Introduction
Gastric cancer was the fourth most prevalent cancer
and caused the second highest number of cancer-associated
mortalities worldwide in 2012 (1).
Metastasis is the main factor contributing to the poor prognosis of
patients with gastric cancer (2). In
cases of metastasis, patients lose the opportunity to receive a
curative treatment, and the response rate of chemotherapy and
targeted drugs is limited (3).
Therefore, exploring the underlying molecular mechanisms that
facilitate the development of gastric cancer may help to identify
potential molecular targets and biomarkers for therapeutic
intervention in patients with gastric cancer.
Stromal cell-derived factor-1 (SDF-1α), also termed
CXCL12, is a member of the CXC chemokine family, which binds to its
receptor, CXC chemokine receptor-4 (CXCR4), performing an important
role in inflammation, immune surveillance and tissue regeneration
as well as oncogenesis (4,5). The CXCL12/CXCR4 axis modulates a number
of downstream signaling pathways associated with chemotaxis, tumor
cell proliferation and metastasis (5). Previous clinical studies have revealed
that the overexpression of CXCL12/CXCR4 is associated with poor
prognosis and is involved in the lymph node and distant metastasis
of gastric carcinoma (6,7). However, CXCL12/CXCR4 axis-mediated
signaling pathways in gastric cancer cells are yet to be fully
elucidated. A number of previous studies have established that
G-protein-coupled receptors (GPCRs), including CXCR4, are able to
transactivate epidermal growth factor receptor (EGFR) via a
disintegrin and metalloprotease (ADAM) domain (8,9). This
cross-activation is involved in prostate cancer cell proliferation
through CXCL12/CXCR4-induced ectodomain shedding of EGFR ligands
(10). Yasumoto et al
(11) reported that CXCL12 and
heparin-binding epidermal growth factor-like growth factor (HB-EGF)
collaboratively stimulate the secretion of amphiregulin from
gastric cancer cells and promote peritoneal metastasis. However,
the underlying mechanism by which other cross-activation accounts
for the regulation of CXCL12/CXCR4-induced EGFR activation in
gastric cancer requires further investigation.
In cross-talk studies, GPCRs have also been
established to transactive receptor pathways through
ligand-independent mechanisms, involving a number of key mediators
of growth factor signalling, including SHC, growth factor receptor
bound 2 (GRB2) and SOS, in addition to mitogen-activated protein
kinase activation (12,13). Fischer et al (14) reported that the GPCR-mediated
activation of c-MET occurs via NADPH-induced release of reactive
oxygen species. In prostate cancer cells, lipid rafts were reported
as the key site of CXCR4 transactivation of the human epidermal
growth factor receptor 2 (HER2) receptor (15). SRC, a non-receptor tyrosine kinase, is
expressed ubiquitously in human malignancies and is involved in
numerous signaling pathways (16).
SRC contributes to CXCL12/CXCR4-induced breast and prostate cancer
bone metastasis (15,17). SRC may also promote the
phosphorylation of protein-tyrosine kinases, including EGFR, HER2
and c-MET, at the plasma membrane through its intracellular domain
(18–20), as well as mediate tumor cell
proliferation and resistance to HER2 or EGFR inhibitors (10,21,22). Our
previous study demonstrated that SRC combines with EGFR to regulate
EGFR activation in gastric cancer cells and antagonizes apoptosis
induced by tumor necrosis factor-related apoptosis-inducing ligand
(23). However, it is unknown whether
the CXCL12/CXCR4 axis-regulated transactivation of EGFR is mediated
in an SRC-dependent manner.
The present study demonstrated that the formation of
the SRC/EGFR heterodimer contributes to constitutive EGFR
activation, and in turn, activated EGFR causes the activation of
ERK/Akt signaling pathways and promotes gastric cancer cell
migration.
Materials and methods
Cells and cell culture
Human gastric MGC-803, BGC-823, SGC-7901 cell lines
were obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin in a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Reagents and antibodies
Recombinant SDF-1α was purchased from PeproTech
(Rocky Hill, NJ, USA). The CXCR4 antagonist AMD3100 (cat. no.
A5602), the phosphoinositide 3-kinase (PI3K)/Akt inhibitor LY294002
(cat. no. L9908) and the SRC inhibitor PP2 (cat. no. P0042) were
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
ERK inhibitor PD98059 (cat. no. V1191) was obtained from Promega
Corporation (Madison, WI, USA). Dimethyl sulfoxide was used to
dilute CXCR4 and PP2. Mouse anti-SRC (cat. no. SC-24621; dilution,
1:500) and rabbit anti-β-actin (cat. no. SC-1616; dilution,
1:1,000) antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Anti-phosphorylated (p)-EGFR (Tyr1068; cat.
no. 2234; 1:250), anti-EGFR (cat. no. 2646; 1:1,000), rabbit
anti-Akt (cat. no. 9272; 1:1,000), anti-p-Akt (Ser473; cat.
no.9271; 1:500), anti-ERK1/2 (cat. no.9102; 1:2,000), anti-p-ERK1/2
(Thr202/Tyr204; cat. no.7263; 1:500) and anti-p-SRC (Y416; cat.
no.6943T; 1:500) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Rabbit anti-CXCR4 antibodies
were obtained from Abcam (Cambridge, UK).
Small interfering RNA (siRNA)
transfection
The MGC-803 cells were seeded at a density of
2×105 cells/well on 6-well plates and incubated
overnight at 37°C. The cells were transfected with siRNAs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The siRNA sequence
(Genepharm, Inc., Sunnyvale, CA, USA) for EGFR was as follows:
5′-GCCUUUGAGAACCUAGAAATT-3′, and the control sequence was:
5′-AATTCTCCGAACGTGTCACGT-3′. After 72 h of transient transfection
at 37°C, the cells were analyzed using western blotting to examine
the effect of EGFR siRNA.
Western blot analysis
Cells were seeded at a density of 2×105
cells/well on 6-well plates and incubated overnight at 37°C. Cells
were treated with CXCL12 (100 ng/ml) for 5 min, 30 min or 3 h.
Cells were lysed in lysis buffer (1% Triton X-100, 50 mM Tris-HCl
pH 7.4, 150 mM NaCl, 10 mM EDTA, 100 mM NaF, 1 mM
Na3VO4, 1 mM PMSF and 2 µg/ml aprotinin) on
ice. The method of western blot analysis was described in our
previous study (23).
Chemotaxis assay
Transwell migration assays were performed in 24-well
chemotaxis chambers (8-µm pore size; Corning Incorporated, Corning,
NY, USA). The pretreated cells (2×104 cells/well) with
C225 (10 µg/ml), LY294002 (50 µM) and PD98059 (25 µM) were loaded
onto the upper chamber with 200 µl serum-free RPMI-1640 medium. The
lower chambers contained 500 µl of RPMI-1640 with 2.5% FBS, with or
without 100 ng/ml of CXCL12. The cells in the chambers were
incubated for 24 h at 37°C. Non-migrated cells were removed from
the upper surface of the chamber with a wet cotton swab and cells
on the lower surface of the chamber were stained using the
Giemsa-Wright method, as described previously (24). A total of 5 random fields per well
were captured and counted in brightfield microscopy (magnification,
×200; DMI3000 B; Leica Microsystems, Wetzlar, Germany).
Co-immunoprecipitation
Mouse anti-SRC, as previously described (1:200), or
control IgG mixed with protein G agarose beads (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and 200 µg of MGC-803 cell
lysate were incubated for 6 h at 4°C. Immunoprecipitates were
washed 4 times with lysis buffer as described previously. The
method of immunoprecipitation was detailed in our previous study
(23).
In situ proximity ligation assay
(PLA)
A PLA was performed to detect the SRC-EGFR
heterodimer. MGC-803 cells were seeded at a density of
1×105 cells/well on 6-well plates and incubated
overnight at 37°C. The cells were treated with CXCL12 and incubated
for a further 3 h at 37°C. Duolink in situ PLA (Olink AB,
Uppsala, Sweden) was used according to the manufacturer's protocol.
The mouse anti-SRC antibody and rabbit anti-EGFR antibody, as
previously described, were used as primary antibodies, at a
dilution of 1:100. ProLong Gold Antifade reagent with DAPI
(Molecular Probes; Thermo Fisher Scientific, Inc.) was used as the
mounting medium. The method of PLA was otherwise as discussed in
our previous study (23).
Statistical analysis
All the experimental data are expressed as the mean
± standard deviation, and the mean values were calculated from
>3 independent experiments. SPSS software (version 18; SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis.
Statistical comparisons were made with Student's two-tailed
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
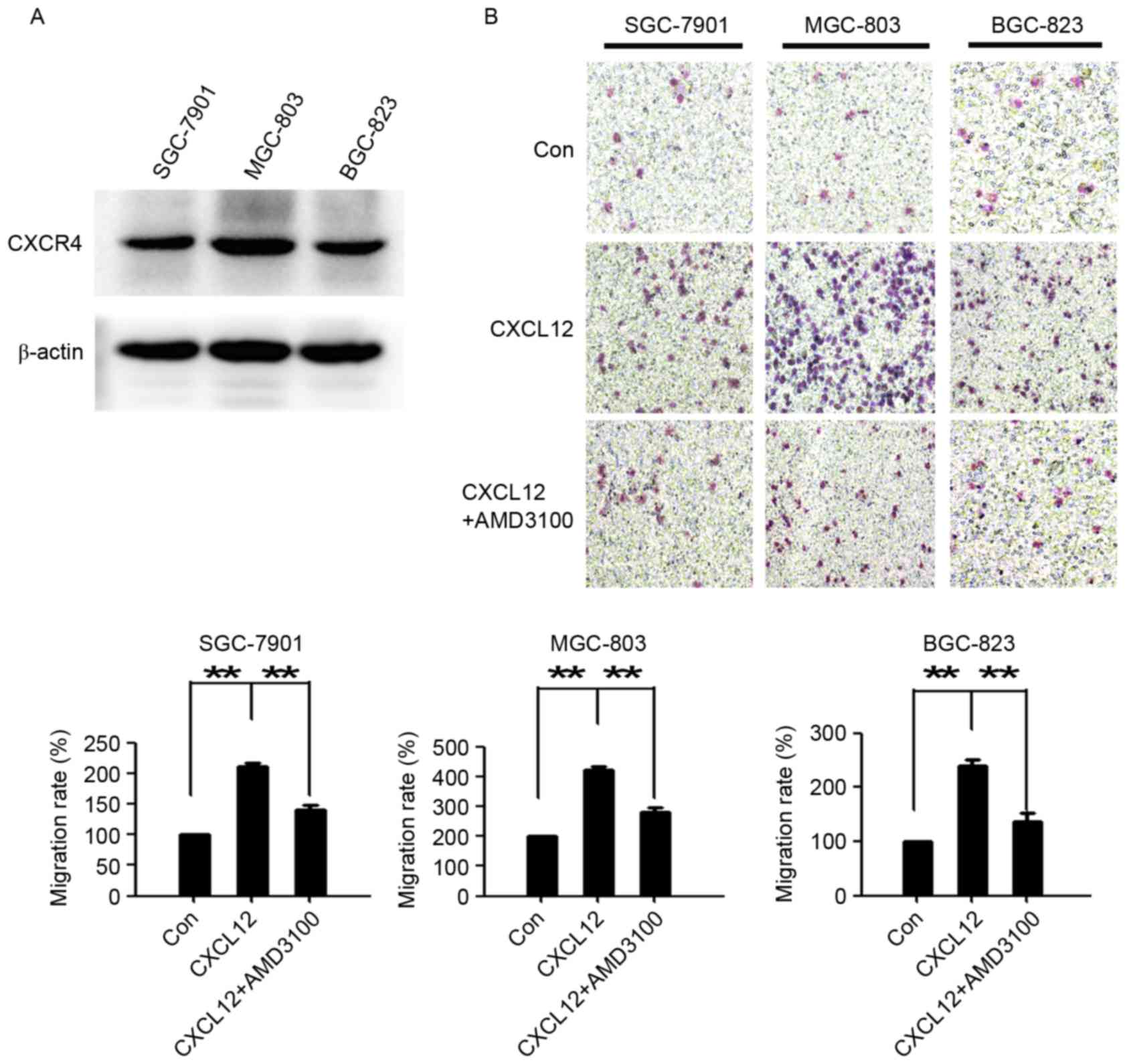
CXCL12/CXCR4 induces gastric cancer
cell migration
To investigate the role of the CXCL12/CXCR4 axis in
gastric cancer cells, western blotting was used to examine the
expression of CXCR4 in 3 gastric cancer cell lines (SGC-7901,
MGC-803 and BGC-823). As presented in Fig. 1A, all 3 gastric cancer cell lines
expressed CXCR4. Compared with the untreated cells, CXCL12
evidently induced gastric cancer cell migration. AMD3100, a highly
specific chemokine receptor CXCR4 antagonist (25), significantly reduced CXCL12-induced
cell migration (Fig. 1B). These data
demonstrated that the CXCL12/CXCR4 axis performs an important role
in the migration of gastric cancer cells.
EGFR, ERK and Akt are involved in
CXCL12-induced gastric cancer cell migration
To examine which pathways are involved in
CXCL12-induced gastric cancer cell migration, cells were treated
with CXCL12. Following treatment with 100 ng/ml CXCL12 for 5 min,
the levels of p-EGFR/Akt/ERK were upregulated (Fig. 2A). The migration ability induced by
CXCL12 was partially suppressed with PD98059 (25 µM), LY294002 (50
µM) and anti-EGFR monoclonal antibody C225 (10 µg/ml; Fig. 2B). These data indicated that CXCL12
induces gastric cancer cell migration due to the activation of
EGFR/Akt/ERK signaling pathways.
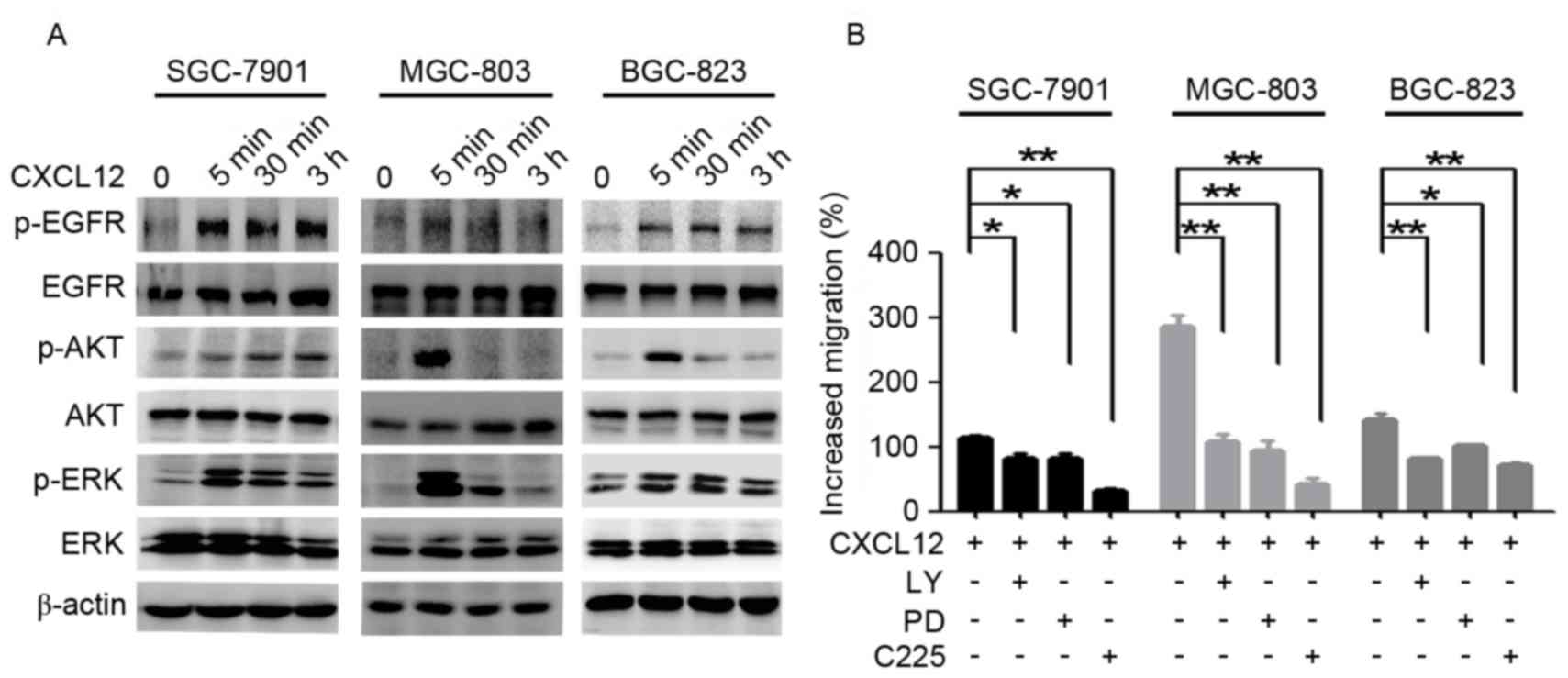 | Figure 2.EGFR, ERK and Akt are involved in
CXCL12-induced gastric cancer cell migration. (A) Serum-starved
SGC-7901, MGC-803 and BGC-823 cells were treated with CXCL12 (100
ng/ml) for 5 min, 30 min or 3 h, and the total protein levels of
EGFR/Akt/ERK and p-EGFR/p-Akt/p-ERK were detected using western
blot analysis. (B) The SGC-7901, MGC-803 and BGC-823 cells were
treated with CXCL12 (100 ng/ml) with or without C225 (10 µg/ml),
LY294002 (50 µM) and PD98059 (25 µM), and cell migration was
assessed using a Transwell assay. Data are presented as the mean ±
standard deviation of 3 independent experiment. *P<0.05,
**P<0.01. CXCL12, stromal cell-derived factor 1α, SDF-1α; EGFR,
epidermal growth factor receptor; p-EGFR, phosphorylated-EGFR;
C225, cetuximab; LY, LY294002; PD, PD98059; ERK, extracellular
signal-regulated kinase; p-ERK, phosphorylated-ERK. |
EGFR regulates the activation of
ERK/Akt pathways in CXCL12-induced gastric cancer cell
migration
To understand the role of EGFR in CXCL12-induced
gastric cancer cell migration, it was investigated whether the
activation of ERK/Akt is dependent on EGFR. The MGC-803 cells were
pretreated with anti-EGFR monoclonal C225 antibody (10 µg/ml) for 2
h (Fig. 3A) or knockdown of the EGFR
gene (Fig. 3B) was performed prior to
stimulation with CXCL12 (100 ng/ml). A marked inhibition of
CXCL12-induced activation of ERK and Akt signaling was observed at
the 30 min and 3 h time points following EGFR inhibitor or si-EGFR
treatment. These results demonstrated that CXCL12-induced gastric
cancer cell migration is EGFR-Akt/ERK-dependent in MGC-803
cells.
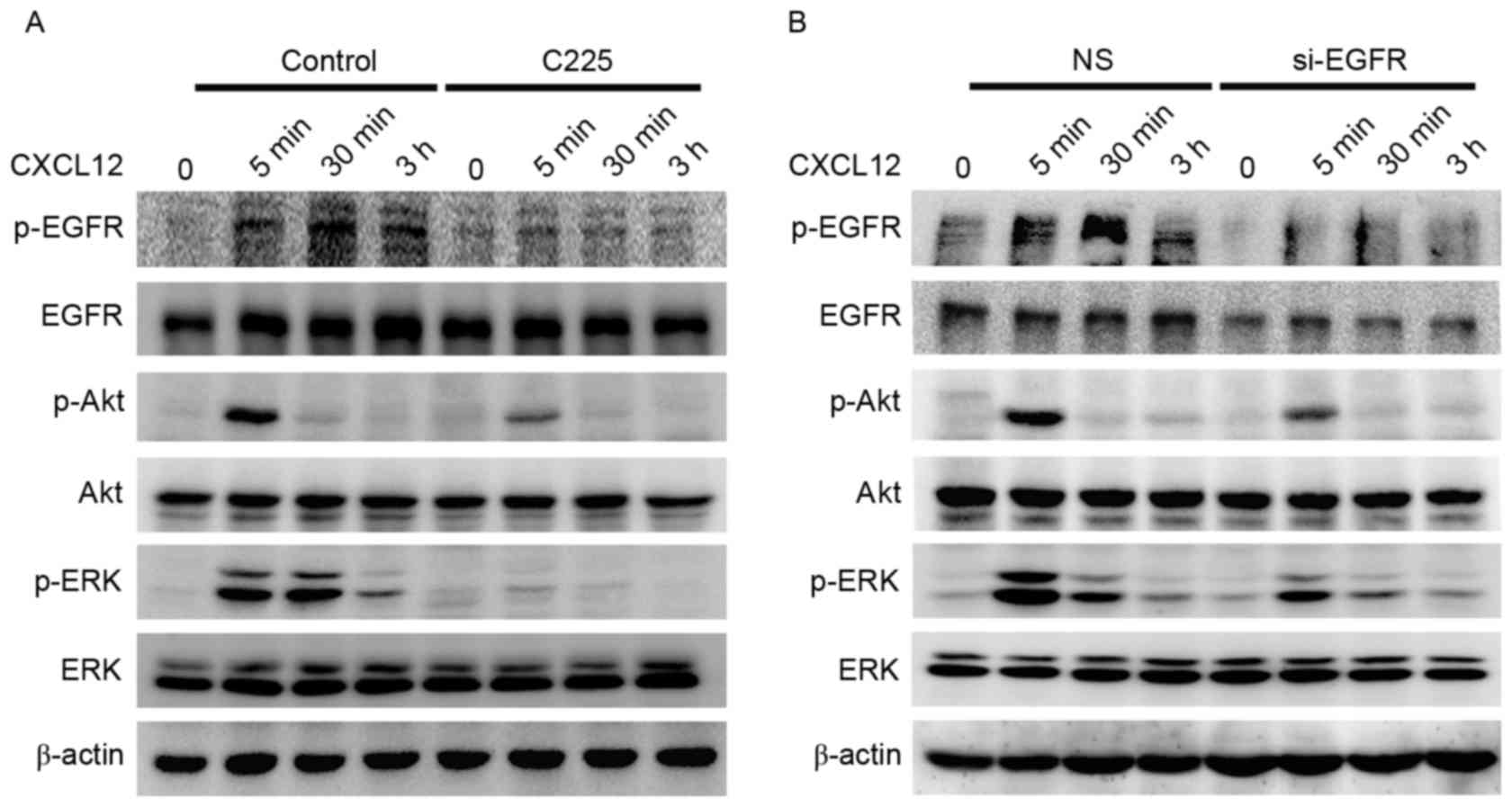 | Figure 3.EGFR-regulated activation of the
ERK/Akt signaling pathway in CXCL12-induced gastric cancer cell
migration. (A) Serum-starved MGC-803 cells were pretreated with or
without C225 (10 µg/ml) for 2 h, cells were treated with CXCL12
(100 ng/ml) for 5 min, 30 min or 5 h, and the total levels of
EGFR/Akt/ERK and p-EGFR/p-Akt/p-ERK proteins were detected using
western blot analysis. (B) Transient knockdown of the EGFR gene
using EGFR siRNA for 72 h, followed by 100 ng/ml CXCL12 for 5 min,
30 min or 3 h. Western blot analysis was used to detect the total
EGFR/Akt/ERK and p-EGFR/p-Akt/p-ERK protein levels. CXCL12, stromal
cell-derived factor 1α, SDF-1α; C225, cetuximab; siRNA, small
interfering RNA; NS, non-silenced; EGFR, epidermal growth factor
receptor; p-EGFR, phosphorylated-EGFR; ERK, extracellular
signal-regulated kinase; p-ERK, phosphorylated-ERK. |
CXCL12/CXCR4 effects the activation of
EGFR and ERK/Akt via SRC
To assess which molecules modulate the activation of
EGFR and the ERK/Akt signaling pathway, the effects of SRC on
CXCL12-induced migration activity in MGC-803 cells was
investigated. Rapid phosphorylation of SRC in MGC-803 cells was
observed following stimulation with CXCL12 (100 ng/ml; Fig. 4A). The CXCL12-induced migration was
inhibited by the SRC family kinase inhibitor PP2 (10 µM; Fig. 4B), as was the activation of EGFR, ERK
and Akt pathways (Fig. 4C). To
determine whether CXCR4 contributes to the phosphorylation of SRC
and EGFR, MGC-803 cells were pretreated with AMD3100 (10 µg/ml), a
highly specific chemokine receptor CXCR4 antagonist (25), 2 h prior to stimulation with CXCL12.
The results demonstrated that the inhibition of CXCR4 reduced the
activation of SRC, EGFR and the downstream ERK/Akt signaling
induced by CXCL12 (Fig. 4D).
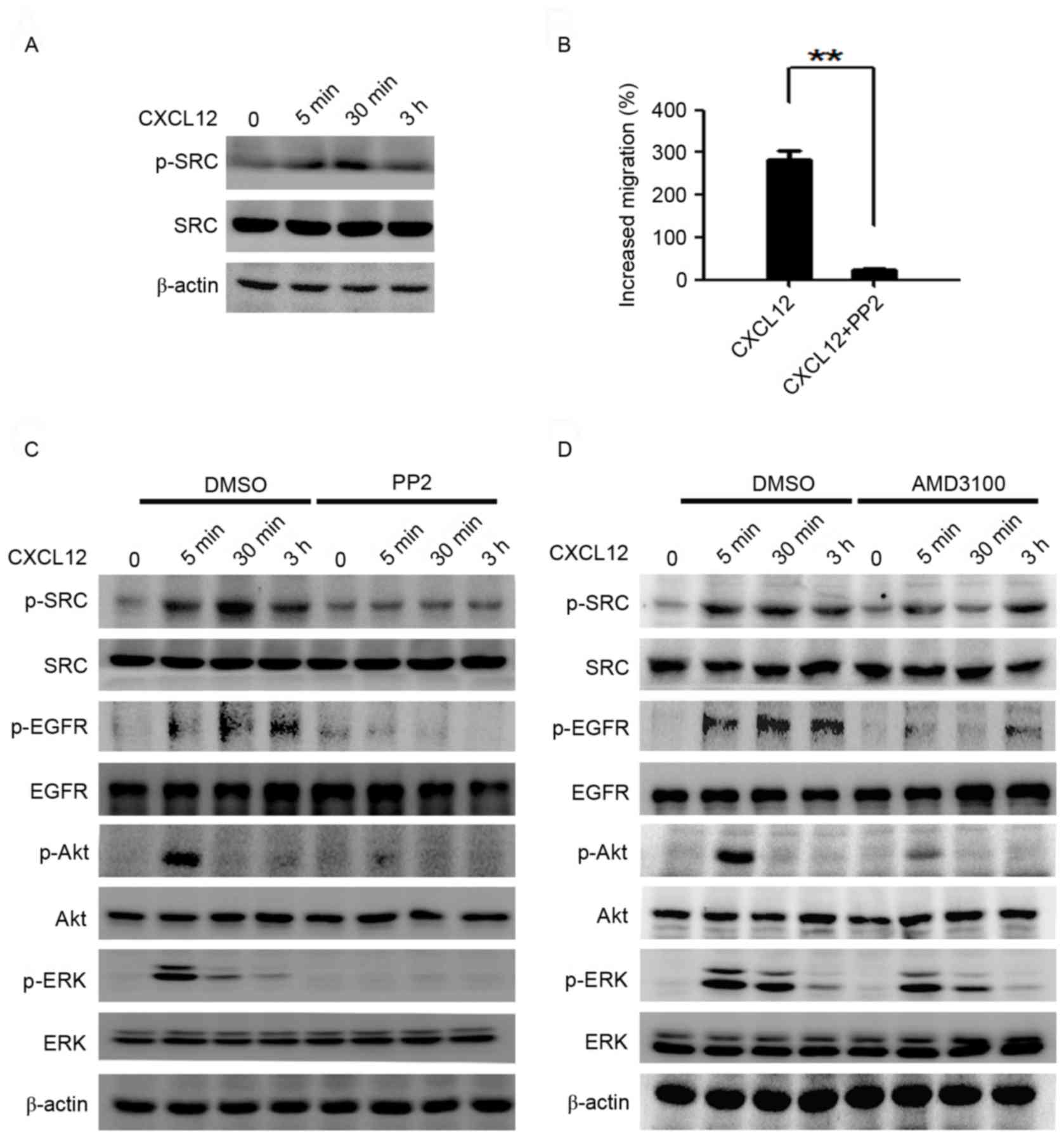 | Figure 4.CXCL12 effects the activation of EGFR
and ERK/Akt via SRC. (A) Serum-starved MGC-803 cells were treated
with CXCL12 (100 ng/ml) for 5 min, 30 min or 3 h, and p-SRC was
analyzed using western blot analysis. (B) The MGC-803 cells were
treated with CXCL12 (100 ng/ml) with or without PP2 (10 µM), and
cell migration was assessed using a Transwell assay. Data are
presented as the mean ± standard deviation of 3 independent
experiment. **P<0.01. (C) Serum-starved MGC-803 cells were
pretreated with or without PP2 for 2 h, then cells were treated
with CXCL12 (100 ng/ml) for the indicated times and western blot
analysis was used to examine the total levels of EGFR/Akt/ERK and
p-EGFR/p-Akt/p-ERK protein. (D) Serum-starved MGC-803 cells were
pretreated with or without AMD3100 for 2 h, cells were incubated
with CXCL12 (100 ng/ml) for 5 min, 30 min or 3 h, and the total
EGFR/Akt/ERK and p-EGFR/p-Akt/p-ERK protein levels were examined
using western blot analysis. CXCL12, stromal cell-derived factor
1α, SDF-1α; PP2, SRC inhibitor; EGFR, epidermal growth factor
receptor; p-EGFR, phosphorylated-EGFR; ERK, extracellular
signal-regulated kinase; p-ERK, phosphorylated-ERK; DMSO, dimethyl
sulfoxide. |
Formation of the SRC/EGFR heterodimer
is induced by CXCL12
To elucidate the interaction between SRC and EGFR, a
co-immunoprecipitation assay was performed following CXCL12
treatment. Co-immunoprecipitation experiments demonstrated that the
interaction between EGFR and SRC was enhanced by CXCL12 (Fig. 5A). The formation of SRC/EGFR
heterodimers by Duolink in situ PLA was also evaluated. This
method confirmed the promotion of the interaction between SRC and
EGFR by CXCL12, with red fluorescent signals indicating the
presence of the SRC-EGFR heterodimer (Fig. 5B). Compared with CXCL12 alone, PP2
reversed the complex of SRC/EGFR promoted by CXCL12 (Fig. 5C). These results indicated that
CXCL12/CXCR4 induced gastric cancer migration via the formation of
SRC/EGFR heterodimers.
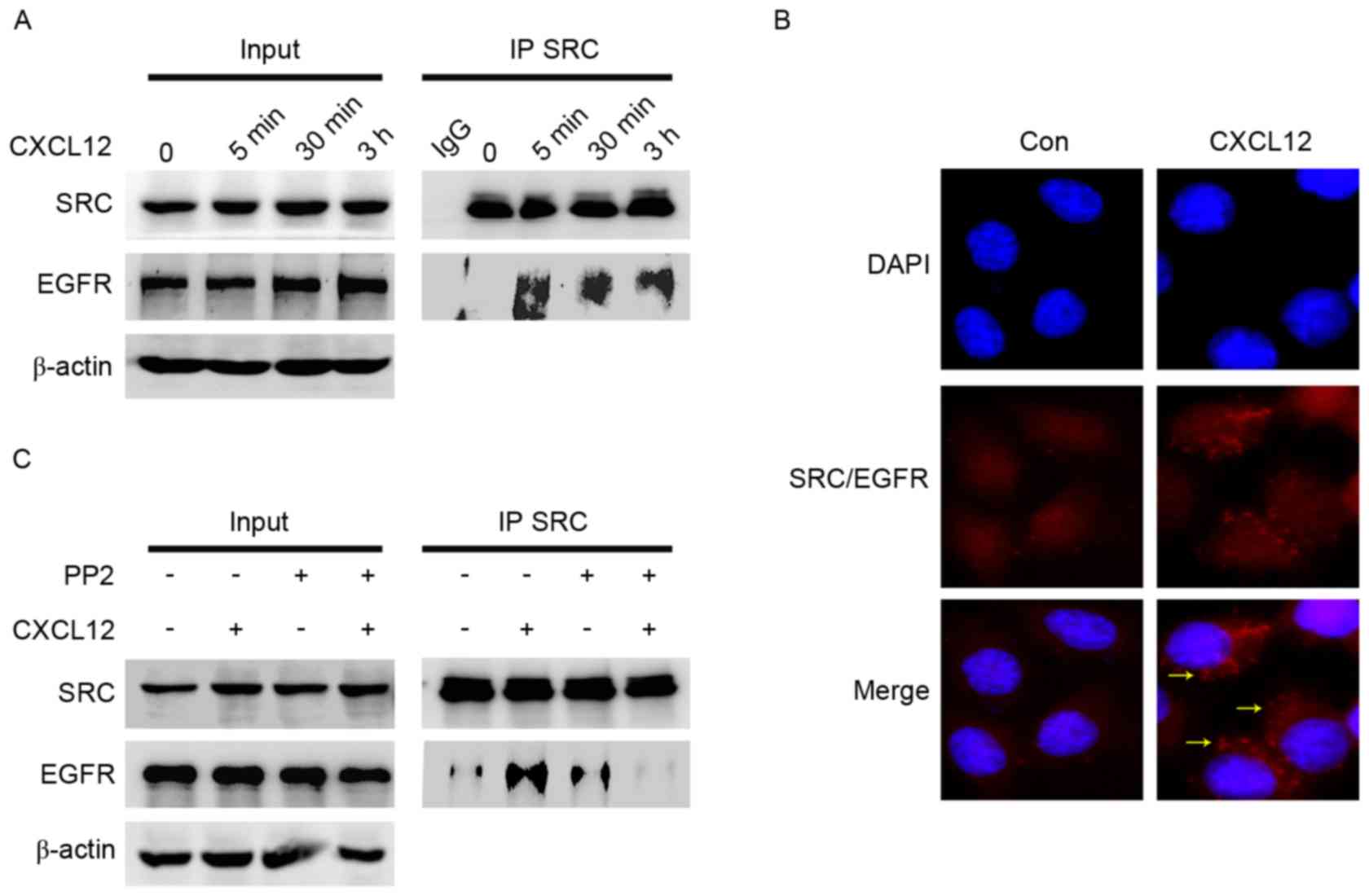 | Figure 5.Formation of the SRC/EGFR heterodimer
is induced by CXCL12. (A) Serum-starved MGC-803 cells were treated
with CXCL12 for 5 min, 30 min or 3 h. Whole cell lysates were
immunoprecipitated with anti-SRC antibody. SRC and EGFR levels were
analyzed using western blot analysis. Input represents cell lysates
that were not subjected to immunoprecipitation or antibodies as an
IP control. (B) The SRC/EGFR complex was detected using Duolink
in situ proximity ligation assay following stimulation with
100 ng/ml CXCL12 for 3 h in MGC-803 cells. Red fluorescence,
interaction of SRC with EGFR; blue, nucleus; yellow arrows, foci of
interaction between SRC and EGFR on the merged image
(magnification, ×60). (C) Serum-starved MGC-803 cells were
pretreated with 10 µM SRC inhibitor PP2 for 2 h and then treated
with 100 ng/ml CXCL12 for 3 h. The interaction between SRC and EGFR
was detected using immunoprecipitation. CXCL12, stromal
cell-derived factor 1α, SDF-1α; PP2, SRC inhibitor; EGFR, epidermal
growth factor receptor; IP, immunoprecipitation; Con, control. |
Discussion
CXCL12/CXCR4 signaling was initially established as
a regulator of B lymphocyte chemoattractant (26). Later, it was reported that CXCR4
performs an important role in tumor cell survival, proliferation,
migration and stemness (27). The
binding of CXCL12 to CXCR4 leads to activation of numerous
downstream signaling pathways, including MAPK-ERK, PI3K-Akt-NF-κB
and c-Jun N-terminal kinase, and also modulates tumor progression
(5). In the present study, it was
observed that 3 gastric cancer cell lines express different levels
of CXCR4. Using AMD3100, a highly specific chemokine receptor CXCR4
antagonist (25), the activation of
ERK/Akt signaling and the migration ability promoted by CXCL12 were
reduced. This supports the central role of the CXCL12/CXCR4 axis in
gastric cancer cell migration.
Aberrant activation or overexpression of EGFR
contributes to tumor progression (28). CXCR4 is a seven-transmembrane trimeric
GPCR, which is a type of receptor that may transactivate EGFR via
ligand-dependent and ligand-independent mechanisms (29). Firstly, CXCL12/CXCR4 stimulates
ectodomain shedding of EGFR ligands mediated by ADAM, including
amphiregulin, EGF, epiregulin and HB-EGF, and mediates the
activation of EGFR (10). A previous
study established that HB-EGF and CXCL12 together enhance the
amphiregulin shedding from NUGC4 cells, serving an important role
in peritoneal carcinomatosis from gastric cancer (11). Secondly, SRC promotes the formation of
the Shc/Grb2/SoS complex and activation of the intracellular
tyrosine kinase domain of EGFR (18).
In breast and ovarian cancer cells, the interaction of CXCL12 to
CXCR4 activates EGFR in a SRC kinase-dependent mechanism (10,30). In
the present study, following exposure to CXCL12, there was a
gradual increase in the phosphorylation of SRC, alongside the
activation of EGFR. PP2 inhibition of SRC suppressed the migration
ability and activation of EGFR. In addition, treatment with C225 or
the knockdown of the EGFR gene inhibited CXCL12-induced ERK/Akt
phosphorylation, indicating that these two signaling pathways are
regulated by CXCL12/CXCR4-induced EGFR transactivation. Therefore,
these results suggested that inclusive of the first hypothesis,
there may be a potential SRC-dependent mechanism of EGFR
intracellular activation in CXCL12/CXCR4-induced gastric cancer
cell migration.
To elucidate the underlying regulatory mechanisms of
SRC involved in EGFR activation in CXCL12/CXCR4-induced gastric
cancer cell migration, the interaction between SRC and EGFR was
investigated. SRC interacts with multiple receptor tyrosine kinases
via its SH2 domain and promotes the activation of multiple
signaling pathways (19,31,32). EGFR
and SRC form a stable complex when exposed to irradiation in lung
cancer cells (33). Our previous
study reported the formation of the Met/SRC/EGFR complex was
induced by cetuximab in colon cancer cells as a resistance
mechanism, and the interaction of SRC/EGFR heterodimers antagonize
apoptosis induced by tumor necrosis factor-related
apoptosis-inducing ligand in gastric cancer cells (23,34).
However, the function of the interaction of the SRC and EGFR
heterodimer in the regulation of CXCL12-induced EGFR activation
remains to be elucidated. Co-immunoprecipitation results
demonstrated that CXCL12 induced the formation of SRC/EGFR
heterodimers. In addition, SRC inhibitors reduced the formation of
the SRC/EGFR complex stimulated by CXCL12. To the best of our
knowledge, the present study is the first to identify that CXCL12
induces EGFR activation, at least partially, via the SRC-EGFR
heterodimer complex.
AMD3100 is a highly specific chemokine receptor
CXCR4 antagonist, which is also termed plerixafor or Mozobil®. In
2008, AMD3100 was approved by the Food and Drug Administration for
patients with non-Hodgkin's lymphoma or multiple myeloma (25). Numerous clinical trials using the
anti-CXCR4 antibody have been initiated in preclinical, phase I or
phase II trials for solid tumor treatment with encouraging results
(35). In addition, SRC kinase
inhibitors, including saracatinib, dasatinib and bosutinib, are
administered to patients with solid tumors (36). Montero et al (37) supported the use of dasatinib in
combination with a number of treatments for solid tumors.
Plerixafor combined with SRC inhibitors and chemotherapy or
radiotherapy may aid individualized patient management,
particularly in patients with metastatic gastric cancer that have
high expression levels of CXCR4 or CXCL12. However, further
experimental and clinical studies are required to confirm the
results of the present study.
In conclusion, the present study indicated that SRC
mediates EGFR activation through the formation of SRC/EGFR
heterodimers, and that activated EGFR stimulates ERK/Akt pathways
in CXCL12/CXCR4-induced gastric cancer cell migration. These
results provide novel insight into the molecular network of the
CXCL12/CXCR4 pathway in gastric cancer cell migration.
Acknowledgements
The present study was supported by the Chinese
National Foundation of National Sciences (grant nos. 81572374,
81201615, 81401938 and 81372547) and the Natural Science Foundation
of Liaoning Province (grant nos. 2014021069, 2014021089 and
LZ2015073).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah MA: Update on metastatic gastric and
esophageal cancers. J Clin Oncol. 33:1760–1769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou YR, Kottmann AH, Kuroda M, Taniuchi I
and Littman DR: Function of the chemokine receptor CXCR4 in
haematopoiesis and in cerebellar development. Nature. 393:595–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cojoc M, Peitzsch C, Trautmann F,
Polishchuk L, Telegeev GD and Dubrovska A: Emerging targets in
cancer management: Role of the CXCL12/CXCR4 axis. Onco Targets
Ther. 6:1347–1361. 2013.PubMed/NCBI
|
|
6
|
Ying J, Xu Q, Zhang G, Liu B and Zhu L:
The expression of CXCL12 and CXCR4 in gastric cancer and their
correlation to lymph node metastasis. Med Oncol. 29:1716–1722.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Zhang H, He H, Shen Z, Tang Z, Xu
J and Sun Y: Prognostic value of stromal cell-derived factor 1
expression in patients with gastric cancer after surgical
resection. Cancer Sci. 105:1447–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohtsu H, Dempsey PJ and Eguchi S: ADAMs as
mediators of EGF receptor transactivation by G protein-coupled
receptors. Am J Physiol Cell Physiol. 291:C1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hart S, Fischer OM, Prenzel N,
Zwick-Wallasch E, Schneider M, Hennighausen L and Ullrich A:
GPCR-induced migration of breast carcinoma cells depends on both
EGFR signal transactivation and EGFR-independent pathways. Biol
Chem. 386:845–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasina S, Scherle PA, Hall CL and Macoska
JA: ADAM-mediated amphiregulin shedding and EGFR transactivation.
Cell Prolif. 42:799–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasumoto K, Yamada T, Kawashima A, Wang W,
Li Q, Donev IS, Tacheuchi S, Mouri H, Yamashita K, Ohtsubo K and
Yano S: The EGFR ligands amphiregulin and heparin-binding egf-like
growth factor promote peritoneal carcinomatosis in CXCR4-expressing
gastric cancer. Clin Cancer Res. 17:3619–3630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Natarajan K and Berk BC: Crosstalk
coregulation mechanisms of G protein-coupled receptors and receptor
tyrosine kinases. Methods Mol Biol. 332:51–77. 2006.PubMed/NCBI
|
|
13
|
Hopkins MM, Liu Z and Meier KE: Positive
and negative cross-talk between lysophosphatidic acid receptor 1,
free fatty acid receptor 4, and epidermal growth factor receptor in
human prostate cancer cells. J Pharmacol Exp Ther. 359:124–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer OM, Giordano S, Comoglio PM and
Ullrich A: Reactive oxygen species mediate Met receptor
transactivation by G protein-coupled receptors and the epidermal
growth factor receptor in human carcinoma cells. J Biol Chem.
279:28970–28978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinni SR, Yamamoto H, Dong Z, Sabbota A,
Bonfil RD and Cher ML: CXCL12/CXCR4 transactivates HER2 in lipid
rafts of prostate cancer cells and promotes growth of metastatic
deposits in bone. Mol Cancer Res. 3:446–457. 2008. View Article : Google Scholar
|
|
16
|
Martin GS: The hunting of the Src. Nat Rev
Mol Cell Biol. 2:467–475. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bendinelli P, Maroni P, Matteucci E and
Desiderio MA: Comparative role of acetylation along c-SRC/ETS1
signaling pathway in bone metastatic and invasive mammary cell
phenotypes. Biochim Biophys Acta. 1813:1767–1776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luttrell LM, Ferguson SS, Daaka Y, Miller
WE, Maudsley S, Rocca GJ Della, Lin F, Kawakatsu H, Owada K,
Luttrell DK, et al: Beta-arrestin-dependent formation of beta2
adrenergic receptor-Src protein kinase complexes. Science.
283:655–661. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Huang WC, Li P, Guo H, Poh SB,
Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stabile LP, He G, Lui VW, Thomas S, Henry
C, Gubish CT, Joyce S, Quesnelle KM, Siegfried JM and Grandis JR:
c-Src activation mediates erlotinib resistance in head and neck
cancer by stimulating c-Met. Clin Cancer Res. 19:380–392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peiró G, Ortiz-Martínez F, Gallardo A,
Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ, Tibau A, López-Vilaro
L, Escuin D, Adrover E, et al: SRC, a potential target for
overcoming trastuzumab resistance in HER2-positive breast
carcinoma. Br J Cancer. 111:689–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boerner JL: Role of SRC family kinases in
acquired resistance to EGFR therapies in cancer. Cancer Biol Ther.
8:704–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Qu X, Li H, Li C, Liu J, Zheng H and
Liu Y: Src/caveolin-1-regulated EGFR activation antagonizes
TRAIL-induced apoptosis in gastric cancer cells. Oncol Rep.
32:318–324. 2014.PubMed/NCBI
|
|
24
|
Zhao L, Liu S, Che X, Hou K, Ma Y, Li C,
Wen T, Fan Y, Hu X, Liu Y and Qu X: Bufalin inhibits TGF-β-induced
epithelial-to-mesenchymal transition and migration in human lung
cancer A549 cells by downregulating TGF-β receptors. Int J Mol Med.
36:645–652. 2015.PubMed/NCBI
|
|
25
|
DiPersio JF, Uy GL, Yasothan U and
Kirkpatrick P: Plerixafor. Nat Rev Drug Discov. 8:105–106. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagasawa T, Hirota S, Tachibana K,
Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H and
Kishimoto T: Defects of B-cell lymphopoiesis and bone-marrow
myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature.
382:635–638. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo G, Gong K, Wohlfeld B, Hatanpaa KJ,
Zhao D and Habib AA: Ligand-independent EGFR signaling. Cancer Res.
75:3436–3441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhola NE and Grandis JR: Crosstalk between
G-protein-coupled receptors and Epidermal growth factor receptor in
cancer. Front Biosci. 13:1857–1865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porcile C, Bajetto A, Barbieri F, Barbero
S, Bonavia R, Biglieri M, Pirani P, Florio T and Schettini G:
Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates
ovarian cancer cell growth through the EGF receptor
transactivation. Exp Cell Res. 308:241–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bromann PA, Korkaya H and Courtneidge SA:
The interplay between Src family kinases and receptor tyrosine
kinases. Oncogene. 23:7957–7968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parsons JT and Parsons SJ: Src family
protein tyrosine kinases: Cooperating with growth factor and
adhesion signaling pathways. Curr Opin Cell Biol. 9:187–192. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dittmann K, Mayer C, Kehlbach R, Rothmund
MC and Rodemann H Peter: Radiation-induced lipid peroxidation
activates src kinase and triggers nuclear EGFR transport. Radiother
Oncol. 92:379–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song N, Liu S, Zhang J, Liu J, Xu L, Liu Y
and Qu X: Cetuximab-induced MET activation acts as a novel
resistance mechanism in colon cancer cells. Int J Mol Sci.
15:5838–5851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Nigris F, Schiano C, Infante T and
Napoli C: CXCR4 inhibitors: Tumor vasculature and therapeutic
challenges. Recent Pat Anticancer Drug Discov. 7:251–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mayer EL and Krop IE: Advances in
targeting SRC in the treatment of breast cancer and other solid
malignancies. Clin Cancer Res. 16:3526–3532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Montero JC, Seoane S, Ocaña A and
Pandiella A: Inhibition of SRC family kinases and receptor tyrosine
kinases by dasatinib: Possible combinations in solid tumors. Clin
Cancer Res. 17:5546–5552. 2011. View Article : Google Scholar : PubMed/NCBI
|