolved in Tris-buffered saline plus Tween-20 [TBS-T; 0.1%
Tween-20; (pH 8.3)] at room temperature for 1 h. The membranes were
then incubated overnight at 4°C with primary antibodies (all
1:1,000 dilution). Following washing with Tween-20 [TBS-T; 0.1%
Tween-20 (pH 8.3)], the membranes were incubated with horseradish
peroxidase-labeled secondary antibodies for 60 min at room
temperature. The immunoreactive bands were visualized using an
enhanced chemiluminescence kit (GE Healthcare Life Sciences).
β-actin was used as a loading control.
Statistical analysis
GraphPad Prism 5.02 (GraphPad Software, Inc., La
Jolla, CA, USA) and Microsoft Excel (Microsoft Corporation,
Redmond, WA, USA) were used for statistical analysis. Statistical
analysis of data for multiple groups was performed using one-way
analysis of variance and Dunnett's test. All experiments were
performed at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
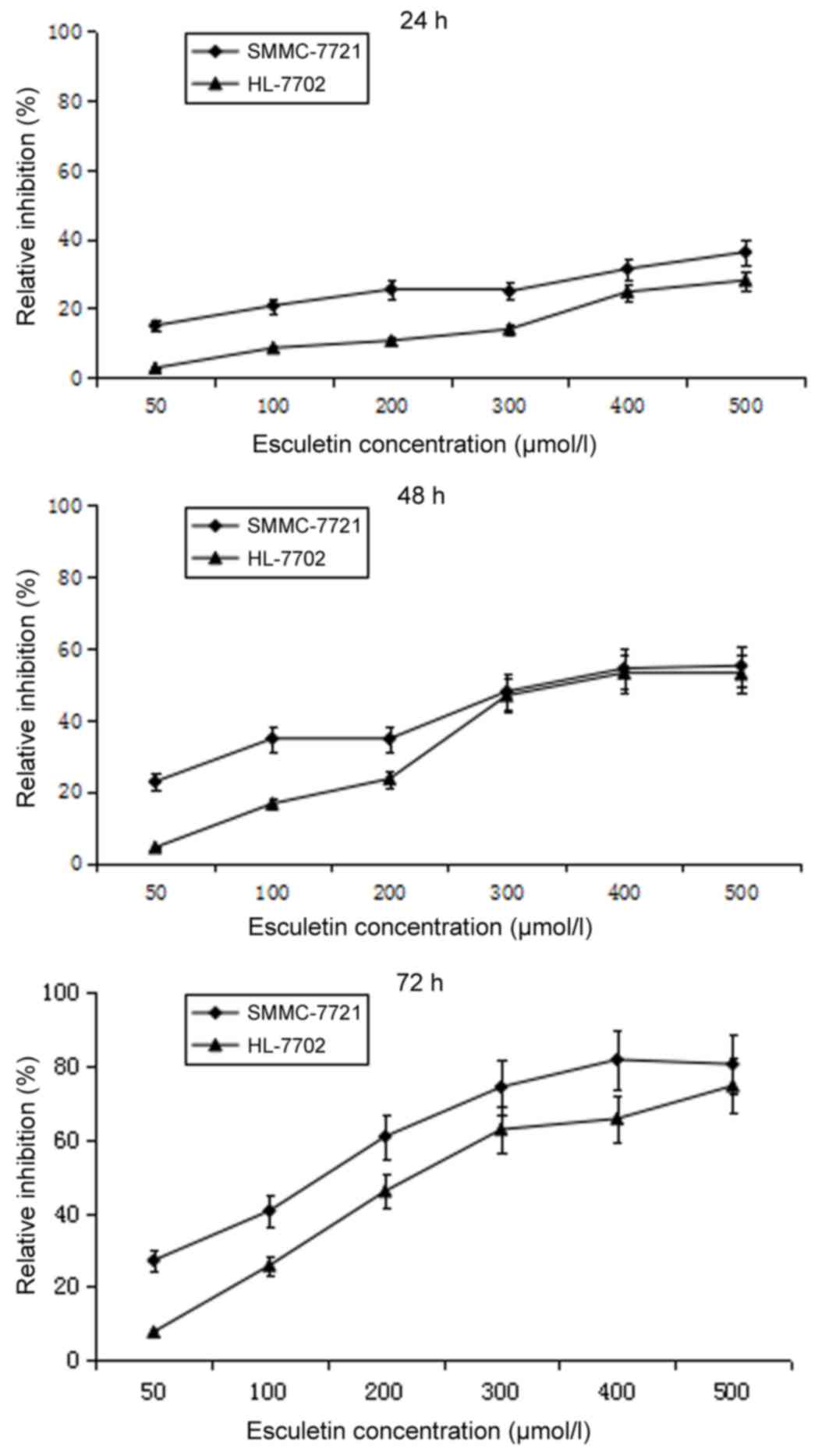
Esculetin dose- and time-dependently
inhibits the proliferation of SMMC-7721 and HL-7702 cells
To test the cytotoxicity of esculetin, SMMC-7721
cells and normal liver HL-7702 cells were treated with different
concentrations of esculetin (50, 100, 200, 300, 400 or 500 µmol/l)
for 24, 48 and 72 h, and the cell viability was assessed using an
MTT assay. Compared with the 0.5% DMSO control, esculetin inhibited
the proliferation of SMMC-7721 and HL-7702 cells in a dose- and
time-dependent manner (Fig. 1). A
pre-experiment was carried out to define the experiment density of
DMSO, and it was found that 0.5% DMSO has no effect on SMMC-7721 or
HL-7702 cells.
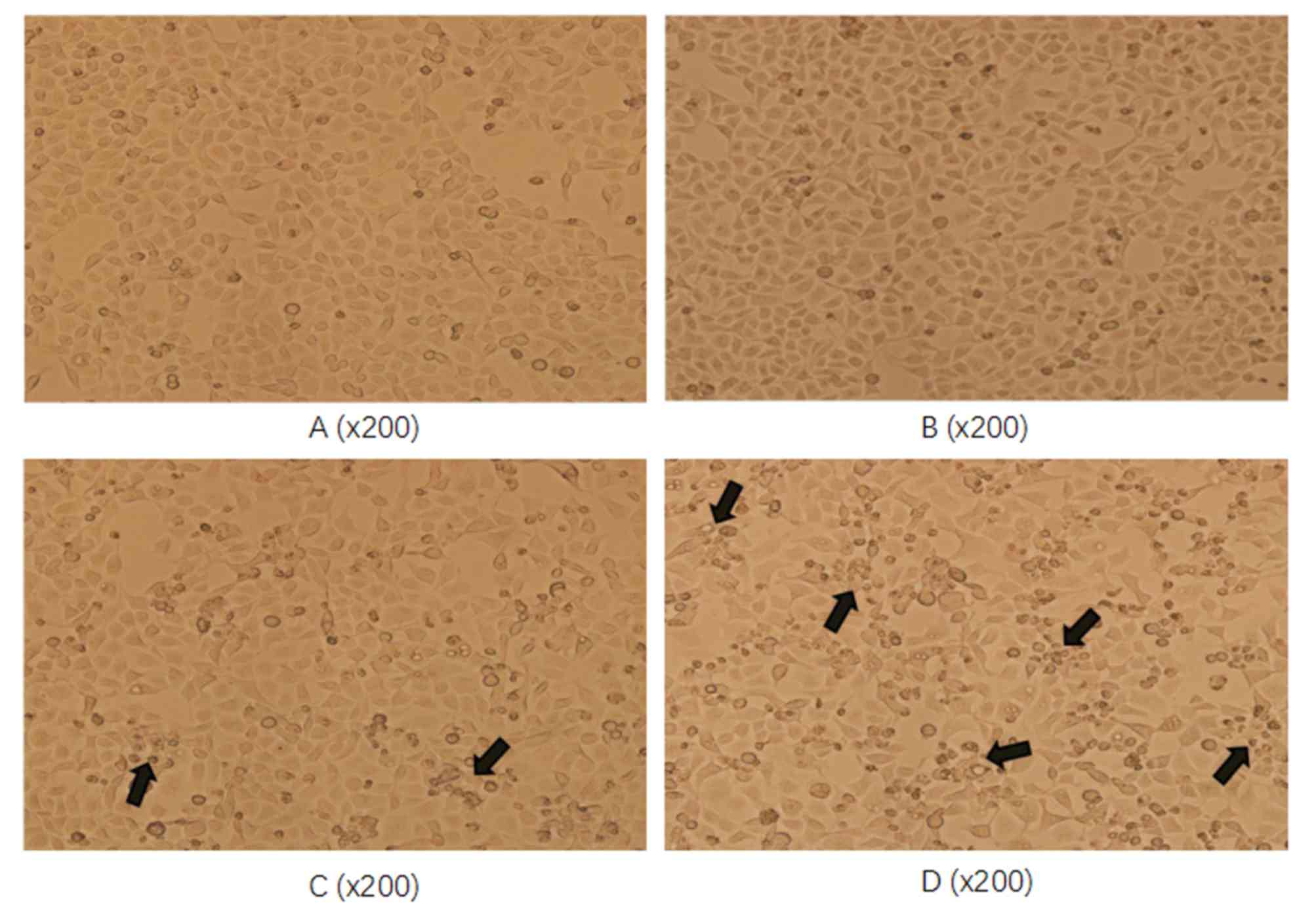
Esculetin dose- and time-dependently
damages the cell morphological structure of SMMC-7721 and HL-7702
cells
To assess whether esculetin damages the cell
morphological structure, SMMC-7721 cells were treated with 100 or
300 µmol/l esculetin for 72 h and the cell morphology was assessed
under a microscope. Compared with the blank and DMSO-treated
control cells, SMMC-7721 cells treated with esculetin exhibited
reduced adherence to the bottom of the bottle and poor refraction
(as indicated by the arrows in Fig.
2). The degree of change was more evident at the higher
esculetin concentration (300 µmol/l). Together, these results
demonstrate that esculetin inhibited the proliferation of SMMC-7721
cells in a dose-dependent manner.
Esculetin downregulates the mRNA and
protein levels of β-catenin, c-Myc and cyclin D1
In order to assess whether esculetin affects the
activity of the Wnt/β-catenin signaling pathway, SMMC-7721 cells
were treated with different concentrations of esculetin (100 or 300
µmol/l) for 24, 48 and 72 h. The mRNA and protein levels of
β-catenin and its downstream targets c-Myc and cyclin D1 were
measured using RT-qPCR and immunoblotting. Esculetin at either 100
or 300 µmol/l, for 48 and 72 h, significantly reduced the mRNA
level of β-catenin, with a stronger effect at 300 µmol/l
(P<0.05; Fig. 3A). Furthermore,
treatment of cells with 300 µmol/l esculetin for 24 h resulted in a
significant reduction in the mRNA levels of c-Myc (P<0.05;
Fig. 3B) and cyclin D1 (P<0.05;
Fig. 3C), which were further enhanced
following treatment for 48 and 72 h. Treatment of cells with 100
µmol/l esculetin for 48 and 72 h also resulted in a significant
reduction in the level of c-Myc (P<0.05; Fig. 3B) and cyclin D1 (P<0.05; Fig. 3C) mRNA, with a stronger reduction
following treatment for 72 h. Similar to the alteration of mRNA
levels, esculetin downregulated the protein levels of β-catenin and
its downstream targets, c-Myc and cyclin D1, in a dose-dependent
manner (Fig. 3D). These results
indicate that esculetin inhibited the Wnt/β-catenin signaling
pathway in SMMC-7721 cells.
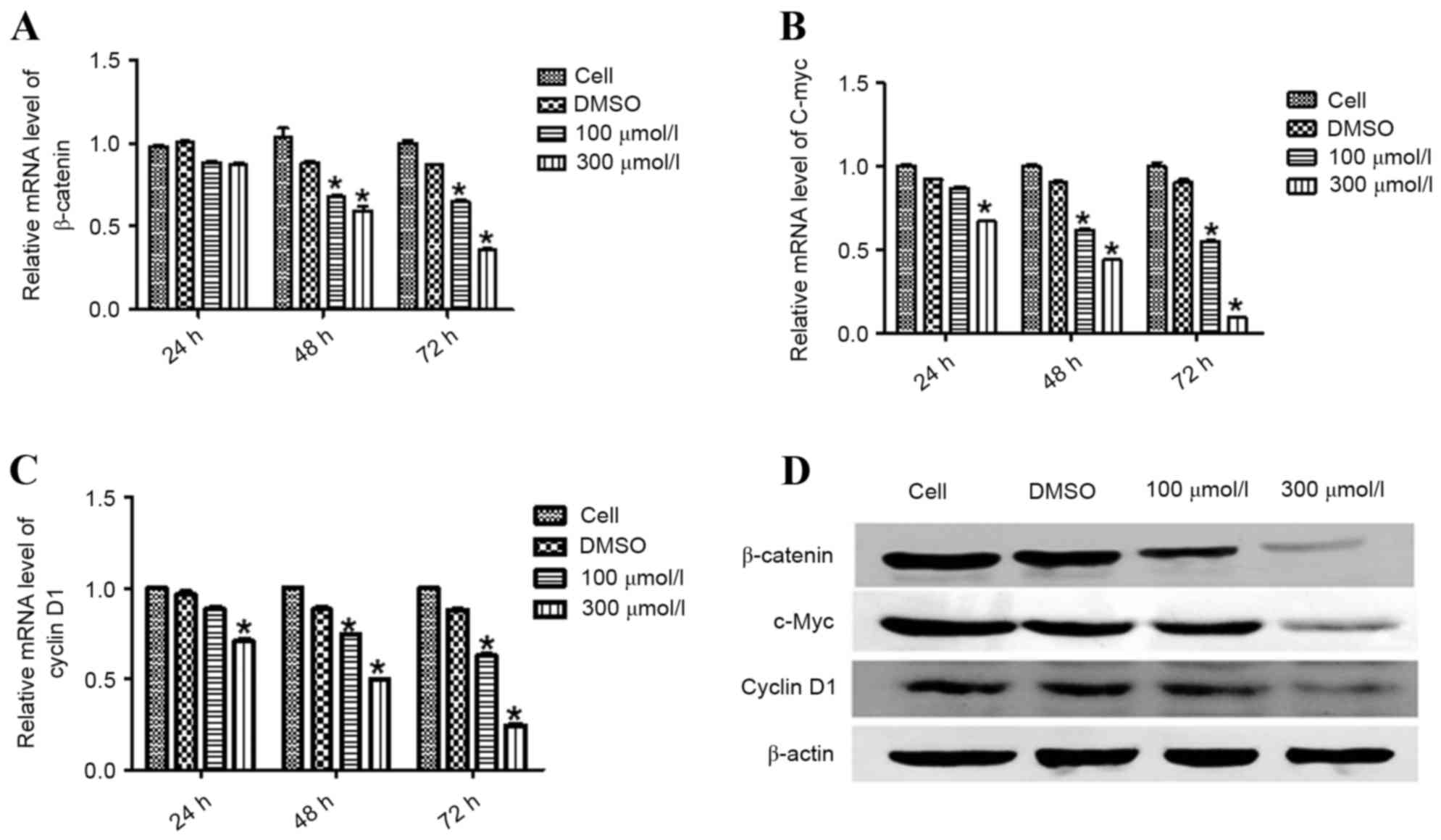 | Figure 3.Esculetin suppresses the activation of
Wnt/β-catenin signaling in SMMC-7721 cells. SMMC-7721 cells were
treated with the indicated concentration of esculetin for 24, 48,
and 72 h. Total RNA was extracted for reverse
transcription-quantitative polymerase chain reaction analysis to
determine the mRNA levels of β-catenin (A), c-Myc (B), and cyclin
D1 (C), with β-actin serving as an internal control. Each value
represents the mean ± standard deviation of three experiments. (D)
SMMC-7721 cells were treated for 72 h with the indicated
concentration of esculetin, with DMSO vehicle for or not treated.
Total protein was extracted for western blot analysis of β-catenin,
c-Myc, and cyclin D1, with β-actin as the loading control.
*P<0.05 vs. DMSO. DMSO, dimethyl sulfoxide; c-Myc, Myc
proto-oncogene. |
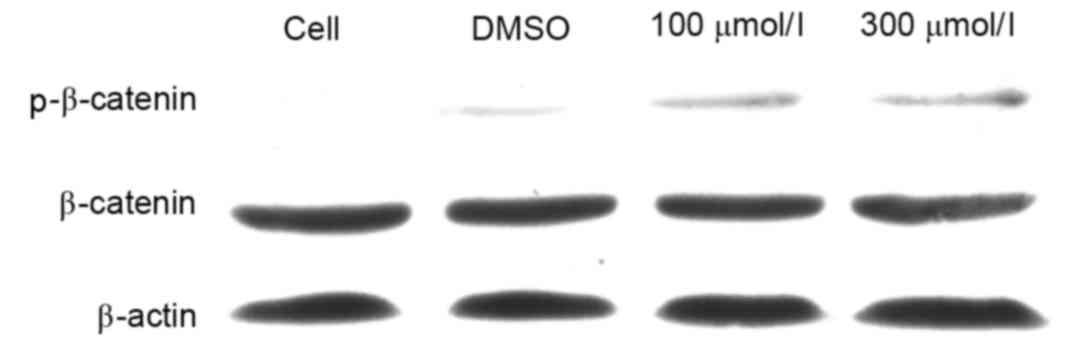
Esculetin induces the phosphorylation
of β-catenin
In order to investigate the mechanism by which
esculetin inhibits the Wnt signaling pathway, SMMC-7721 cells were
treated with 100 or 300 µmol/l esculetin for 24 h and the
phosphorylation level of β-catenin was determined by
immunoblotting. The results revealed that the total β-catenin
protein level was not evidently decreased following 100 or 300
µmol/l esculetin treatment for 24 h. However, the phospho-β-catenin
level (Ser33/Ser37/Thr41) was evidently increased following 100 or
300 µmol/l esculetin treatment for 24 h (Fig. 4). Since it has been established that
phosphorylation of β-catenin (Ser33/Ser37/Thr41) leads to
degradation of the cytoplasmic β-catenin (17), β-catenin cannot accumulate in the
nucleus and will not activate the transcription of downstream
target genes of the Wnt/β-catenin signaling pathways so will not
promote cancer cell growth, proliferation and metastasis (18). These results suggest that esculetin
promotes the phosphorylation of β-catenin, thereby leading to
suppression of Wnt/β-catenin signaling in SMMC-7721 cells.
Discussion
In the present study, esculetin was demonstrated to
inhibit the proliferation of SMMC-7721 and HL-7702 cells in a dose-
and time-dependent manner. Furthermore, treatment of SMMC-7721
cells with esculetin resulted in cell shrinkage, membrane blebbing
and vacuolization in the cytoplasm. Esculetin downregulated the
mRNA and protein expression levels of β-catenin, and its downstream
targets c-Myc and cyclin D1. Notably, treatment of SMMC-7721 cells
with esculetin resulted in the phosphorylation of β-catenin. The
present results suggest that esculetin inhibits the viability of
SMMC-7721 by enhancing the phosphorylation of β-catenin
(Ser33/Ser37/Thr41) to inhibit Wnt signaling pathway.
The Wnt signaling pathway serves a key role in
normal embryonic development and the central nervous system; in
addition, it regulates cell growth, migration and differentiation.
Evidence suggests that the Wnt signaling pathway is frequently
dysregulated in the majority of cancer types (19); therefore, blocking the Wnt signaling
pathway is a promising strategy for cancer treatment. β-catenin is
a key component of the Wnt signaling pathway; cytoplasmic β-catenin
is normally maintained at a low level by proteasomal degradation.
The binding of Wnt proteins to Frizzled receptors results in the
accumulation of unphosphorylated β-catenin, which translocates into
the nucleus and activates the expression of Wnt target genes,
including c-Myc and cyclin D1 (19–21). c-Myc
is the most commonly overexpressed oncogene in human cancer and is
mutated in ~20% of human cancers (22). Cyclin D1 is a key regulator of the G1
to S phase transition in the normal cell cycle, and the excess
expression of cyclin D1 is observed in numerous types of human
cancer, including those of the lymphatic system, breast, esophagus,
lung and bladder (23–27). c-Myc and cyclin D1 are target genes of
the Wnt signaling pathway and contribute to regulation of cell
cycle progression by Wnt signaling. In the present study, esculetin
reduced the mRNA and protein levels of c-Myc and cyclin D1,
suggesting that one of the mechanisms by which esculetin inhibits
the proliferation of SMMC-7721 cells is through suppression of
c-Myc and cyclin D1 expression.
Unphosphorylated β-catenin avoids proteasomal
degradation and subsequently translocates into the nucleus. A high
concentration of nuclear β-catenin is associated with a poor
prognosis in certain cancer types. It has been reported that
cytoplasmic β-catenin can be degraded though phosphorylation at
Ser33, Ser37, and Thr41 (28).
Accumulating evidence indicates that numerous natural products can
inhibit the activity of Wnt signaling. Haraguchi et al
(29) reported that Ajuba can
effectively reduce β-catenin expression in human cervical cancer
cells by inducing the phosphorylation of β-catenin via glycogen
synthase kinase (GSK)-3β. In addition, Feng et al (30) reported that sulindac reduced the
phosphorylation level of GSK-3β, thereby increasing the
phosphorylation levels of β-catenin. To confirm that the increased
phosphorylation levels of β-catenin (at Ser33/Ser37/Thr41) were not
due to alteration of total β-catenin levels, total β-catenin levels
following treatment with esculetin for 24 h was observed. Results
showed that treatment of cells with esculetin for 24 h did not
alter the amount of β-catenin protein, but markedly increased the
phosphorylation of β-catenin (at Ser33/Ser37/Thr41) in a
concentration-dependent manner. As a result, we hypothesized that
esculetin enhanced the phosphorylation of β-catenin (at
Ser33/Ser37/Thr41), thereby leading to its degradation.
Subsequantly, we hypothesized that esculetin inhibits
transcriptional activity of β-catenin by inducing β-catenin
degradation. Although the mechanism by which the phosphorylation of
β-catenin is induced by esculetin remains to be determined, the
findings of the present study suggest that inhibition of Wnt
signaling is an important mechanism for natural products to prevent
tumorigenesis and inhibit the proliferation of cancer cells.
In conclusion, the present study found that
esculetin inhibited the Wnt signaling pathway in SMMC-7721 cells,
which was accompanied by increased phosphorylation of total
β-catenin. The results of the present study suggest that one of the
molecular mechanisms of the antitumor effects of esculetin may be
through inhibition of the Wnt signaling pathway.
Acknowledgements
This study was supported by a grant from the
Agricultural Science and Technology Achievements Transformation
Project (no. 2014GB2A300003).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, risk factors, and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossi L, Zoratto F, Papa A, Iodice F,
Minozzi M, Frati L and Tomao S: Current approach in the treatment
of hepatocellular carcinoma. World J Gastrointest Oncol. 2:348–359.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson PJ: Hepatocellular carcinoma: Is
current therapy really altering outcome? Gut. 51:459–462. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoshida Y, Toffanin S, Lachenmayer A,
Villanueva A, Minguez B and Llovet JM: Molecular classification and
novel targets in hepatocellular carcinoma: Recent advancements.
Semin Liver Dis. 30:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi-Yanaga F and Sasaguri T: The
Wnt/beta-catenin signaling pathway as a target in drug discovery. J
Pharmacol Sci. 104:293–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiang DY, Villanueva A, Hoshida Y, Peix
J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M,
et al: Focal gains of VEGFA and molecular classification of
hepatocellular carcinoma. Cancer Res. 68:6779–6788. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boyault S, Rickman DS, de Reyniès A,
Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti
J, Franco D, et al: Transcriptome classification of HCC is related
to gene alterations and to new therapeutic targets. Hepatology.
45:42–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tubaro A, Del Negro P, Ragazzi E, Zampiron
S and Loggia R Della: Anti-inflammatory and peripheral analgesic
activity of esculetin in vivo. Pharmacol Res Commun. 20 Suppl
5:S83–S85. 1988. View Article : Google Scholar
|
|
12
|
Kaneko T, Tahara S and Takabayashi F:
Suppression of lipid hydroperoxide-induced oxidative damage to
cellular DNA by esculetin. Biol Pharm Bull. 26:840–844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SY, Lim TG, Chen H, Jung SK, Lee HJ,
Lee MH, Kim DJ, Shin A, Lee KW, Bode AM, et al: Esculetin
suppresses proliferation of human colon cancer cells by directly
targeting beta-catenin. Cancer Prev Res (Phila). 6:1356–1364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim AD, Han X, Piao MJ, Hewage SR, Hyun
CL, Cho SJ and Hyun JW: Esculetin induces death of human colon
cancer cells via the reactive oxygen species-mediated mitochondrial
apoptosis pathway. Environ Toxicol Pharmacol. 39:982–989. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Lu ML, Dai HL, Zhang SP, Wang HX
and Wei N: Esculetin, a coumarin derivative, exerts in vitro and in
vivo antiproliferative activity against hepatocellular carcinoma by
initiating a mitochondrial-dependent apoptosis pathway. Braz J Med
Biol Res. 48:245–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HH, Uen YH, Tian YF, Sun CS, Sheu MJ,
Kuo HT, Koay LB, Lin CY, Tzeng CC, Cheng CJ, et al: Wnt-1 protein
as a prognostic biomarker for hepatitis b-related and hepatitis
C-related hepatocellular carcinoma after surgery. Cancer Epidemiol
Biomarkers Prev. 18:1562–1569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi-Yanaga F and Kahn M: Targeting
Wnt signaling: Can we safely eradicate cancer stem cells? Clin
Cancer Res. 16:3153–3162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nesbit CE, Tersak JM and Prochownik EV:
MYC oncogenes and human neoplastic disease. Oncogene. 18:3004–3016.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Landis MW, Pawlyk BS, Li T, Sicinski P and
Hinds PW: Cyclin D1-dependent kinase activity in murine development
and mammary tumorigenesis. Cancer Cell. 9:13–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YM and Sicinski P: Targeting cyclins
and cyclin-dependent kinases in cancer: Lessons from mice, hopes
for therapeutic applications in human. Cell Cycle. 5:2110–2114.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Wang C, Prendergast GC and Pestell
RG: Cyclin D1 functions in cell migration. Cell Cycle. 5:2440–2442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 2005.cm1PubMed/NCBI
|
|
29
|
Haraguchi K, Ohsugi M, Abe Y, Semba K,
Akiyama T and Yamamoto T: Ajuba negatively regulates the Wnt
signaling pathway by promoting GSK-3beta-mediated phosphorylation
of beta-catenin. Oncogene. 27:274–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng XC, Wang XM, Zhang Y, Li J and Shen
ZH: Sulindac induces SMMC-7721 cells apoptosis through inhibiting
Wnt pathway. Fudan Univ J Med Sci. 1–526. 2007.
|