Introduction
Glioma has been confirmed as a lethal malignant
tumor due to high mortalities caused by gliomas in recent years
(1). Modrek et al showed that
gliomas account for 29% of primary tumors of system diseases, which
constitutes 80% of malignant tumors (2). The incidence of gliomas is approximately
52,400/100,000 individuals (3).
Previous findings have shown that approximately 0.602% of Chinese
individuals exhibit varying degrees of increased primary system
diseases; thus, there is a high incidence of patients with gliomas
in China (4,5). Gliomas are caused by the interaction
between human genetic material and the external environment;
however, the related genes causing brain glioma have yet to be
identified. Therefore, glioma pathogenesis remains to be determined
(6).
In recent years, advances in the research on glial
stem cells, and the study of the pathogenesis of brain tumors by
glial stem cells, have become imperative in the study of gliomas.
Robinson et al showed that transient axonal glycoprotein-1
(TAG1) is important in the development of the central nervous
system in the human body (7).
Previous findings showed that the physiological function of TAG1 is
mainly expressed in the human body as a cell adhesion molecule to
guide the nerve cells in the adhesion, migration, and increase of
axon growth (8). Huang et al
found a correlation between TAG1 and glioma (9). Previous results have also shown that
amyloid β precursor protein (APP) is an important, widely
distributed protein in brain, and plays important roles in the
promotion of nerve growth, regulation of neuronal migration and
differentiation (10). In the present
study, we investigated the role of the expression of TAG1/APP
signaling pathway in the proliferation and differentiation of
glioma stem cells to provide a reference for the study of the
genetic mechanism and treatment of brain glioma.
Materials and methods
Chemicals, cell lines and
reagents
In this study, U373 glioma cell lines with
hepatocellular function were purchased from the American Type
Culture Collection (Manassas, VA, USA). The main components of the
serum-free medium included DMEM/F12 + Bfgf 20 ng/ml, EGF 20 ng/ml,
and B27 0.2%. Cells were cultured at 37°C with 5% CO2.
Differentiation medium comprised DMEM/F12 culture medium containing
10% fetal bovine serum, and cells were cultured at 37°C with 5%
CO2. The TAG1/APP primary antibodies were purchased from
Roche (Mannheim, Germany).
RT-PCR and RNA extraction
RNA extraction was operated in accordance with
AXYGEN kit instructions (10).
Briefly, 500 ng RNA was collected and added to 2.0 ml 5X g DNA
eraser Buffer, 1.0 µg DNA eraser, and RNase-free ddH2O
to supplement the whole system to 10 ml and the DNA was eliminated
from RNA, followed by the addition of 5 ml of the above reaction
liquid, 0.5 ml PrimeScript RT Enzyme mix, 2.0 ml 5X Prime Script
Buffer and 2.0 ml RT Primer mix. RNase-free ddH2O was
added to supplement the whole system to 10.0 ml. Fluorescent
quantitative PCR reaction system was as follows: 5.0 ml SYBR Premix
Ex Taq TMII (2X), 0.3 ml PCR forward primer (10 mmol/l), 0.3 ml PCR
reverse primer (10 mmol/l), 1.0 ml cDNA, and H2O to
supplement the whole system to 20 ml. The system was detected as
previously described (11).
Fluorescent quantitative PCR
The fluorescence quantitative PCR kit used in the
present study was purchased from Takara Biotechnology Co., Ltd.
(Dalian, China). The experiment was carried out in triplicate.
Specific steps were conducted with reference to the specification,
resulting in disease amelioration (Table
I).
 | Table I.Fluorescence quantitative PCR
primer. |
Table I.
Fluorescence quantitative PCR
primer.
| Gene | Primer sequence |
|---|
| TAG1 | F:
5′-AGTCACACCTGTCCTCTAG-3′ |
|
| R:
5′-ATCTGCCTATGCCTTGGTTG-3′ |
| APP | F:
5′-GTGGCTGAGGAGATTCAAG-3′ |
|
| R:
5′-AAAGAAGGCATGAGAGCATC-3′ |
| GAPDH | F:
5′-TCATGGGTGTGAACCATGAGAA-3′ |
|
| R:
5′-GGCAGGACTGTGGTCATGAG-3′ |
Western blot analysis
Roche's animal cell protein extraction kit was used
to extract the total protein in the sample (specific operation
according to the specification) and the operation was optimized
(12). Rabbit monoclonal TAG1
antibody (dilution, 1:500; cat. no. ab133498) and rabbit monoclonal
APP antibody (dilution, 1:500; cat. no. ab180140) were purchased
from Abcam (Cambridge, MA, USA).
ELISA detection
The double antibody sandwich method was used to
detect the expression of TAG1/APP gene as previously
described (11). Briefly, pH 9.0 PBS
buffer was used to dilute the antibody protein, at a concentration
of approximately 1–10 µg/ml. Then, 0.1 ml of the sample was added
in the 96-well plate and the sample was treated at 4°C overnight.
The following day, the liquid in the well was discarded and the
plate was washed with PBS five times for 2 min. Subsequently, 0.1
ml of the treated serum sample was added into the 96-well plate and
incubated at 37°C for 1 h. The plate was washed five times with PBS
for 2 min. Of note, the blank well was used for the negative and
positive controls. After washing, 0.1 ml of the secondary antibody
was added to the 96-well plate and incubated at 37°C for 0.5–1.2 h.
After staining with red, the plate was washed five times with PBS
for 2 min. After washing, 0.1 ml new configured chromogenic
substrate, TMB, was added to the 96-well plate and incubated at
37°C for 30 min, followed by the addition of 0.005 ml of 0.2 M
sulfuric acid stop solution.
For qualitative detection, the 96-well plate above
was placed on blank paper. By reading the color depth, a
qualitative observation was conducted, i.e., a deeper color
indicated a higher positive degree, suggesting higher TAG1/APP
protein content. The negative control hole was colorless. The
96-well plate was arranged on the enzyme standard instrument for
quantitative detection with 450 nm as the wavelength. The blank
well was adjusted to zero. If the OD value was >1.2-fold of the
negative control value, a positive state was confirmed (12).
Statistical analysis
SPSS 20.2 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis in the experiment.
Measurement data were presented as mean ± standard deviation.
Countable data were tested using the Chi-square test. P<0.05 was
considered statistically significant.
Results
Gene expression of TAG1/APP signaling
pathway in glioma stem cell proliferation
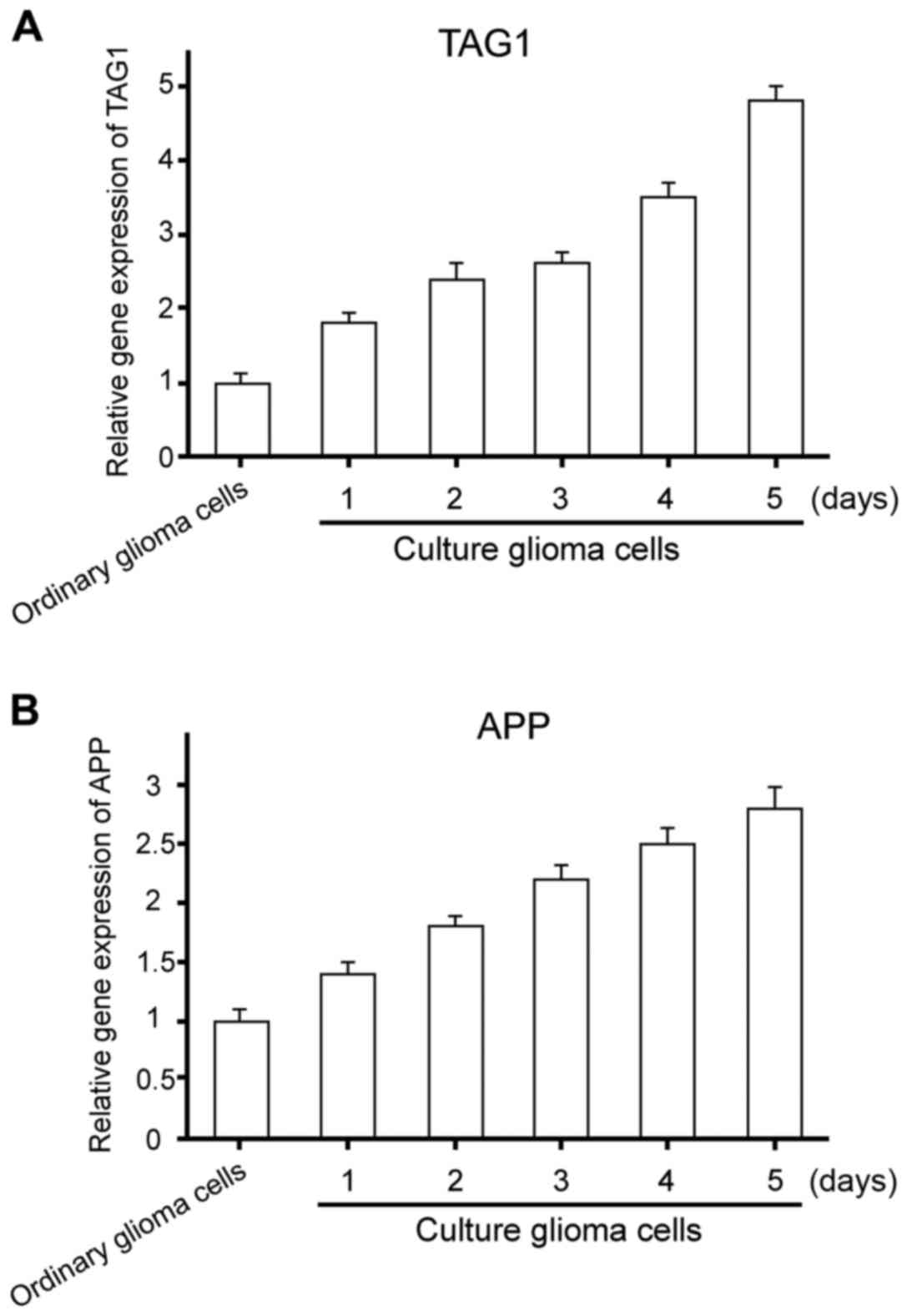
To explore the gene expression of TAG1/APP signaling
pathway in glioma stem cell proliferation, ordinary glioma and
glioma stem cells were collected to extract the RNA.
TAG1/APP gene expression status was detected (Fig. 1). The expression of TAG1/APP increased
significantly in glioma stem cells. Differences were of statistical
significance (t1=−3.427, P=0.018; t2=−4.201, P=0.032).
Protein expression of TAG1/APP
signaling pathway in glioma stem cell proliferation
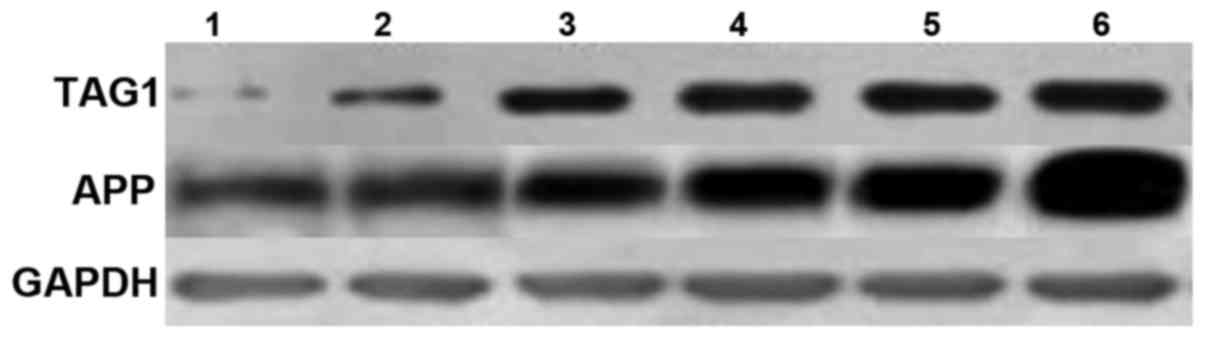
The protein expression levels of TAG1/APP protein
were studied using ELISA and western blotting, and the results are
shown in Fig. 2. The expression of
protein of TAG1/APP in glioma stem cells was significantly higher
in comparison to the ordinary cells (t3=−3.49, P=0.021; t4=−9,782,
P=0.012).
Gene expression of TAG1/APP in the
differentiation of glioma stem cells
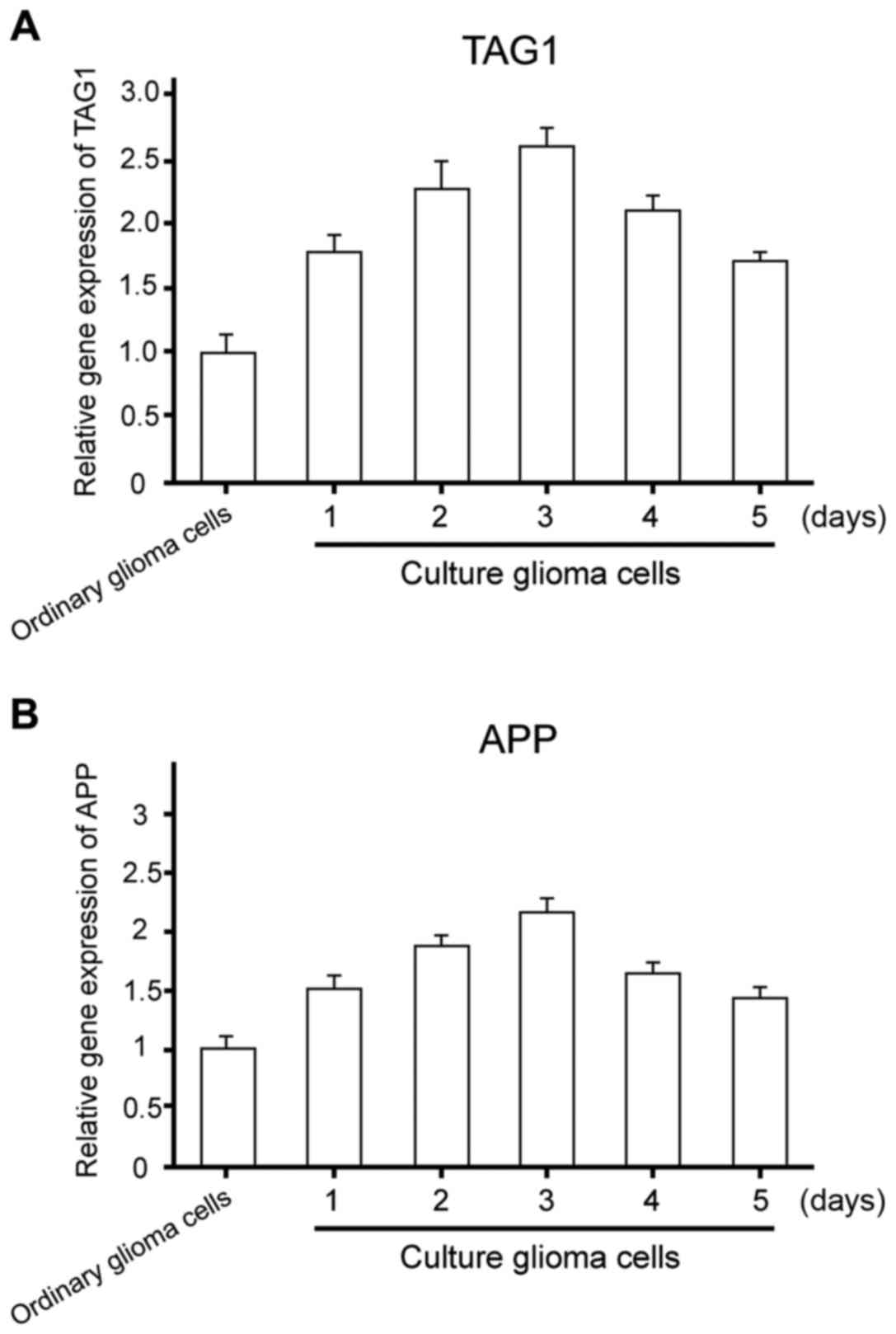
To further examine the gene expression of TAG1/APP
signaling pathway and the differentiation of glioma stem cells, the
protein of ordinary glioma and glioma stem cells cultured in
differentiation medium was extracted. RNA expression levels of
TAG1/APP were determined by RT-PCR. The results revealed a
significant increase in the RNA expression levels of TAG1/APP in
glioma stem cells (P1=0.003, P2=0.004) (Fig. 3).
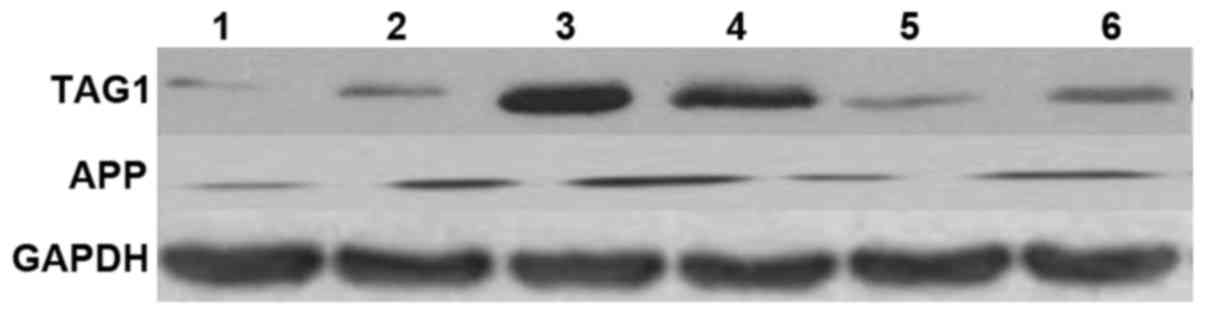
Protein expression of TAG1/APP signaling pathway in
the differentiation of glioma stem cells. The protein expression of
TAG1/APP signaling pathway in glioma stem cell differentiation was
examined following protein extraction in ordinary glioma and glioma
stem cells cultured in differentiation medium, using ELISA and
western blotting. The results shown in Fig. 4 suggest that, the protein expression
of TAG1/APP was significantly higher in glioma stem cells
(P1=0.002, P2=0.001).
Discussion
The recent increase in the incidence of cerebral
gliomas results in the deepening of the research on brain glioma
(13). Researchers are focused on
important genes, which are able to affect gliomas such as TAG1 and
APP. However, research on the relevant signaling pathway in glioma
is rarely reported (14). The present
study has focused on this aspect of glioma research and has
explored the expression profiles of TAG1/APP signaling pathway in
the proliferation and differentiation of glioma stem cells.
TAG1 gene is crucial in the signal pathway and can promote
axonal formation and remodeling (15). Furthermore, the interaction of TAG1
with glial cells plays an important role in the regulation of glial
cell migration (16). We observed a
significant increase in the expression profiles of TAG1 in the
present study and the results are consistent with an earlier study
by Liu et al (17).
The APP protein is another important factor that has
signal transfer function (18,19).
Results by Nagai et al (20)
showed that the APP gene is also involved in brain and
nervous system development and maturation. Chen et al
suggested the association of this gene with many types of gliomas
(21). We also observed a significant
increase in its expression profiles, as observed by Mirzayans et
al (22). Therefore, it can be
inferred from the abovementioned studies and results that
TAG1/APP gene may be involved in the development and
maturation of the nervous system, to a certain extent. At present,
there are few reports about the expression of TAG1/APP signal
pathway that is abnormally expressed in glioma cells. In addition,
the TAG1/APP signaling pathway involves glioma stem cell
proliferation and differentiation, promotes glioma stem cell
proliferation, and inhibits glioma differentiation, which provides
certain theoretical and experimental basis to the subsequent
diagnosis and treatment of glioma.
References
|
1
|
Liu S, Yin F, Zhang J, Wicha MS, Chang AE,
Fan W, Chen L, Fan M and Li Q: Regulatory roles of miRNA in the
human neural stem cell transformation to glioma stem cells. J Cell
Biochem. 115:1368–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Modrek AS, Bayin NS and Placantonakis DG:
Brain stem cells as the cell of origin in glioma. World J Stem
Cells. 6:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Stetson L, Virk SM
and Barnholtz-Sloan JS: Epidemiology of gliomas. Cancer Treat Res.
163:1–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ooi YC, Tran P, Ung N, Thill K, Trang A,
Fong BM, Nagasawa DT, Lim M and Yang I: The role of regulatory
T-cells in glioma immunology. Clin Neurol Neurosurg. 119:125–132.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Xu S, Cao C, Dong J, Chu Y, He G
and Xu Z: Evidence from a large-scale meta-analysis indicates
eczema reduces the incidence of glioma. Oncotarget. 7:62598–62606.
2016.PubMed/NCBI
|
|
6
|
Trazzi S, Fuchs C, Valli E, Perini G,
Bartesaghi R and Ciani E: The amyloid precursor protein (APP)
triplicated gene impairs neuronal precursor differentiation and
neurite development through two different domains in the Ts65Dn
mouse model for Down syndrome. J Biol Chem. 288:20817–20829. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson A, Grösgen S, Mett J, Zimmer VC,
Haupenthal VJ, Hundsdörfer B, Stahlmann CP, Slobodskoy Y, Müller
UC, Hartmann T, et al: Upregulation of PGC-1α expression by
Alzheimer's disease-associated pathway: Presenilin 1/amyloid
precursor protein (APP)/intracellular domain of APP. Aging Cell.
13:263–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Binello E, Mormone E, Emdad L, Kothari H
and Germano IM: Characterization of fenofibrate-mediated
anti-proliferative pro-apoptotic effects on high-grade gliomas and
anti-invasive effects on glioma stem cells. J Neurooncol.
117:225–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Q, Zhang QB, Dong J, Wu YY, Shen YT,
Zhao YD, Zhu YD, Diao Y, Wang AD and Lan Q: Glioma stem cells are
more aggressive in recurrent tumors with malignant progression than
in the primary tumor, and both can be maintained long-term in
vitro. BMC Cancer. 8:3042008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Zhang H, Wu J, Xu L, Xu D, Sun J,
He Y, Zhou X, Wang Z, Wu L, et al: Promotion of the induction of
cell pluripotency through metabolic remodeling by thyroid hormone
triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials.
33:5514–5523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji X, Qiang H and Qing L: Morphological,
marker and cell proliferation kinetics of brain tumor stem cells
differentiation in vitro. Chin Med J (Engl). 86:1604–1609.
2006.
|
|
12
|
Sharp J, Frame J, Siegenthaler M, Nistor G
and Keirstead HS: Human embryonic stem cell-derived oligodendrocyte
progenitor cell transplants improve recovery after cervical spinal
cord injury. Stem Cells. 28:152–163. 2010.PubMed/NCBI
|
|
13
|
Macas J, Ku MC, Nern C, Xu Y, Bühler H,
Remke M, Synowitz M, Franz K, Seifert V, Plate KH, et al:
Generation of neuronal progenitor cells in response to tumors in
the human brain. Stem Cells. 32:244–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi R, Li Y, An H, Yu Y, Yang X and Cao X:
The effect of Notch1 gene transfection on the growth of Hep3B
hepatocarcinoma cells and the related mechanisms. Chin J of Cancer
Biother. 10:180–184. 2003.(In Chinese).
|
|
15
|
Clement V, Sanchez P, de Tribolet N,
Radovanovic I and i Altaba A Ruiz: HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and
tumorigenicity. Curr Biol. 17:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang L, Li W and Chen H: Breeding of the
resistant strain of the Bacillus pneumoniae and the construction of
the mutant strain of the signal label. J Microbiol. 48:73–79.
2007.
|
|
17
|
Liu ZJ, Xiao M, Balint K, Smalley KS,
Brafford P, Qiu R, Pinnix CC, Li X and Herlyn M: Notch1 signaling
promotes primary melanoma progression by activating
mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt
pathways and up-regulating N-cadherin expression. Cancer Res.
66:4182–4190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Esteban MA, Wang T, Qin B, Yang J, Qin D,
Cai J, Li W, Weng Z, Chen J, Ni S, et al: Vitamin C enhances the
generation of mouse and human induced pluripotent stem cells. Cell
Stem Cell. 6:71–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyoshi N, Ishii H, Nagano H, Haraguchi N,
Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, et
al: Reprogramming of mouse and human cells to pluripotency using
mature microRNAs. Cell Stem Cell. 8:633–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai K, Ishii H, Miyoshi N, Hoshino H,
Saito T, Sato T, Tomimaru Y, Kobayashi S, Nagano H, Sekimoto M, et
al: Long-term culture following ES-like gene-induced reprogramming
elicits an aggressive phenotype in mutated cholangiocellular
carcinoma cells. Biochem Biophys Res Commun. 395:258–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Fu X, Wan Y, Wang Z, Jiang D and
Shi L: miR-125b inhibitor enhance the chemosensitivity of
glioblastoma stem cells to temozolomide by targeting Bak1. Tumour
Biol. 35:6293–6302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirzayans R, Andrais B, Scott A and Murray
D: New insights into p53 signaling and cancer cell response to DNA
damage: Implications for cancer therapy. J Biomed Biotechnol.
2012:1703252012. View Article : Google Scholar : PubMed/NCBI
|