Introduction
Metaplastic breast carcinoma (MBC) is a rare and
heterogeneous type of neoplasm characterized by the histological
presence of ≥2 cellular types, commonly a mixture of epithelial and
mesenchymal components (1). The World
Health Organization (WHO) recognized this subtype of breast cancer
as a unique pathological entity in 2000, and its incidence is
<1% of all breast malignancies (2,3). The key
concept in the pathogenesis and development of MBC may be that
epithelial to mesenchymal transition (EMT)-associated genes are
differentially upregulated (4) and
enriched in tumor-initiating cells (5).
Only a few large studies on MBC have been published
to date (6,7); however, MBC has long been recognized as
a distinct histological type of breast cancer (8). Due to its low incidence rate and
pathological variability, the clinical characteristics, prognostic
significance and optimal treatment modalities are unclear and
controversial. Certain reports have suggested that the prognosis of
MBC is favorable, with survival similar to that of adenocarcinoma
of a comparable stage (9,10), whereas others consider that MBC may
exhibit an aggressive course, with worse outcomes than those of
triple-negative invasive ductal carcinoma (11,12). MBCs
usually present as large tumors that are negative for hormone
receptors and rarely benefit from conventional chemotherapy or
hormonal therapy (13). Although the
incidence of axillary lymph node (LN) involvement is low, these
tumors exhibit an increased risk of developing distant organ
metastasis compared with infiltrating ductal carcinoma (IDC)
(14). In clinical practice, MBC is
usually treated based on the guidelines developed for IDC (15). The ideal treatment paradigm for MBC is
unknown; thus, potential predictors of treatment efficacy must be
explored.
The present study reviewed the clinical,
pathological and biological characteristics of 69 patients with MBC
who were diagnosed and treated at Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China), and evaluated the
clinicopathological features, different therapeutic strategies and
prognostic factors of survival.
Patients and methods
Patients
Among 30,053 patients who underwent surgery for
breast cancer at Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China) from January 1, 2002 to January 1, 2015,
69 (0.23%) patients were identified as MBC. All patients were
females and their ages ranged from 28 to 89 years, with a median
age of 53 years. Details concerning clinicopathological
characteristics, surgical treatment, chemotherapy and radiation
therapy (RT) were also gathered from the medical records.
In total, 53 cases received modified radical
mastectomy (MRM), 9 cases received radical mastectomy (RM), 2 cases
received segmental mastectomy and axillary dissection, and 5 cases
received segmental mastectomy only. A total of 50 cases had
received chemotherapy, 14 cases had received radiotherapy, and 8
cases with positive estrogen receptor (ER) or progesterone receptor
(PR) expression had received adjuvant hormonal therapy. The
pathological diagnosis was performed in accordance with the
histological classification of tumors developed by the WHO
(16) and clinical staging was based
on the tumor-node-metastasis staging of breast cancer developed by
the American Joint Committee on Cancer (AJCC, 7th edition)
(17).
The diagnosis of MBC was conducted in the absence of
an associated primary metaplastic cell type in a secondary site and
in the absence of skin involvement. The pathologic material for
each case of MBC was retrieved from the archive of the Department
of Breast Cancer at the Tianjin Medical University Cancer Institute
and Hospital (Tianjin, China) and reviewed.
Immunohistochemistry (IHC) and
fluorescent in situ hybridization (FISH)
Formalin-fixed paraffin embedded tissue sections
were employed in each case using a standard protocol. IHC staining
and FISH analysis were performed on tissue sections (3–4 µm) and
tissue microarray slides. IHC staining (Benchmark XT; Ventana
Medical Systems, Tucson, AZ, USA) was performed on tumor sections
from 69 patients using the avidin-biotin-immunoperoxidase technique
for ER (cat no. NCL-L-PGR-312; dilution, 1:100), PR (cat no.
NCL-L-ER-6F11; dilution, 1:80) [both from Novocastra, Leica
Biosystems (Newcastle) Ltd., Newcastle Upon Tyne, UK] and human
epidermal growth factor receptor 2 (HER-2; cat no. 800-2996;
dilution 1:300; Ventana Medical Systems). An iView DAB detection
kit (Ventana Medical Systems) was used for secondary antibody. The
FISH test was performed according to the Abbott/Vysis PathVysion
HER2 DNA Probe kit (cat no. 30161060/02J01-030; Abbott Molecular
Inc., Des Plaines, IL, USA) manufacturers protocol. The
SpectrumOrange fluorophore-labeled DNA probe for the HER-2/neu gene
locus and SpectrumGreen fluorophore-labeled α-satellite DNA probe
for chromosome 17 from this kit were used. In total 2 separate
fields of ≥20 cells were counted and an average of the results from
the preselected tumor areas were used to create mean average gene
and chromosomal counts, which were used to calculate the HER2:CEP17
signal ratio. Tumor cells from matching sites of IHC were scored
for the number of red (HER2) and green (chromosome 17) signals. The
slides were evaluated using an Olympus BX51 microscope (Olympus,
Tokyo, Japan) with an oil-immersion objective lens and an
appropriate filter set at magnification, ×100. The immunoreaction
was evaluated independently by ≥2 pathologists.
The threshold used for positive ER and PR expression
was 1% of nuclear staining in the total number of tumor cells
stained. HER-2 immunoreactivity was evaluated on a standardized
scale from 0–3 based on the intensity of staining of the cell
membrane and the proportion of invasive tumor cells stained. Strong
complete staining of the membrane in >10% of tumor cells (score,
3+) was considered positive. Intensity patterns with scores 0–1+
were considered negative, and samples scored as 2+ were further
assessed by FISH test where HER2/CEP17 ratio of >2.0 was
considered positive for HER2 gene amplification.
Follow-up
Overall survival (OS) was defined as the time from
the date of first surgery to the date of mortality or last
follow-up. Disease-free survival (DFS) was defined as the duration
of time between the date of first surgery and the date of first
local recurrence or distant metastasis or last follow-up. For all
patients, follow-up started from the date of operation. The
patients were followed up in the Outpatients Department of Tianjin
Medical University Cancer Institute and Hospital at 3 months
intervals for the first year, at 6 months intervals for the
following 2 years and then annually. All patients were followed up
until mortality or the cut-off date of January 1, 2015. A total of
3 cases were lost to follow-up, and the median follow-up time was
37 months (range, 9–139 months). OS data were obtained from medical
records or by telephone calls or letter communication. This study
was approved by the Institutional Review Board of the Tianjin
Medical University Cancer Institute and Hospital and written
consent was obtained from all participants.
Statistical analysis
All statistical analyses were carried out using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA) for Windows. Cumulative
survival analysis of the patients was used to evaluate possible
associations between survival and patient covariates. The
Kaplan-Meier method and the log-rank test were used for univariate
analysis, while the Cox proportional hazard model was applied for
multivariate analysis. All tests were two sided. P<0.05 or a 95%
confidence interval (CI) that did not include 1 was considered to
indicate a statistically significant difference.
Results
Clinicopathological
characteristics
The clinical characteristics of MBC patients are
summarized in Table I. Among the
patients, squamous and sarcomatoid were the most common
histological subtype, followed by adenosquamous, spindle cell and
osseous/chondroid carcinoma. A total of 11 patients had a family
history of cancer. The most common clinical presentation was a firm
lump, which was noted in 66 patients, among which, 3 patients had a
history of blood nipple discharge and other 3 patients had a
history of blood nipple discharge with pain. Tumor sizes were
determined by gross pathological examination, and ranged from 0.8
to 10.0 cm (mean, 3.7 cm; median, 3.5 cm). LN involvement was
reported in 29.0% of the 69 MBC patients. Among all the MBC cases,
78.2% had triple-negative breast cancer (TNBC) tumors and 69.6% had
basal-like type breast carcinomas.
 | Table I.Clinicopathological features of
patients with metaplastic carcinoma of the breast. |
Table I.
Clinicopathological features of
patients with metaplastic carcinoma of the breast.
|
| Patients |
|---|
|
|
|
|---|
| Variables | No. | % |
|---|
| Age, years |
|
|
|
<50 | 22 | 31.9 |
|
≥50 | 47 | 68.1 |
| Menopausal
status |
|
|
|
Premenopausal | 26 | 37.7 |
|
Postmenopausal | 43 | 63.3 |
| Tumor stage |
|
|
| T1 | 10 | 14.5 |
| T2 | 42 | 60.9 |
| T3 | 12 | 17.4 |
| T4 | 5 | 7.2 |
| Lymph node
stage |
|
|
| N0 | 44 | 63.8 |
| N1 | 14 | 20.3 |
| N2 | 5 | 7.2 |
| N3 | 1 | 1.4 |
| Unknown
ER status | 5 | 7.2 |
|
Negative | 59 | 85.5 |
|
Positive | 10 | 14.5 |
| PR status |
|
|
|
Negative | 63 | 91.3 |
|
Positive | 6 | 8.70 |
| HER-2 status |
|
|
|
Negative | 60 | 87.0 |
|
Positive | 9 | 13.0 |
| Subtype |
|
|
|
Squamous cell | 22 | 31.9 |
|
Adenosquamous cell | 14 | 20.3 |
| Spindle
cell | 9 | 13.0 |
|
Chondroid | 2 | 2.9 |
|
Sarcomatoid | 22 | 31.9 |
Treatment modalities
All patients received surgical treatment. The most
common surgical procedure was MRM, which was performed in 53
patients. If required, patients would receive this treatment in
combination with chemotherapy, radiotherapy and/or hormonal therapy
post-surgery (Table II). All
chemotherapy regimens and their outcomes in 50 patients are
summarized in Table III. The
remaining 19 patients did not receive chemotherapy due to poor
health or economic problems.
 | Table II.Treatment modalities. |
Table II.
Treatment modalities.
|
| Patients |
|---|
|
|
|
|---|
| Treatment
modalities | No. | % |
|---|
| Breast surgery |
|
|
|
Modified-radical
mastectomy | 53 | 76.8 |
| Radical
mastectomy | 9 | 13.0 |
|
Breast-conserving surgery
with | 2 | 2.9 |
|
Axillary lymph nodes
dissection |
|
|
|
Segmental mastectomy | 5 | 7.2 |
| Chemotherapy |
|
|
|
Yes | 50 | 72.5 |
| No | 19 | 27.5 |
| Radiation
therapy |
|
|
|
Yes | 14 | 20.3 |
| No | 55 | 79.7 |
| Adjuvant hormonal
therapy |
|
|
|
Yes | 8 | 11.6 |
| No | 61 | 88.4 |
| Adjuvant
trastuzumab |
|
|
|
Yes | 2 | 2.9 |
| No | 67 | 97.1 |
 | Table III.Adjuvant chemotherapy regimens and
their outcomes. |
Table III.
Adjuvant chemotherapy regimens and
their outcomes.
| Chemotherapy
regimens | Patients, no. | 5-year DFS, % | MST |
|---|
| CMF | 9 | 62.2 | 6 relapse free at
122, 89, 73, 34, 27 and 22 months, respectively; 3 relapsed |
| AT/ET/TAC/TEC | 31 | 52.3 | 14 relapse free at
133, 99, 89, 76, 67, 66, 63, 62, 61, 60, 46, 41, 26 and 20 months,
respectively; 17 relapsed |
| NP/CP/TP | 10 | 70.0 | 6 relapse free at
139, 105, 79, 76, 60 and 24 months, respectively; 4 relapsed |
Outcome, recurrence and prognosis
Up to the cut-off date for the analysis, 3 patients
were lost to follow-up, 35 patients were still alive without
recurrence and 28 patients succumbed to disease progression. In
total, 20 patients experienced locoregional recurrence during the
follow-up subsequent to surgery. Of the 20 patients with
locoregional relapse, 13 relapses occurred in the chest wall, 4
relapses occurred in the ipsilateral breast and 3 relapses occurred
in the ipsilateral axilla. Among 28 patients who developed distant
metastasis during the follow-up, the most common organs involved
were the lung (n=16), liver (n=9), bone (n=7), supraclavicular LNs
(n=3) and brain (n=4). The 5-year DFS and OS rates were 52.2 and
60.2%, respectively.
Univariate and multivariate analysis
of OS and DFS
The univariate and multivariate analyses for the
association between 5-year DFS and OS rates and clinicopathological
characteristics are shown in Table
IV. Kaplan-Meier analysis and log-rank test revealed that T
stage and LN status were significant predictors for DFS and OS in
univariate analysis. Chemotherapy could significantly improve the
5-year OS rate in univariate analysis, whereas no significantly
difference for the 5-year DFS rate was observed. The 5-year OS rate
was 68.7% in the chemotherapy group and 37.2% in the
non-chemotherapy group. When the above variables were analyzed by a
Cox proportional hazard model, T stage and LN status remained
significant independent predictors for DFS and OS. The hazard ratio
for patients subjected to chemotherapy was 0.27 (95% CI, 0.11–0.67)
for OS.
 | Table IV.Analysis of the prognostic factors
for DFS and OS. |
Table IV.
Analysis of the prognostic factors
for DFS and OS.
|
| 5-year DFS | 5-year OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.213 |
|
|
| 0.380 |
|
|
|
<50 | 1 |
|
|
| 1 |
|
|
|
|
≥50 | 1.63
(0.76–3.51) |
|
|
| 1.45
(0.63–3.31) |
|
|
|
| Menopausal
status |
| 0.153 |
|
|
| 0.165 |
|
|
|
Premenopausal | 1 |
|
|
| 1 |
|
|
|
|
Postmenopausal | 1.70
(0.82–3.51) |
|
|
| 1.76
(0.79–3.93) |
|
|
|
| T stage |
| 0.001 |
| 0.001 |
| 0.003 |
| 0.001 |
|
T1-2 | 1 |
| 1 |
| 1 |
| 1 |
|
|
T3-4 | 3.18
(1.57–6.42) |
| 4.09
(1.84–9.06) |
| 3.27
(1.51–7.07) |
| 3.90
(1.71–8.90) |
|
| LN stage |
| 0.026 |
| 0.016 |
| 0.013 |
| 0.028 |
|
LN− | 1 |
| 1 |
| 1 |
| 1 |
|
|
LN+ | 2.21
(1.01–4.45) |
| 2.62
(1.20–5.72) |
| 2.68
(1.24–5.81) |
| 2.45
(1.10–5.44) |
|
| Hormone receptor
expression status |
| 0.289 |
|
|
| 0.250 |
|
|
|
Negative | 1 |
|
|
| 1 |
|
|
|
|
Positive | 0.57
(0.20–1.62) |
|
|
| 0.49
(0.25–1.65) |
|
|
|
| Chemotherapy |
| 0.096 |
|
|
| 0.010 |
| 0.005 |
| No | 1 |
|
|
| 1 |
| 1 |
|
|
Yes | 0.52
(0.24–1.12) |
|
|
| 0.33
(0.14–0.77) |
| 0.27
(0.11–0.67) |
|
| Radiotherapy |
| 0.539 |
|
|
| 0.582 |
|
|
| No | 1 |
|
|
| 1 |
|
|
|
|
Yes | 1.27
(0.59–2.74) |
|
|
| 1.26
(0.55–2.89) |
|
|
|
| Hormonal
therapy |
| 0.240 |
|
|
| 0.359 |
|
|
| No | 1 |
|
|
| 1 |
|
|
|
|
Yes | 0.49
(0.15–1.61) |
|
|
| 0.57
(0.17–1.90) |
|
|
|
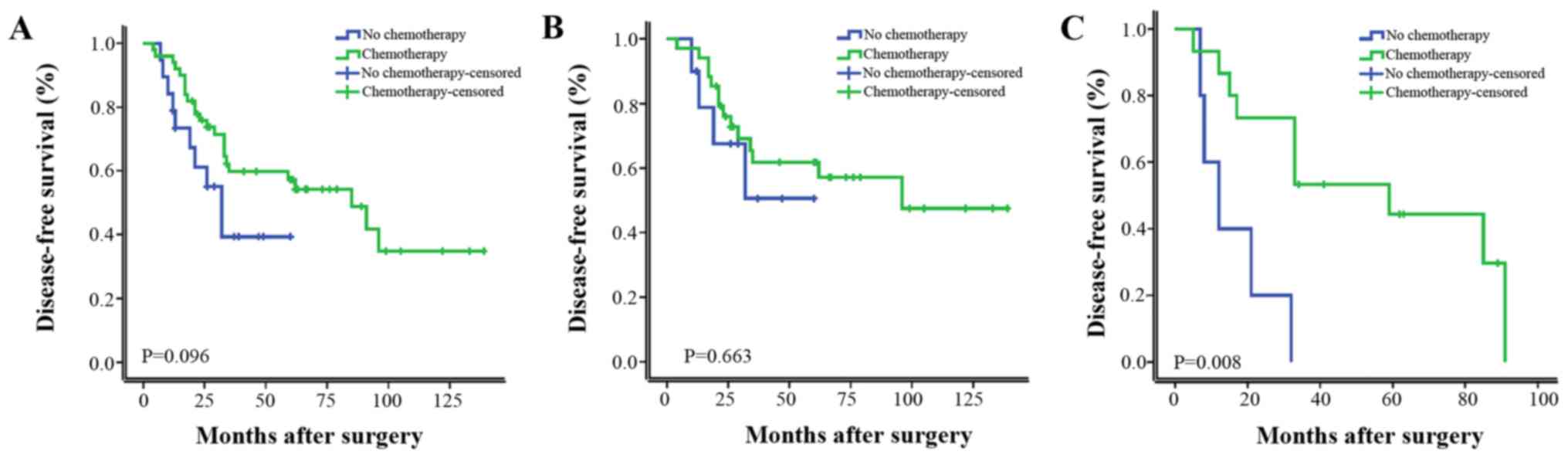
Furthermore, patients were stratified as LN positive
(LN+) and LN negative (LN−). As shown in
Fig. 1, chemotherapy significantly
improved the DFS in univariate analysis. In a subgroup analysis of
the patients with LN+ group and LN− group,
chemotherapy significantly improved the DFS of LN+
patients, LN− patients did not gain a significant
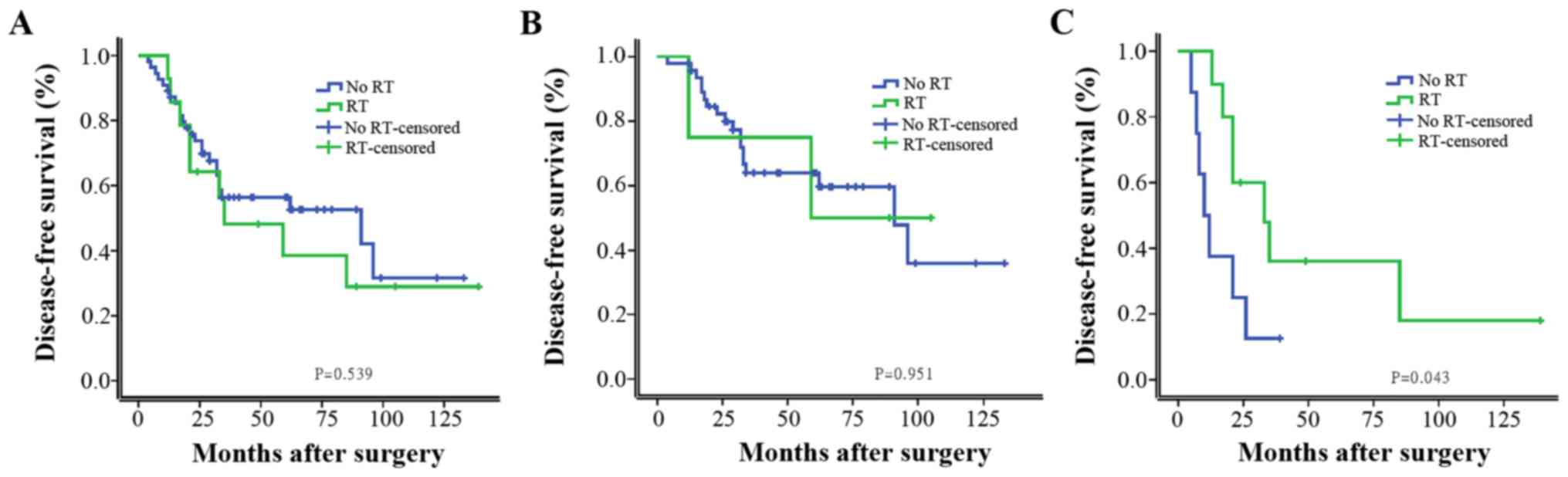
survival benefit from chemotherapy. As shown in Fig. 2, RT did not significantly improve the
DFS in univariate analysis; however, it significantly improved the
DFS of patients with tumors ≥5 cm or with >4 metastatic axillary
LNs.
Discussion
MBC is an extremely rare malignancy and the current
WHO 2012 classification distinguishes five subtypes of MBC
(16). Previous studies reported that
no significant difference was observed in terms of clinical outcome
among patients with different MBC subtypes (18,19). In
the present study, there was no significant difference in OS or DFS
among squamous cell carcinoma, spindle cell carcinoma,
adenosquamous carcinoma or carcinosarcoma. As previously reported,
MBC tends to occur in older females, and usually presents as a
palpable mass that grows rapidly, which may indicate that MBC is an
aggressive disease (20). A previous
study reported that tumor size was best correlated with prognosis
(21), whereas this finding was not
consistent with another study (22).
In the current study, 24.6% of patients presented with a palpable
tumor at stage T3-4. Among them, 14 experienced relapse. By
contrast, of the 52 cases with a palpable tumor at stage T1-2, 25
relapsed, and had a significant difference in their 5-year DFS and
OS rates in univariate analysis. The tumor size acted as an
independent prognostic factor in multivariate analysis for MBC
patients. Previous studies have demonstrated axillary LN metastases
in 22–31% of patients with MBC (14,23).
Despite this low rate, MBC patients with LN metastasis had a
greater risk of developing metastatic disease and a poorer
prognosis than IDC patients (24,25). This
may also support the concept that MBC is an aggressive tumor with a
high risk of recurrence following primary therapy. In the current
study, 29.0% of patients had axillary LN metastases, which is
consistent with the majority of previous reports (23). In univariate analysis, LN status was a
significant prognostic factor, while in multivariate analysis, LN
positivity remained an independent risk factor for the 5-year DFS
and OS rates. MBC patients with a large tumor size and LN
positivity had a poor survival outcome. Therefore, early diagnosis
and treatment of this rare entity is critical to patient
prognosis.
The majority of MBC cases have similar
characteristics. MBCs have a basal-like immunophenotype, and rarely
overexpress hormone receptors and HER-2 (26). In a study on 2,338 patients with MBC,
21.0% were hormone receptor positive, and hormone receptor
positivity does not improve prognosis (27). According to the present study, 78.2%
of patients had TNBC tumors and 69.6% had basal-like type breast
carcinomas, which may help to explain the commonly observed higher
grade and increased rapid growth of MBCs compared with IDCs. In
addition, there was no significant difference in the 5-year DFS or
OS rates between the TNBC group and the non-TNBC group in the
present study.
As MBC patients typically present with large tumors,
>70% of patients with MBC present with AJCC stage II (14) and a higher percentage of patients with
MBC receive mastectomy rather than lumpectomy (3,9). However,
a previous study observed no difference in OS or DFS in MBC
patients treated with mastectomy compared with those treated with
lumpectomy, even upon controlling for known prognostic factors
(22). In the present study, the
majority of cases were stage II and received MR/MRM. There was no
significant difference between MRM/RM and other type of operation.
Taking into account the large tumor size, and the somewhat
refractory nature of the tumor to standard chemotherapy and
hormonal therapy, MRM/RM was selected as an optimal surgical
treatment; however, breast conservative surgery and segmental
mastectomy cannot be precluded in certain eligible patients.
Several MBC cases have been shown to exhibit a good
response to chemotherapy (28).
Takuwa et al (29) reported
that a patient had a good response to platinum combined with taxane
or anthracycline therapy. The majority of previous studies,
however, have revealed an ineffective response of MBC to
chemotherapy (30). Compared with the
response rates of stage-matched female patients with IDC, those
with MBC receiving chemotherapy had lower response rates to the
chemotherapy regimens (30,31). A single-institute retrospective study
reported that tumor response to systemic chemotherapy remains
generally poor, since only 17.6% of patients who received
taxane-based chemotherapy exhibited a positive response (32). The reason for the ineffective response
to chemotherapy may be that MBCs are part of the spectrum of
basal-like breast carcinomas and display a myoepithelial and
EMT-like molecular makeup (26).
Basal-like tumors and breast cancer 1 (BRCA1)-associated breast
cancer tumors were reported to be similar according to microarray
and immunohistochemical analyses (33,34). In
BRCA1-associated breast cancer and basal-like tumors, current
standard anthracycline- and taxanes-containing chemotherapy
regimens were prone to ineffectiveness (35). However, these regimens were able to
sensitize tumors with homologous recombination DNA repair defects
to platinum salts and poly ADP-ribose polymerase (PARP) inhibitors
(36). The results from two
randomized trials indicated that cyclophosphamide, methotrexate and
5-fluorouracil (CMF) chemotherapy significantly improved treatment
outcome in patients with triple-negative, node-negative breast
cancer (37). In the present study,
MBC patients with LN− did not benefit from chemotherapy.
By contrast in MBC patients with LN+, the 5-year OS and
DFS rates significantly improved with chemotherapy. Among the
different chemotherapy regimens, CMF and cisplatin-based regimens
may be effective to certain subgroups of MBC patients, as suggested
by their relatively long median 5-year DFS, although no significant
survival benefit from different regimens was observed in these
patients. These findings would suggest that a subset of MBC
patients may potentially experience a curative benefit from
systemic chemotherapy, but they also support the chemorefractory
behavior of these tumors. Platinum salts combined with PARP
inhibitors could be used for clinical treatment. The limitations of
the present study include: i) The majority of patients who did not
receive chemotherapy were elderly patients with a relative poor
health; ii) a small sample was analyzed; and iii) the positive rate
of LN was low. Therefore, additional clinical data and multicenter
studies are required to confirm the present results.
Tseng and Martinez (38) described patients with MBC who had
received RT and experienced a benefit in terms of OS and DFS, which
suggests that patients undergoing breast conservation surgery and
those with tumors ≥5 cm or with >4 metastatic axillary LNs
undergoing mastectomy should receive RT. Another study suggested
that RT, regardless of the type of surgery, should be considered as
a part of the therapy for patients with MBC (15). In the present study, survival analysis
did not reveal a significant survival benefit from RT, since no
significant differences were observed in the risk of recurrence for
those patients treated with adjuvant RT and not treated with RT.
However, in MBC patients with tumors ≥5 cm or with >4 metastatic
axillary LNs who received RT, the 5-year OS and DFS rates
significantly improved. Genomic profiling of MBC tumors has shown
downregulation of DNA repair pathways, including the BRCA1,
phosphatase and tensin homolog and topoisomerase 2-α pathways,
which may explain the lower incidence of LN spread and sensitivity
towards RT of MBC (39). The present
results demonstrated that RT should be considered as a component of
multimodality therapy for MBC patients with tumors ≥5 cm or with
>4 metastatic axillary LNs. However, since the present study is
a retrospective analysis with a low LN metastasis rate and a small
sample receiving RT, a larger multicenter study is required to draw
definitive conclusions.
In MBC patients, the positive expression of hormone
receptors has been reported to be low (40). However, hormone receptor positivity
does not improve the prognosis of MBC patients (27), since in a retrospective study, the
non-TNBC group of MBC patients had a poor prognosis compared with
that of the TNBC group (41). MBC
patients often show little or no response to adjuvant hormonal
therapy or HER-2-targeted treatment (trastuzumab) (42). In the present study, only 8 patients
had been prescribed tamoxifen/aromatase inhibitors therapy, as they
had ER- and/or PR-positive tumors. Of these patients, 3 relapsed at
17, 28 and 62 months after surgery, respectively. The tumors in 9
patients were HER-2+; however, only 2 patients were
treated with trastuzumab due to financial reasons. Of these 2
patients, 1 developed lung and brain metastases 33 months after
surgery and succumbed to the disease 7 months later, while the
other patient is still alive and free of recurrence at 43 months
after surgery. Due to the high incidence of TNBC in MBC, hormonal
therapy or HER-2-targeted treatment is unlikely to influence the
survival of MBC patients.
In conclusion, MBC is a clinically aggressive
subtype of breast cancer associated with large tumor size and a
high proportion of TNBC and basal-like tumors. Hormonal therapy or
HER-2-targeted treatment is unlikely to influence survival due to
the high incidence of TNBC tumors. Chemotherapy may be recommended
for certain subtype of MBC patients with LN positivity.
Cisplatin-based regimens and CMF regimens may be effective in
certain subgroups. MBC patients with tumors ≥5 cm or with >4
metastatic LNs could benefit from RT. Other therapeutic strategies,
including mechanistic target of rapamycin, androgen receptor and
transforming growth factor-β, should strongly be considered in
clinical trials of innovative therapeutic regimens. However, the
present study possess certain limitations: This is a retrospective
analysis with a small sample size and not a prospective study; a
multicenter study would be preferable in the future. There may have
also been a lack of uniformity as the surgery was performed by
different surgeons. To additionally clarify the characteristics and
prognosis of MBC patients, and in order to improve treatment, the
systematic study of a large number of cases with long-term
follow-up will be necessary.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81472472).
References
|
1
|
Yigit S, Pehlivan FS, Evcim G and Etit D:
Clinicopathologic features of the mixed epithelial and mesenchymal
type metaplastic breast carcinoma with myoepithelial
differentiation in a subset of six cases. Pathol Res Pract.
208:147–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Velasco M, Santamaría G, Ganau S, Farrús
B, Zanón G, Romagosa C and Fernández PL: MRI of metaplastic
carcinoma of the breast. AJR Am J Roentgenol. 184:1274–1278. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al Sayed AD, El Weshi AN, Tulbah AM, Rahal
MM and Ezzat AA: Metaplastic carcinoma of the breast clinical
presentation, treatment results and prognostic factors. Acta Oncol.
45:188–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lien HC, Hsiao YH, Lin YS, Yao YT, Juan
HF, Kuo WH, Hung MC, Chang KJ and Hsieh FJ: Molecular signatures of
metaplastic carcinoma of the breast by large-scale transcriptional
profiling: Identification of genes potentially related to
epithelial-mesenchymal transition. Oncogene. 26:7859–7871. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Toy KA and Kleer CG: Metaplastic
breast carcinomas are enriched in markers of tumor-initiating cells
and epithelial to mesenchymal transition. Mod Pathol. 25:178–184.
2012.PubMed/NCBI
|
|
6
|
Langlands F, Cornford E, Rakha E, Dall B,
Gutteridge E, Dodwell D, Shaaban AM and Sharma N: Imaging overview
of metaplastic carcinomas of the breast: A large study of 71 cases.
Br J Radiol. Jun 21–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherwell-Cabello S, Maffuz-Aziz A,
Hernández-Hernández B, Bautista-Piña V, Labastida-Almendaro S and
Rodríguez-Cuevas S: Metaplastic carcinoma of the breast and the
impact of the p63 and cytokeratin 5/6: Experience of 40 patients.
Ginecol Obstet Mex. 84:127–135. 2016.(In Spanish). PubMed/NCBI
|
|
8
|
Grechi G and Pagnini P: Study of mammary
gland neoplasms with an osteocartilaginous component. I.
Cartilaginous metaplastic epiphenomena in the course of connective
tissue malignancy. Arch De Vecchi Anat Patol. 46:277–303. 1965.(In
Italian).
|
|
9
|
Gibson GR, Qian D, Ku JK and Lai LL:
Metaplastic breast cancer: Clinical features and outcomes. Am Surg.
71:725–730. 2005.PubMed/NCBI
|
|
10
|
Chao TC, Wang CS, Chen SC and Chen MF:
Metaplastic carcinomas of the breast. J Surg Oncol. 71:220–225.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung SY, Kim HY, Nam BH, Min SY, Lee SJ,
Park C, Kwon Y, Kim EA, Ko KL, Shin KH, et al: Worse prognosis of
metaplastic breast cancer patients than other patients with
triple-negative breast cancer. Breast Cancer Res Treat.
120:627–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson RA, Guye ML, Luu T and Lai LL:
Survival outcomes of metaplastic breast cancer patients: Results
from a US population-based analysis. Ann Surg Oncol. 22:24–31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beatty JD, Atwood M, Tickman R and Reiner
M: Metaplastic breast cancer: Clinical significance. Am J Surg.
191:657–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pezzi CM, Patel-Parekh L, Cole K, Franko
J, Klimberg VS and Bland K: Characteristics and treatment of
metaplastic breast cancer: Analysis of 892 cases from the National
Cancer Data Base. Ann Surg Oncol. 14:166–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah DR, Tseng WH and Martinez SR:
Treatment options for metaplastic breast cancer. ISRN Oncol.
2012:7061622012.PubMed/NCBI
|
|
16
|
Frank GA, Danilova NV, Andreeva Iulu and
Nefedova NA: WHO classification of tumors of the breast, 2012. Arkh
Patol. 75:53–63. 2013.(In Russian). PubMed/NCBI
|
|
17
|
Sinn HP, Helmchen B and Wittekind CH: TNM
classification of breast cancer: Changes and comments on the 7th
edition. Pathologe. 31:361–366. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okada N, Hasebe T, Iwasaki M, Tamura N,
Akashi-Tanaka S, Hojo T, Shibata T, Sasajima Y, Kanai Y and
Kinoshita T: Metaplastic carcinoma of the breast. Hum Pathol.
41:960–970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi R, Horii R, Maeda I, Suga S,
Makita M, Iwase T, Oguchi M, Ito Y and Akiyama F: Clinicopathologic
study of 53 metaplastic breast carcinomas: Their elements and
prognostic implications. Hum Pathol. 41:679–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi BB and Shu KS: Metaplastic carcinoma
of the breast: Multimodality imaging and histopathologic
assessment. Acta Radiol. 53:5–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oberman HA: Metaplastic carcinoma of the
breast. A clinicopathologic study of 29 patients. Am J Surg Pathol.
11:918–929. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dave G, Cosmatos H, Do T, Lodin K and
Varshney D: Metaplastic carcinoma of the breast: A retrospective
review. Int J Radiat Oncol Biol Phys. 64:771–775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HS, Park S, Kim JH, Lee JH, Choi SY,
Park BW and Lee KS: Clinicopathologic features and outcomes of
metaplastic breast carcinoma: Comparison with invasive ductal
carcinoma of the breast. Yonsei Med J. 51:864–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae SY, Lee SK, Koo MY, Hur SM, Choi MY,
Cho DH, Kim S, Choe JH, Lee JE, Kim JH, et al: The prognoses of
metaplastic breast cancer patients compared to those of
triple-negative breast cancer patients. Breast Cancer Res Treat.
126:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH,
Park IH, Lee KS, Lee S, Kim SW, Kang HS, et al: Metaplastic breast
cancer: Clinicopathological features and its prognosis. J Clin
Pathol. 65:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cakir A, Gönül II and Uluoğlu O:
Metaplastic breast carcinomas and their relationship with
basal-like phenotype. Turk Patoloji Derg. 28:134–141.
2012.PubMed/NCBI
|
|
27
|
Wright G Paul, Davis AT, Koehler TJ,
Melnik MK and Chung MH: Hormone receptor status does not affect
prognosis in metaplastic breast cancer: A population-based analysis
with comparison to infiltrating ductal and lobular carcinomas. Ann
Surg Oncol. 21:3497–3503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hennessy BT, Giordano S, Broglio K, Duan
Z, Trent J, Buchholz TA, Babiera G, Hortobagyi GN and Valero V:
Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann
Oncol. 17:605–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takuwa H, Ueno T, Ishiguro H, Mikami Y,
Kanao S, Takada M, Sugie T and Toi M: A case of metaplastic breast
cancer that showed a good response to platinum-based preoperative
chemotherapy. Breast Cancer. 21:504–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rayson D, Adjei AA, Suman VJ, Wold LE and
Ingle JN: Metaplastic breast cancer: Prognosis and response to
systemic therapy. Ann Oncol. 10:413–419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moulder S, Moroney J, Helgason T, Wheler
J, Booser D, Albarracin C, Morrow PK, Koenig K and Kurzrock R:
Responses to liposomal doxorubicin, bevacizumab, and temsirolimus
in metaplastic carcinoma of the breast: Biologic rationale and
implications for stem-cell research in breast cancer. J Clin Oncol.
29:e572–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen IC, Lin CH, Huang CS, Lien HC, Hsu C,
Kuo WH, Lu YS and Cheng AL: Lack of efficacy to systemic
chemotherapy for treatment of metaplastic carcinoma of the breast
in the modern era. Breast Cancer Res Treat. 130:345–351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lakhani SR, Reis-Filho JS, Fulford L,
Penault-Llorca F, van der Vijver M, Parry S, Bishop T, Benitez J,
Rivas C, Bignon YJ, et al: Prediction of BRCA1 status in patients
with breast cancer using estrogen receptor and basal phenotype.
Clin Cancer Res. 11:5175–5180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foulkes WD, Stefansson IM, Chappuis PO,
Bégin LR, Goffin JR, Wong N, Trudel M and Akslen LA: Germline BRCA1
mutations and a basal epithelial phenotype in breast cancer. J Natl
Cancer Inst. 95:1482–1485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Banerjee S, Reis-Filho JS, Ashley S,
Steele D, Ashworth A, Lakhani SR and Smith IE: Basal-like breast
carcinomas: Clinical outcome and response to chemotherapy. J Clin
Pathol. 59:729–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ashworth A: A synthetic lethal therapeutic
approach: Poly(ADP) ribose polymerase inhibitors for the treatment
of cancers deficient in DNA double-strand break repair. J Clin
Oncol. 26:3785–3790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colleoni M, Cole BF, Viale G, Regan MM,
Price KN, Maiorano E, Mastropasqua MG, Crivellari D, Gelber RD,
Goldhirsch A, et al: Classical cyclophosphamide, methotrexate, and
fluorouracil chemotherapy is more effective in triple-negative,
node-negative breast cancer: Results from two randomized trials of
adjuvant chemoendocrine therapy for node-negative breast cancer. J
Clin Oncol. 28:2966–2973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tseng WH and Martinez SR: Metaplastic
breast cancer: To radiate or not to radiate? Ann Surg Oncol.
18:94–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weigelt B, Kreike B and Reis-Filho JS:
Metaplastic breast carcinomas are basal-like breast cancers: A
genomic profiling analysis. Breast Cancer Res Treat. 117:273–280.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luini A, Aguilar M, Gatti G, Fasani R,
Botteri E, Brito JA, Maisonneuve P, Vento AR and Viale G:
Metaplastic carcinoma of the breast, an unusual disease with worse
prognosis: The experience of the European institute of oncology and
review of the literature. Breast Cancer Res Treat. 101:349–353.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim KH, Oh DY, Chie EK, Han W, Im SA, Kim
TY, Park IA, Noh DY, Ha SW and Bang YJ: Metaplastic breast
carcinoma: Clinicopathologic features and prognostic value of
triple negativity. Jpn J Clin Oncol. 40:112–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu Q, Chen WX, Zhong SL, Li J, Luo Z, Tang
JH and Zhao JH: Current progress in the treatment of metaplastic
breast carcinoma. Asian Pac J Cancer Prev. 14:6221–6225. 2013.
View Article : Google Scholar : PubMed/NCBI
|