Introduction
Superficial soft tissue masses frequently occur and
primarily manifest as benign lesions (including lipoma and
hemangioma) that typically do not require treatment. Although
malignant masses are rare, prompt surgical resections are required
following the confirmation of a diagnosis (1). Therefore, differentiating between benign
and malignant masses is important to prevent delays in the
treatment of the malignant masses and avoid unnecessary surgical
treatments for the benign masses (2).
As the most effective method, pathological diagnosis is typically
obtained from a needle biopsy. However, it is an invasive
inspection that is uncomfortable for patients and impractical for
all types of soft tissue masses (3).
Ultrasound is the primary examination method for superficial soft
tissue masses to confirm their size, location and association
between the masses and the surrounding structures. Through
observations of the borders of the tissue masses, internal echo
characteristics and internal blood flow signals, ultrasounds may
provide a preliminary diagnosis that is inaccurate (4). Stiffness of the tissue structures may be
accessed using ultrasound strain elastography (USE) (5), which is an effective tool for
differentiating malignant and benign masses (6). Stiffness of a malignant tumor is
typically higher compared with a benign tumor. Previously, the
differential diagnosis was primarily based on palpations by the
physicians, which was indirect and could be limited in patients
with obesity, mass sizes and depths, and physicians’ experiences
(7). Following the first application
at the end of the last century, USE has been widely accepted as an
effective method for differentiating between malignant and benign
tumors, in particular the differential diagnosis for breast cancer
(8). In addition, USE has been
successfully applied in the diagnoses of thyroid, liver and kidney
tumors (9–11). However, differentiation of malignant
and benign soft tissue masses using USE has rarely been
investigated (12). The current study
aimed to assess the importance of strain elastography (SE) for the
differentiation of malignant and benign soft tissue masses.
Materials and methods
Patients and treatments
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Fujian Medical
University and written informed consent was obtained from all
patients. Between October 2012 and November 2014, 66 patients (34
males and 32 females) admitted at the Second Affiliated Hospital of
Fujian Medical University (Quanzhou, China) due to palpable
superficial masses were enrolled onto the current study. The mean
age was 45.9±15.9 years (range, 6–74). Conventional ultrasound and
USE were performed on all patients. Surgical resections were
sequentially performed on 48 patients with normal clinical
histopathology carried out by professionals of our hospital (0.2%
hematoxylin for 5 min and 0.5% eosin stained for 2 min), whilst no
treatments were administered to 13 patients with benign masses
following a comprehensive diagnosis. During the >1-year
follow-up period, no significant alterations in the masses were
observed. In total 5 patients were lost to follow-up and therefore
excluded from the study. The ultrasound instrument was VISION
Preirus (Hitachi, Ltd., Tokyo, Japan) with a 3–13 MHz linear
transducer and the examination was performed by a radiologist with
10 years of experience in ultrasound examination and >5 years of
expertise in USE examination. Based on the locations of the masses,
different patient positions were adopted to ensure that the body
surfaces, where the masses were located, were parallel to the
examination table. Gray-scale and color Doppler examinations were
initially performed to observe the locations and sizes of the
masses and their association with the adjacent structures and
subsequently, the SE mode was initiated. The probe was repeatedly
and mildly pressed then released to obtain the elastic images.
Every site was examined 3 times and the images were captured for
further analysis.
USE analysis
Colors of the images represented different strain
rates in a decreasing order from red to green to blue. Higher
strain rates indicated greater deformation tendencies and lower
stiffness of the tissues. Based on the image colors, tissue
elasticity was classified into 4 scores representing different
stiffness: score 1, completely red or green; score 2, blue and
green, with green as the dominant color; score 3, blue and green,
with blue as the dominant color; and score 4, completely blue.
Strain rates of samples from inside and outside the masses were
measured to calculate the strain ratios (SRs).
Statistical analysis
SPSS software (version 19.0; IBM SPSS, Armonk, NY
USA) was adopted for the statistical analysis. The non-parametric
test was used to compare the elastic scores and SR values between
the benign and malignant masses. The receiver operating
characteristic (ROC) curve of elastic score and SR value was
generated to calculate the area under the curve (AUC), determine
the optimal threshold values and measure the sensitivity and
specificity. P<0.05 was considered to indicate a statistically
significant difference.
Results
Of the 61 patients with complete follow-up data, 31
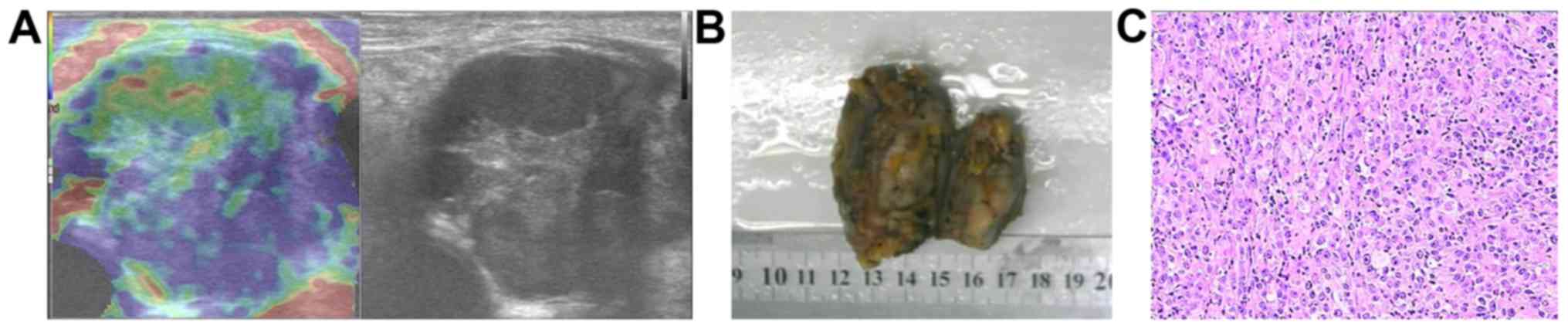
had benign soft tissue masses, including 11 lipomas (Fig. 1), 6 hemangiomas, 4 fibromas, 4
inflammatory masses (Fig. 2), 3
epidermoid cysts and 3 neurofibromas, and 13 other benign masses
confirmed by the unchanged status during the >1-year follow-up.
In total 17 patients had malignant masses, comprising of 10
metastatic carcinomas, 3 lymphomas, 2 malignant melanomas (Fig. 3), a liposarcoma and a myeloma. The
elastic scores and SR values of the benign and malignant masses
were 2.03±0.99 and 3.13±0.34 (P<0.001), respectively, and
1.80±2.10 and 5.42±3.47 (P<0.001), respectively. Area under
receiver operating characteristic curve (AUROC) values of the SRs
and elastic scores were 0.87 (P<0.001; 95% confidence interval
of 0.775–0.968) and 0.805 (P=0.001; 95% confidence interval of
0.688–0.922), respectively (Fig. 4).
There were no significant differences identified between the AUROCs
of the 2 methods (P>0.05). Using analysis of the ROC data, the
optimal SR threshold value for determining a malignant mass was
2.295, with a sensitivity of 93.8%, specificity of 80.5%, positive
predictive value of 65.2% and negative predictive value of 97.1%,
whereas adopting an elastic score ≥3 (Fig. 5A and B) as the optimal threshold
value, the sensitivity, specificity, positive predictive value and
negative predictive value for diagnosing a malignant mass were 100,
51.6, 51.6 and 100%, respectively.
Discussion
By applying pressure to the inspection sites, USE
acquires response information resulting from the pressure and
determines the tissue stiffness. As malignant tumors are typically
harder compared with benign tumors, USE may be used to
differentiate between them (13). The
two most frequently used USE methods are SE and shear wave
elastography (SWE) (14). SE acquires
the deformation information of the tissues under pressure, with
greater deformations indicating lower tissue stiffness and less
deformations representing greater tissue stiffness, and presents
the results in different colors or differing degrees of brightness.
SWE obtains the shear wave information from the tissues under
pressure, with faster propagation velocities of shear wave
indicating greater tissue stiffness, and also presents the results
in different colors or differing degrees of brightness. In
addition, SWE also measures and quantifies the shear wave
propagation velocities at the regions of interest, and therefore
provides more information compared with SE. However, SWE is a novel
technique with an inadequate number of published studies, and its
advantages have not been conclusively demonstrated. Chang et
al (15) and Youk et al
(16) compared the importance of SWE
and SE for differentiating between malignant and benign breast
masses, and did not identified any significant differences between
the AUROCs of these 2 methods. Carlsen et al (17) assessed the elastic scores of targets
with different diameters and depths using SE, SWE and strain
histogram, and observed that SE and strain histogram AUCs were
higher compared with the SWE AUC, and target diameter influenced
all 3 methods, whilst depth only influenced shear-wave velocity.
Mass depths do not significantly differ in small organs, including
the thyroid (18), but in the current
study, masses had greater depth ranging from the subcutaneous layer
to the muscular layer, which may result in an increased frequency
in errors in the SWE examination. As SE is primarily unaffected by
the mass depths, it is potentially advantageous compared with SWE
in the differentiation of soft tissue masses.
Riishede et al (12) applied SE to predict malignancy in 60
patients with a total of 61 soft tissue tumors and identified
significant differences between the mean SR values for malignant
and benign tumors, with significantly higher SR in the malignant
tumors, but no significant differences were observed for strain
histograms or elastic scores. The results of the present study
indicated significant differences in the SR values and elastic
scores between the malignant and benign masses. Setting an SR of
>2.295 and an elastic score of ≥3 as thresholds was highly
sensitive for the diagnosis of a malignant mass (sensitivities,
93.8 and 100%, respectively). If a mass is diagnosed as benign by
the 2 methods, possibility of malignancy may be excluded with the
aid of two-dimensional and color Doppler examinations, and needle
biopsies may be avoided. The specificities of SR and elastic score
for diagnosing a malignant tumor were comparatively low
(specificities were 80.5 and 51.6%, respectively), which may be as
certain benign masses also have high stiffness. For instance, a
particular patient with calcinosis has an SR value of 9.4 and
elastic score of 3, whilst another patient with epidermoid cyst
complicated by foreign body giant cell reaction had SR value of 4.4
and elastic score of 3. The resected tissue samples from these
patients exhibited high stiffness.
The current study has certain limitations. As the
origins of the soft tissue tumors are diverse, only the elasticity
between the malignant and benign masses were compared and not the
masses from different pathological types due to the small sample
size. Further studies with larger sample sizes are required. The
degree of motion is also an effective way to differentiate between
the malignancy and benignancy of a mass. During the physical
examination, the degree of motion may be determined by palpations,
which is not sufficiently achieved by USE. This disadvantage of USE
highlights the simplicity and effectiveness of palpation in
clinical practice.
In conclusion, SR values and elastic scores of the
malignant soft tissue masses were significantly higher compared
with those of the benign tissues. With a high sensitivity, SE may
be used to differentiate between the malignant and benign soft
tissue masses. Setting an SR value of >2.295 and elastic score
of ≥3 as the threshold for diagnosing a malignant tumor, is highly
sensitive but not sufficiently specific.
References
|
1
|
Kransdorf MJ: Benign soft-tissue tumors in
a large referral population: Distribution of specific diagnoses by
age, sex, and location. AJR Am J Roentgenol. 164:395–402. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumral TL, Yildirim G, Önol SD, Ataç E,
Uyar Y and Coşkun ZÜ: Real-time ultrasound elastography for the
differentiation of malignant and benign masses in the head and
neck. J Craniofac Surg. 25:1971–1974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manaster BJ: Soft-tissue masses: Optimal
imaging protocol and reporting. AJR Am J Roentgenol. 201:505–514.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwok HC, Pinto CH and Doyle AJ: The
pitfalls of ultrasonography in the evaluation of soft tissue
masses. J Med Imaging Radiat Oncol. 56:519–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hahn S, Lee YH, Lee SH and Suh JS: Value
of the strain ratio on ultrasonic elastography for differentiation
of benign and malignant soft tissue tumors. J Ultrasound Med.
36:121–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun J, Cai J and Wang X: Real-time
ultrasound elastography for differentiation of benign and malignant
thyroid nodules: A meta-analysis. J Ultrasound Med. 33:495–502.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dargar S, Akyildiz AC and De S:
Development of a soft tissue elastography robotic arm (STiERA).
Stud Health Technol Inform. 220:77–83. 2016.PubMed/NCBI
|
|
8
|
Kim YS, Park JG, Kim BS, Lee CH and Ryu
DW: Diagnostic value of elastography using acoustic radiation force
impulse imaging and strain ratio for breast tumors. J Breast
Cancer. 17:76–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi WJ, Park JS, Koo HR, Kim SY, Chung MS
and Tae K: Ultrasound elastography using carotid artery pulsation
in the differential diagnosis of sonographically indeterminate
thyroid nodules. AJR Am J Roentgenol. 204:396–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Q, Ling W, Lu C, Li J, Ma L, Quan J, He
D, Liu J, Yang J, Wen T, et al: Hepatocellular carcinoma: Stiffness
value and ratio to discriminate malignant from benign focal liver
lesions. Radiology. 275:880–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Göya C, Daggulli M, Hamidi C, Yavuz A,
Hattapoglu S, Cetincakmak MG and Teke M: The role of quantitative
measurement by acoustic radiation force impulse imaging in
differentiating benign renal lesions from malignant renal tumours.
Radiol Med. 120:296–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riishede I, Ewertsen C, Carlsen J,
Petersen MM, Jensen F and Nielsen MB: Strain elastography for
prediction of malignancy in soft tissue tumours-preliminary
results. Ultraschall Med. 36:369–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onur MR, Poyraz AK, Bozgeyik Z, Onur AR
and Orhan I: Utility of semiquantitative strain elastography for
differentiation between benign and malignant solid renal masses. J
Ultrasound Med. 34:639–647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwak JY and Kim EK: Ultrasound
elastography for thyroid nodules: Recent advances. Ultrasonography.
33:75–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang JM, Won JK, Lee KB, Park IA, Yi A
and Moon WK: Comparison of shear-wave and strain ultrasound
elastography in the differentiation of benign and malignant breast
lesions. AJR Am J Roentgenol. 201:W347–W356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youk JH, Son EJ, Gweon HM, Kim H, Park YJ
and Kim JA: Comparison of strain and shear wave elastography for
the differentiation of benign from malignant breast lesions,
combined with B-mode ultrasonography: Qualitative and quantitative
assessments. Ultrasound Med Biol. 40:2336–2344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carlsen JF, Pedersen MR, Ewertsen C,
Săftoiu A, Lönn L, Rafaelsen SR and Nielsen MB: A comparative study
of strain and shear-wave elastography in an elasticity phantom. AJR
Am J Roentgenol. 204:W236–W242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamanaka N, Kaminuma C, Taketomi-Takahashi
A and Tsushima Y: Reliable measurement by virtual touch tissue
quantification with acoustic radiation force impulse imaging:
Phantom study. J Ultrasound Med. 31:1239–1244. 2002. View Article : Google Scholar
|