Introduction
Zinc finger regulator of apoptosis and cell cycle
arrest (ZAC) occurs within the chromosomal region 6q24. The zinc
finger protein encoded by ZAC acts as a transcription factor, and
there is substantial evidence for its role as a tumor suppressor
(1). ZAC inhibits tumor cell
proliferation through the induction of apoptosis and cell cycle
arrest. ZAC has been implicated in tumorigenesis in a number of
types of cancer; loss or downregulation of expression has thus far
been identified in ovarian cancer (2), breast cancer (3), head and neck squamous cell carcinoma
(4), nonfunctioning pituitary
adenomas (5) and extra-skeletal
myxoid chondrosarcoma (6). The
molecular mechanism for the alteration in ZAC gene expression in
gastric cancer tissues is unclear. Downregulation of tumor
suppressor genes in cancer tissue may occur as the consequence of
one or more genetic or epigenetic events. Genetic alterations
include the mutation of the protein-coding region or loss of
heterozygosity (LOH) and microsatellite instability (MSI) in the
coding or non-coding regions. Epigenetic changes typically involve
the methylation of promoter DNA or histone acetylation (7).
Microsatellite sequences are highly polymorphic,
short tandem repeat sequences that occur in the human genome. They
may be situated in the exon, intron or promoter regions. MSI is the
polymorphism in the length of these microsatellite sequences; they
may affect amino acid codons or the regulation of transcription
(8). At present, various MSI
phenotypes have been identified in a number of tumor types
(7,8).
The mechanism of inactivating mutations and deletions of both
alleles of a tumor suppressor gene was involved in the process of
carcinogenesis. Loss of heterozygosity (LOH) may increase cancer
susceptibility, particularly following exposure to environmental
carcinogens (9).
LOH or MSI can cause functional abnormality of the
associated gene when they occur in protein coding regions, or lead
to gene expression abnormalities in non-coding regions. In this
study, four ZAC-associated microsatellite loci were selected for
examination in gastric cancer tissues using a polymerase chain
reaction (PCR)-PAGE-silver staining technique in order to explore
LOH/MSI-associated mechanisms for the downregulation of ZAC
expression in cancer. The association between LOH/MSI of the ZAC
gene and clinicopathological factors in gastric cancer was also
analyzed.
Patients and methods
Sample collection
Samples including cancer tissue, the tissue adjacent
to cancer and normal gastric mucosal tissues, were collected from
30 patients with gastric cancer and preserved in liquid nitrogen.
The gastric cancer samples were obtained from surgical patients of
the Department of Gastroenterology, Cancer Hospital of Henan
(Zhengzhou, China). The gastric cancer tissues were collected from
patients undergoing gastrectomy from March 2014 to December 2014.
All patients were informed of the aims of specimen collection and
provided written consent in accordance with the ethical guidelines
of Cancer Hospital of Henan. The investigations were conducted
according to the principles of the Declaration of Helsinki, and the
Ethical Committee of Zhengzhou University approved the study.
Extraction of genomic DNA
Tissue from each sample (0.5 g) was ground and mixed
with an equal amount of 2X Triton lysate mix. Following
centrifugation (12,000 rpm, 5 min, 4°C), the sediment was incubated
with EDTA-Na2 proteinase K-SDS mixture. It was treated
three times with phenol, phenol-chloroform-isoamyl alcohol mixture
and chloroform-isoamyl alcohol mixture in sequence, respectively.
The DNA product was precipitated with anhydrous isopropanol and
dissolved in Tris-EDTA buffer. It was then stored at −20°C. The
integrity of genomic DNA was evaluated by 1% agarose gel
electrophoresis, and the purity was determined using the
A260/A280 absorbance value measured with a
spectrophotometer.
PCR reaction
Based on the sequences of the ZAC-associated
microsatellite loci CA197, 15AAAG, CA340 and D6S1703 as reported in
GenBank (Gene ID: 5325; PMID:11313869; DOI:
10.1038/sj.onc.1204237), four pairs of PCR primers were designed
using Primer3 online primer design software (Primer 3 Input 0.4.0;
http://frodo.wi.mit.edu/). The PCRs were
performed in the corresponding reaction systems, consisting of 10
µl 2X Taq PCR Mastermix (Shanghai Sangon Biological Engineering
Technology & Services Co., Ltd., Shanghai, China), 0.9 µl
forward primer, 0.9 µl reverse primer, 1 µl template DNA (50 ng/µg)
and 7.2 µl water. The PCR products were run on 2% agarose gel to
examine amplified results.
CA197
For the detection of CA197 (CA)n
fragments, the following primers were used: Forward, TTT ATA TGT
TGC ATT TCC TTT; reverse, CAA ACC ATG GCA CAC ATA TAC C. PCR
conditions were as follows: 95°C for 5 min followed by 38 cycles of
95°C for 30 sec, annealing at 52°C for 30 sec and extension at 72°C
for 45 sec, followed by a final extension step of 72°C for 7
min.
15AAAG
For the detection of 15AAAG (CTTT)n
fragments, the following primers were used: Forward, CCT CAC CTG
CAG TTT TGC T; reverse, GTG ACA GAG AAA GAC CCC AAC T. PCR
conditions: 95°C for 5 min followed by 35 cycles of 95°C for 30
sec, annealing at 58°C for 30 sec and extension at 72°C for 45 sec,
followed by a final extension step of 72°C for 7 min.
CA340
For the detection of CA340 (AT)n
fragments, the following primers were used: Forward, AGA GTT TGC
AGT GAG CCA AGA T; reverse, TCT CCT CAC TCC CTT TCA CTT C. PCR
conditions: 95°C for 5 min followed by 42 cycles of 95°C for 30
sec, annealing at 54°C for 30 sec and extension at 72°C for 45 sec,
followed by a final extension step of 72°C for 7 min.
D6S1703
For the detection of (CA)n fragments, the
following primers were used: Forward, CTG GTG CTG ATG TAT CCA AAA
T; reverse, TTTGGAGGATCAGGAAAGAAAA. PCR conditions: 95°C for 5 min
followed by 35 cycles of 95°C for 30 sec, annealing at 55°C for 30
sec and extension at 72°C for 45 sec, followed by a final extension
step of 72°C for 7 min.
Determination of MSI and LOH
The PCR products were run on a 6% polyacrylamide gel
at 550V for 2.5 h. Following electrophoresis, the gel was fixed
with 10% ethyl alcohol for 10 min at room temperature. The gel was
oxidized with nitric acid for 2 min and stained with silver nitrate
solution 15 min at room temperature. Sodium carbonate-formaldehyde
solution was added to color the gel 3 times, then the staining
reaction was terminated through the addition of acetic acid (10%)
at room temperature. If the density of a band was not present or
lowered to that that of 50% of normal tissue, it was defined as
LOH. If the density of the band was more than that of normal
tissue, it was defined as MSI.
Results
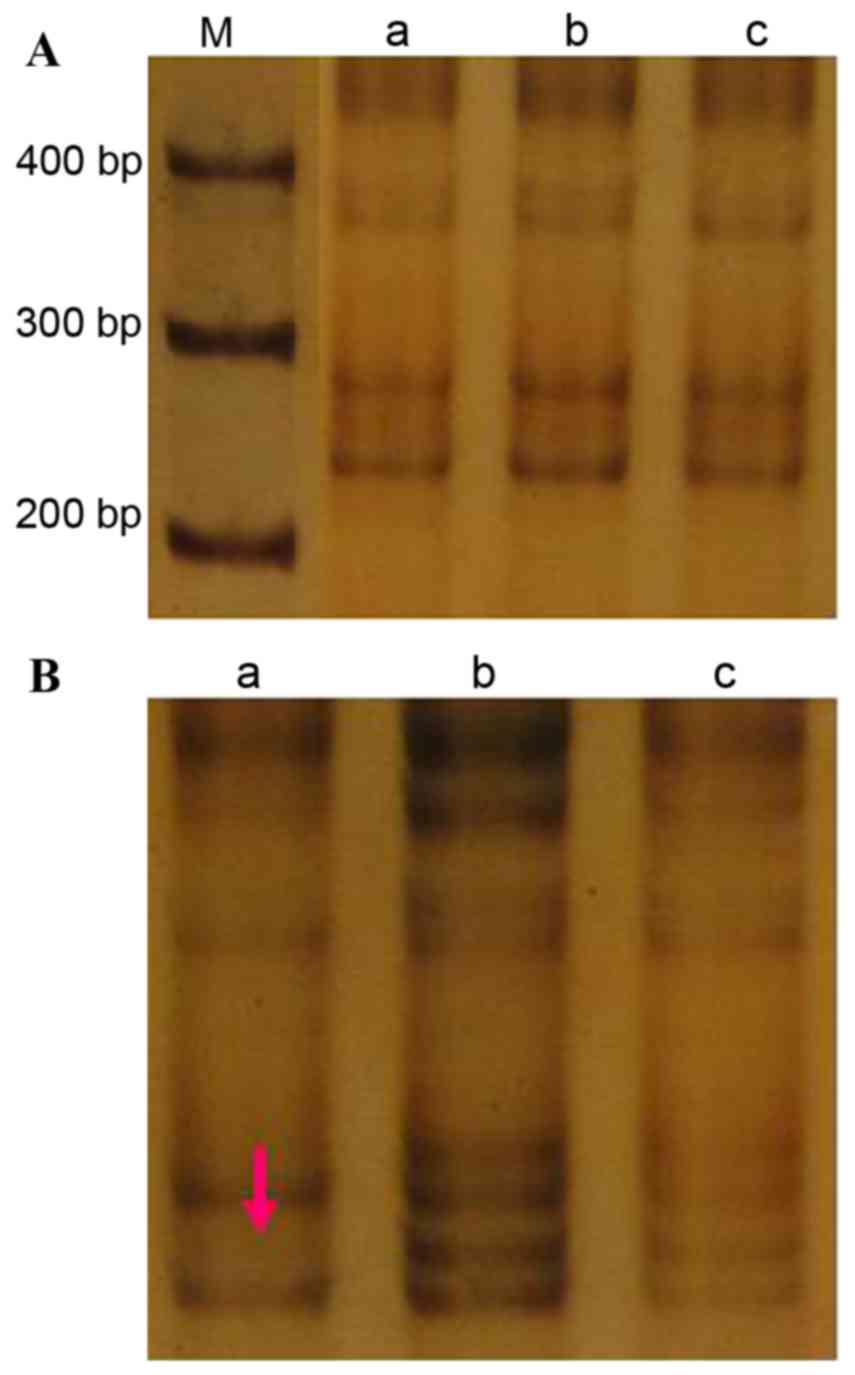
CA197
The amplified (CA)n fragment size was 240
bp (Fig. 1A). A single LOH event was
observed at this locus in the tumor tissue from case 4, equating to
an incidence of 3.3% (1/30; Fig. 1B).
There were no incidences of MSI at this locus in the cancer tissue
samples, or incidences of LOH or MSI in the cancer-adjacent
tissue.
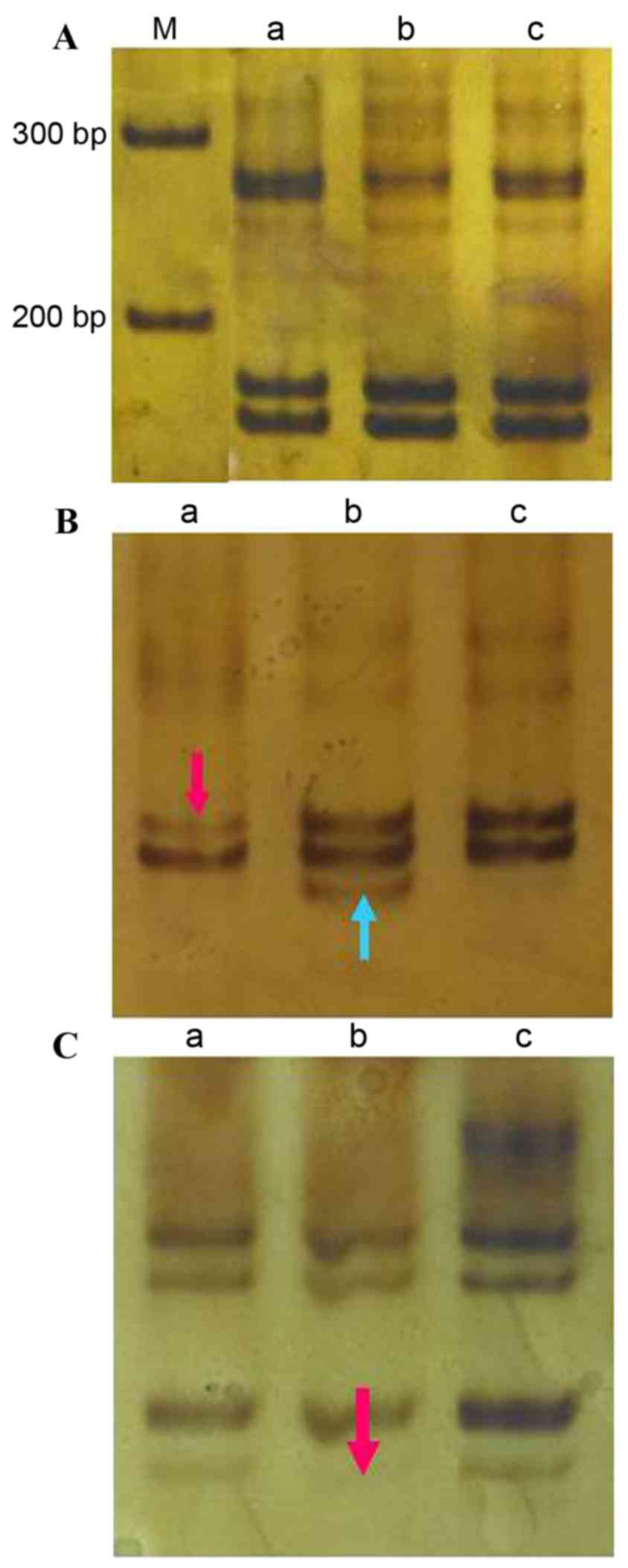
15AAAG
The amplified (CTTT)n fragment size was
173 bp (Fig. 2A). There was a single
LOH event at this locus in sample 27, an incidence of 3.3% (1/30;
Fig. 2B). No incidences of MSI were
detected at this locus in the cancer tissue; however,
cancer-adjacent tissue from sample 27 exhibited MSI at this locus
(incidence, 3.3%; Fig. 2B). In the
cancer-adjacent tissue of case 23 there was one LOH event at this
locus (incidence, 3.3%; Fig. 2C).
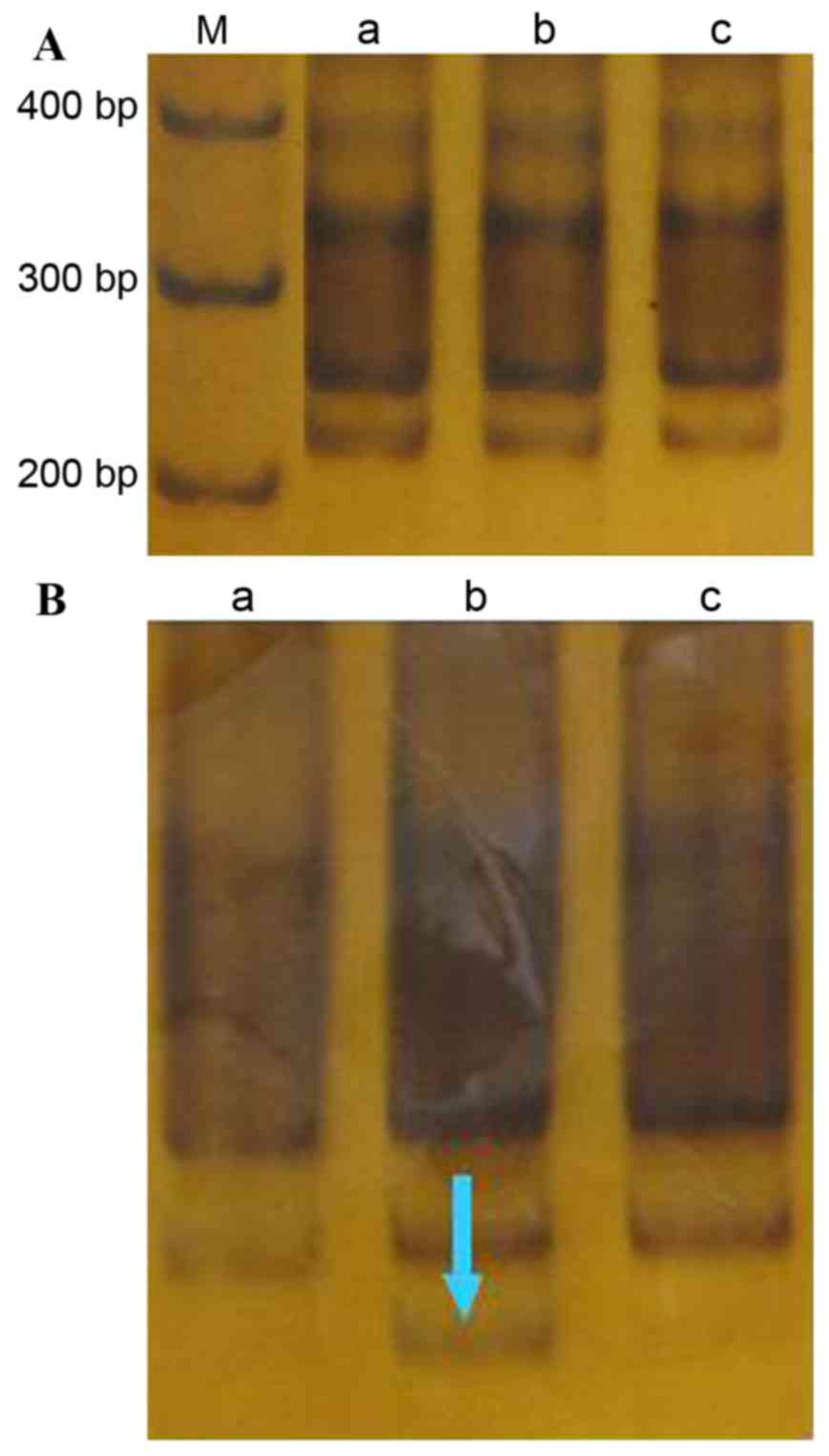
D6S1703
The amplified (CA)n fragment length was
223 bp (Fig. 3A). In the
cancer-adjacent tissue of one case (case 28) an MSI event was
detected (incidence, 3.3%; Fig. 3B).
No other MSI or microsatellite LOH events were detected at this
locus in any tissue.
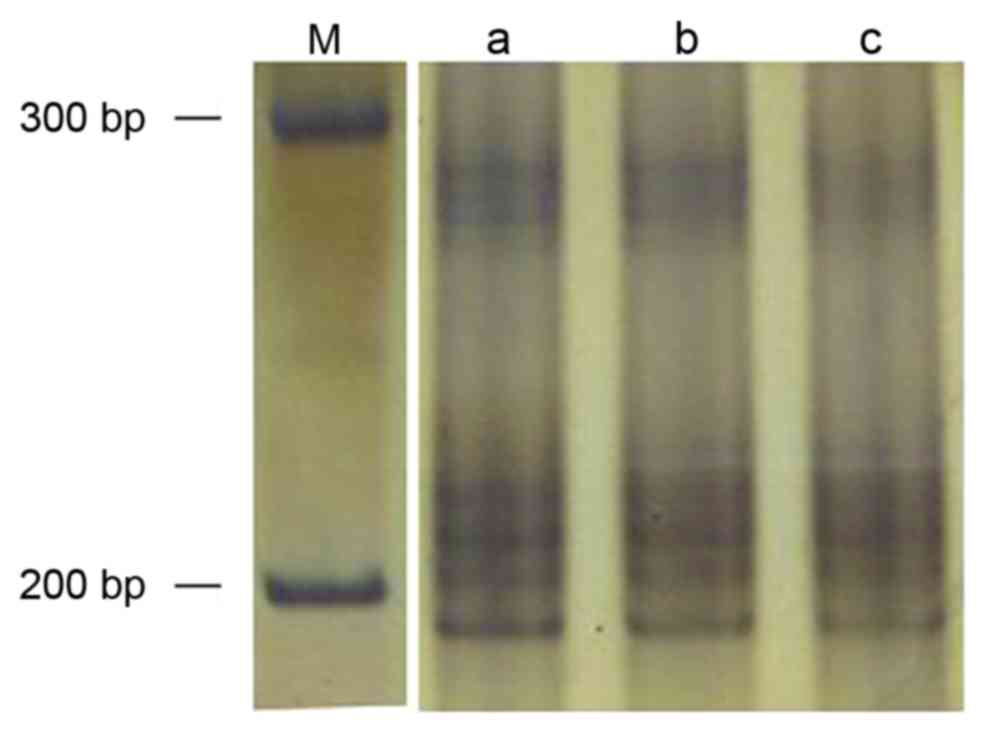
CA340
The amplified (AT)n fragment length was
247 bp (Fig. 4). No cancer or
cancer-adjacent tissue samples exhibited MSI or LOH at this
locus.
Association between MSI/LOH and
clinicopathological variables
As presented in Table
II, all four cases of moderately-differentiated gastric cancer
were negative for MSI/LOH. In the 26 cases of poorly-differentiated
gastric cancer, 4 cases were MSI/LOH positive (Table I), including 1 case at clinical stage
I/II and 3 cases at stage III/IV. These were staged according to
the tumor-node-metastasis classification system and graded into
five groups [the TNM classification UICC 2009, 7th edition
(10)]: Stage 0 (TisN0M0, n=0), Stage
I (T1N0-1M0, T2N0M0, n=8), Stage II (T1N2-3M0, T2N1-2M0, T3N0M0,
T4aN0M0, n=64), Stage III (T2N3M0, T3N2-3M0, T4aN1-3M0, T4bN0-3M0,
n=73), Stage IV (TanyNanyM1, n=8).
 | Table II.ZAC gene microsatellite locus event
association with clinicopathological indicators in gastric
cancer. |
Table II.
ZAC gene microsatellite locus event
association with clinicopathological indicators in gastric
cancer.
| Characteristic | Patients, n | ZAC MSI/LOH cases,
n |
|---|
| Sex |
| Male | 24 | 4 |
|
Female | 6 | 0 |
| Age |
|
<60 | 18 | 2 |
| ≥60 | 12 | 2 |
| Tumor diameter |
| <5
cm | 16 | 2 |
| ≥5
cm | 14 | 2 |
| Histopathological
classification |
|
Moderately differentiated | 4 | 0 |
| Poorly
differentiated | 26 | 4 |
| Clinical stage |
| I or
II | 14 | 1 |
| III or
IV | 16 | 3 |
 | Table I.ZAC-associated MSI and LOH events
detected in 30 paired cancer and cancer-adjacent tissue samples
from patients with gastric cancer. |
Table I.
ZAC-associated MSI and LOH events
detected in 30 paired cancer and cancer-adjacent tissue samples
from patients with gastric cancer.
|
| CA197 | 15AAAG | D6S1703 | CA340 |
|---|
|
|
|
|
|
|
|---|
| Tissue | LOH | MSI | LOH | MSI | LOH | MSI | LOH | MSI |
|---|
| Cancer tissue, n | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Adjacent tissue,
n | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
Discussion
ZAC expression has previously been identified to be
downregulated or eliminated in a variety of solid cancer types,
including breast (3), ovarian
(2,11,4), head
and neck squamous cell carcinoma (4),
pheochromocytoma (12) and pituitary
adenoma (5). Differences in the
frequency of ZAC gene LOH in the tumor lead to the downregulation
of ZAC (13). In previous reports,
the frequency of ZAC gene LOH in ovarian (11), breast (3) and squamous cell carcinoma (4) tumors was 36.4, 40 and 31.4%,
respectively; in B-cell lymphoma, LOH was identified in 23% (3/11)
of cases (8). Screening in colorectal
cancer revealed an LOH for at least one marker in half of the
cases, but no mutations in the ZAC coding region (14). Including all 6q23-25 ZAC-specific
microsatellite markers, which differ to those used in the present
study. LOH or allele imbalance was detected in 50% (9/18) of
pheochromocytomas (5). In the present
study, microsatellite LOH or MSI occurred in 13.3% (4/30) of
gastric cancer cases, demonstrating that ZAC gene microsatellite
LOH/MSI may be one of the molecular mechanisms underlying ZAC gene
downregulation in gastric cancer tissue.
ZAC is a maternally imprinted gene (12). If the paternal allele were lost, ZAC
gene expression would not be influenced; if the maternal allele
were lost, ZAC gene expression would be silenced, causing ZAC
downregulation (12,13). However, it was not possible to check
which allele was lost in the present study.
A study by Jarmalaite et al (12) demonstrated that the rate of ZAC gene
expression in gastric cancer was 33.3%, markedly below the 66.6% of
normal gastric mucosa tissues. A LOH/MSI frequency of 13.3%, as
identified in the present study, would be insufficient to explain
the low levels of ZAC gene expression in gastric cancer, as
observed in the study by Jarmalaite et al (12). The study further determined that that
29.5% of gastric cancer samples exhibited ZAC promoter methylation
and that a high rate ZAC promoter methylation is associated with
the loss of ZAC expression in gastric cancer tissue (12). Therefore, in gastric cancer tissue,
alterations to ZAC function may be a result of genetic and
epigenetic modifications.
A high rate of MSI has been associated with the
development of multiple primary colorectal carcinomas (14). The rate of MSI has also been
demonstrated to be associated with gastric cancer clinical
pathology (15). A previous study
revealed that MSI events in gastric cancer were more likely in
women and with increasing age, and that they were associated with
poor tumor differentiation (16).
Another study reported that MSI was not associated with any index
of clinical pathology in familial gastric cancer, whereas MSI was
more likely in women and with increasing age, and was associated
with the intestinal subtype in sporadic gastric cancer (17). A further study of gastric cancer by
Seo et al (18) identified
associations between a high MSI frequency and increasing age, tumor
size and intestinal subtype. Another previous study demonstrated
that the rate of LOH in p16 microsatellite loci did not
significantly differ between gastric cancer tumors with moderate
and poor levels of differentiation (19).
The present study has demonstrated that LOH and MSI
events may contribute to the downregulation of ZAC; however, it is
unlikely to be the primary cause, as it was only identified in
13.3% of cases. In the present study, all four patients exhibiting
MSI/LOH were male with a poorly-differentiated adenocarcinoma,
including three cases of stage III–IV and one case of stage I–II,
suggesting a trend between MSI/LOH occurrence and
poorly-differentiated, advanced-stage tumors. However, the low
number of participants, including just four female patients and
four moderately-differentiated tissue specimens, limits the present
study. In order to determine whether ZAC gene-associated LOH/MSI is
associated with sex, tumor differentiation and clinical stage in
gastric cancer, further studies with a larger sample size are
required. In order to explore the underlying mechanism of ZAC gene
downregulation in gastric cancer, our future study will aim to
detect the methylation of the ZAC gene promoter, and the levels of
ZAC mRNA and protein expression, and to analyze the relationship
between methylation, MSI/LOH and ZAC gene expression.
References
|
1
|
Spengler D, Villalba M, Hoffmann A,
Pantaloni C, Houssami S, Bockaert J and Journot L: Regulation of
apoptosis and cell cycle arrest by Zac1, a novel zinc finger
protein expressed in the pituitary gland and the brain. EMBO J.
16:2814–2825. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cvetkovic D, Pisarcik D, Lee C, Hamilton
TC and Abdollahi A: Altered expression and loss of heterozygosity
of the LOT1 gene in ovarian cancer. Gynecol Oncol. 95:449–455.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilanges B, Varrault A, Basyuk E,
Rodriguez C, Mazumdar A, Pantaloni C, Bockaert J, Theillet C,
Spengler D and Journot L: Loss of expression of the candidate tumor
suppressor gene ZAC in breast cancer cell lines and primary tumors.
Oncogene. 18:3979–3988. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koy S, Hauses M, Appelt H, Friedrich K,
Schackert HK and Eckelt U: Loss of expression of ZAC/LOT1 in
squamous cell carcinomas of head and neck. Head Neck. 26:338–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pagotto U, Arzberger T, Theodoropoulou M,
Grübler Y, Pantaloni C, Saeger W, Losa M, Journot L, Stalla GK and
Spengler D: The expression of the antiproliferative gene ZAC is
lost or highly reduced in nonfunctioning pituitary adenomas. Cancer
Res. 60:6794–6799. 2000.PubMed/NCBI
|
|
6
|
Poulin H and Labelle Y: The PLAGL1 gene is
down-regulated in human extraskeletal myxoid chondrosarcoma tumors.
Cancer Lett. 227:185–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chatterton Z, Burke D, Emslie KR, Craig
JM, Ng J, Ashley DM, Mechinaud F, Saffery R and Wong NC: Validation
of DNA methylation biomarkers for diagnosis of acute lymphoblastic
leukemia. Clin Chem. 60:995–1003. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valleley EM, Cordery SF, Carr IM,
MacLennan KA and Bonthron DT: Loss of expression of ZAC/PLAGL1 in
diffuse large B-cell lymphoma is independent of promoter
hypermethylation. Genes Chromosomes Cancer. 49:480–486.
2010.PubMed/NCBI
|
|
9
|
Jahng J, Youn YH, Kim KH, Yu J, Lee YC,
Hyung WJ, Noh SH, Kim H, Kim H, Park H and Lee SI: Endoscopic and
clinicopathologic characteristics of early gastric cancer with high
microsatellite instability. World J Gastroenterol. 18:3571–3577.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kikuchi S, Futawatari N, Sakuramoto S,
Katada N, Yamashita K, Shibata T, Nemoto M and Watanabe M:
Comparison of staging system between the old (6th edition) and new
(7th edition) TNM classifications in advanced gastric cancer.
Anticancer Res. 31:2361–2365. 2011.PubMed/NCBI
|
|
11
|
Kamikihara T, Arima T, Kato K, Matsuda T,
Kato H, Douchi T, Nagata Y, Nakao M and Wake N: Epigenetic
silencing of the imprinted gene ZAC by DNA methylation is an early
event in the progression of human ovarian cancer. Int J Cancer.
115:690–700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarmalaite S, Laurinaviciene A,
Tverkuviene J, Kalinauskaite N, Petroska D, Böhling T and
Husgafvel-Pursiainen K: Tumor suppressor gene ZAC/PLAGL1: Altered
expression and loss of the nonimprinted allele in
pheochromocytomas. Cancer Genet. 204:398–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ankolkar M, Salvi V, Warke H, Vundinti BR
and Balasinor NH: Methylation status of imprinted genes DLK1-GTL2,
MEST (PEG1), ZAC (PLAGL1), and LINE-1 elements in spermatozoa of
normozoospermic men, unlike H19 imprinting control regions, is not
associated with idiopathic recurrent spontaneous miscarriages.
Fertil Steril. 99:1668–1673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawakami H, Zaanan A and Sinicrope FA:
Microsatellite instability testing and its role in the management
of colorectal cancer. Curr Treat Options Oncol. 16:302015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita K, Arimura Y, Kurokawa S, Itoh
F, Endo T, Hirata K, Imamura A, Kondo M, Sato T and Imai K:
Microsatellite instability in patients with multiple primary
cancers of the gastrointestinal tract. Gut. 46:790–794. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polom K, Marrelli D, Roviello G, Pascale
V, Voglino C, Rho H, Marini M, Macchiarelli R and Roviello F:
Molecular key to understand the gastric cancer biology in elderly
patients-The role of microsatellite instability. J Surg Oncol.
115:344–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pedrazzani C, Corso G, Velho S, Leite M,
Pascale V, Bettarini F, Marrelli D, Seruca R and Roviello F:
Evidence of tumor microsatellite instability in gastric cancer with
familial aggregation. Fam Cancer. 8:215–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seo HM, Chang YS, Joo SH, Kim YW, Park YK,
Hong SW and Lee SH: Clinicopathologic characteristics and outcomes
of gastric cancers with the MSI-H phenotype. J Surg Oncol.
99:143–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang QX, Ding Y, Le XP and Du P: Studies
on microsatellite instability in p16 gene and expression of hMSH2
mRNA in human gastric cancer tissues. World J Gastroenterol.
9:437–441. 2003. View Article : Google Scholar : PubMed/NCBI
|