Introduction
Mammary tumors are the most common malignancy in
reproductively intact female dogs (1). Malignant neoplasms represent ~50% of
total mammary tumors in canines, and ~50% of these have already
metastasized at the time of clinical diagnosis (2). The enzyme cyclooxygenase (COX) controls
the synthesis of prostaglandins (PG) from arachidonic acid
(3). COX-2, an inducible form of COX,
has been associated with carcinogenesis, cell proliferation,
resistance to apoptosis, tumor tissue invasion, immunosuppression
and angiogenesis (4,5). Several previous studies have suggested
an association between COX-2 expression and the progression of
various human (6,7) and canine (8,9) types of
cancer. In addition, high COX-2 expression has been documented in
canine mammary carcinoma and associated with tumor malignancy
(10,11). There is known to be an association
between cancer cell invasiveness, COX-2 expression and matrix
metalloproteinase (MMP) activity (12). COX-2 induces MMP-2 expression in
breast cancer cells, facilitating tumor motility (13). MMP-2 expression has been associated
with invasive carcinomas, given that it degrades type IV collagen
(14).
Other molecular signaling cascades, including the
Wnt/β-catenin signaling pathway, exert effects on cell survival,
polarity and migration. In the Wnt/β-catenin signaling pathway
binding of Wnt ligands to Frizzled receptors induces the release of
β-catenin from a ubiquitinated cytoplasm complex, enhancing the
translocation of β-catenin to the nucleus where it functions as a
transcription factor (15). Numerous
genes are targeted via β-catenin, which regulates tumor promotion
and progression (15,16). An association between COX-2/PGE2 and
the Wnt/β-catenin signaling pathway has been proposed in human
breast cancer, where COX-2 expression is associated with increased
β-catenin activity, promoting replicative immortality, invasion and
metastasis (6).
Non-steroidal anti-inflammatory drugs (NSAIDs)
inhibit COX activity, thus modulating PGE2 synthesis (17,18). Since
COX-2 serves a role in tumor progression, there is considerable
evidence that NSAIDs may serve a role in inhibiting this process in
mammary tissue (19,20). Various NSAIDs, including piroxicam and
meloxicam, have been studied in cancer. However, the preferential
activity of meloxicam against COX-2 makes it an attractive
therapeutic option compared with non-selective NSAIDs, because it
may reduce side effects (21).
Meloxicam is licensed for medium to long-term pain management in
dogs, in which it induces only minor secondary effects (22). Few in vitro studies have
determined the antiproliferative effect of meloxicam on mammary
tumor cells, and they used high drug concentrations that cannot be
translated in vivo (23,24).
Therefore, the novel analysis of lower
concentrations of meloxicam is required. CF41.Mg is a canine
mammary carcinoma cell line expressing mesenchymal-associated
genes, including vimentin and N-cadherin, and low levels of
E-cadherin (25), indicating that
these cells exhibit invasiveness and may be representative of high
histological grade canine mammary tumors. The aim of the present
study was to analyze the potential antiproliferative and
anti-invasive effects of meloxicam on CF41.Mg canine mammary
carcinoma cells.
Materials and methods
Cell culture
The CF41.Mg (ATCC® CRL-6232™) cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and grown in Dulbecco's modified Eagle medium High Glucose
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 10% fetal
bovine serum (FBS) (Hyclone; GE Healthcare Life Sciences), 100 U/ml
penicillin G, 100 µg/ml streptomycin sulfate and 2 mM L-glutamine.
The Madin-Darby Canine Kidney (MDCK) cell line was provided by Dr
Victor Neira (Laboratory of Animal Virology, University of Chile,
Santiago, Chile) and cultured in minimal essential medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10% FBS, 100
U/ml penicillin G and 100 µg/ml streptomycin sulfate. All cultures
were maintained in a humidified atmosphere with 5% CO2
at 37°C. Evaluation of the growth kinetics of the cell lines under
standard culture conditions was performed prior to starting the
experiments.
Drug preparation
Meloxicam (Selleck Chemicals, Houston, TX, USA) and
doxorubicin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were
prepared in dimethyl sulfoxide (DMSO; 40 mg/ml) and PBS (1 mg/ml),
respectively. Concentrations ranges were chosen on the basis of the
average and maximum serum drug concentrations previously reported
in dogs (26,27). The final concentration of DMSO in the
culture medium was 0.1% in all the experiments where meloxicam was
used.
Indirect immunofluorescence
CF41.Mg and MDCK cells (1×104/well) were seeded and
grown on sterile glass coverslips, and then fixed with absolute
methanol. Cells were washed and blocked with PBS plus 2% bovine
serum albumin (Applichem GmbH, Darmstadt, Germany) for 30 min in a
humidified chamber at room temperature. Subsequently, the cells
were incubated with rabbit anti-COX-2 primary antibody clone SP21
(MA5-145; 1:50; Thermo Fisher Scientific, Inc.) at room temperature
for 1 h as previously described (28,29). Cells
were then incubated with a goat anti-rabbit Alexa Fluor® 488 secary
antibody (1:500; Invitrogen; Thermo Fisher Scientific, Inc.;
A11008) for 1 h at room temperature. Coverslips were mounted using
Vectashield® mounting media with DAPI (Vector Laboratories, Inc.,
Burlingame, CA, USA). Finally, the samples were analyzed with an
epifluorescence microscope fitted with a color charge-coupled
device camera.
Western blot
Cells (2.5×105/100 mm dish) were seeded
and exposed to 0.25 µg/ml meloxicam for 24–48 h and then lysed in
RIPA Buffer with a Protease Inhibitor Cocktail added (both Cell
Signaling Technology, Inc., Danvers, MA, USA), scraped and
sonicated (3 cycles of 5 sec setting 10 of Branson sonifier 150;
Danbury, CT, USA). After measuring protein concentration (Micro BCA
Protein Assay kit, Thermo Fisher Scientific, Inc.), according to
the manufacturer's indications, proteins (30 µg protein/lane) were
resolved on 10% gels using SDS-PAGE. Proteins were then transferred
onto polyvinylidene difluoride membranes, which were incubated
overnight at 4°C with primary antibodies directed against the
following proteins: COX-2 (1:200; Thermo Fisher Scientific, Inc.;
MA5-145), β-catenin (1:1,000; BD Biosciences, Franklin Lakes, NJ,
USA; 610154), phosphorylated (p)-β-catenin (1:1,000; Cell Signaling
Technology, Inc.; 9561) and MMP-2 (1:1,000; Invitrogen; Thermo
Fisher Scientific, Inc.; 35-130-0Z). After washing, the membranes
were incubated with anti-rabbit IgG F (ab')2 fragment
(A6667) and anti-mouse IgG (Fab specific; A9917) peroxidase
antibodies (1:5,000; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. Protein bands were by enhanced chemiluminescence
(Pierce ECL Western Blotting substrate; Thermo Fisher Scientific,
Inc.; 32106). Relative levels of protein were determined by
reprobing the membranes with anti-β-actin antibody (1:1,000; Abcam,
Cambridge, UK; ab8226) for 1 h at room temperature. The bands
obtained were analyzed with ImageJ software version 1.49v (National
Institutes of Health, Bethesda, MD, USA).
Cell viability assay
CF41.Mg and MDCK cells were seeded at a density of
1.5×103 cells/well into 96-well plates, cultured for 24 h and then
exposed to 0–25 µg/ml meloxicam alone or in combination with
doxorubicin, a chemotherapy drug frequently used as an adjuvant
treatment in dogs with mammary carcinoma. To evaluate synergism and
sensitization, doxorubicin was added at the same time and after 24
h, respectively. MDCK cells were exposed only to meloxicam as a
non-tumor negative control. Control groups were cultured without
meloxicam and doxorubicin, but the corresponding amount of DMSO was
added to the medium. Following an incubation period of 24 and 48 h,
cell growth was measured using the MTS assay (CellTiter 96® AQueous
One Solution Cell Proliferation assay system; Promega Corporation,
Madison, WI, USA), according to the manufacturer's instructions,
with the absorbance at 490 nm determined using a microplate reader.
Each experiment was performed 3 times in triplicate.
Flow cytometric apoptosis assay
CF41.Mg cells were cultured at a density of
4.5×105 per 100 cm2 dish. Cells treated with
0.25 µg/ml meloxicam or 500 ng/ml doxorubicin for 24 h were
harvested with 0.25% trypsin-EDTA, washed and resuspended with PBS
plus 2% FBS. A FITC Annexin V Apoptosis Detection kit II (556419)
was used according to the manufacturer's protocol (BD-Pharmingen,
San Diego, CA, USA), to identify cells in the early phases of
apoptosis. Early apoptotic cells were defined as propidium iodide
(PI)−/Annexin V+ and late apoptotic cells
were defined as PI+/Annexin V+ (30). Cell staining was measured using a
FACSCalibur™ flow cytometer and the data was analyzed with
FACSDiva™ software version 6.1.3 (both BD Biosciences).
Experiments were run in triplicate and ~10,000–20,000 events were
analyzed.
Cell migration assay
A scratch wound healing assay was performed to
determine cell migration ability. CF41.Mg cells were seeded at a
density of 1×104 cells/well into 24-well plate and cultured until
80% confluent. Cell monolayers were scratched in a single line
using a 200 µl pipette tip, rinsed with PBS to remove cell debris
and allowed to heal for 24 and 48 h at 37°C in the presence or
absence of 0.25 µg/ml meloxicam. The average extent of wound
closure was evaluated at 0, 24 and 48 h by measuring the width of
the wound, and images were captured using an inverted microscope.
The migration area was calculated with ImageJ software version
1.49v using the following formula: Migration area=(area of original
wound-area of wound after healing)/area of original wound.
Matrigel cell invasion assay
Cell invasion was assayed using Transwell® 24-well
cell culture containing inserts with an 8 µm pore size (BD
Biosciences) coated with Matrigel. A total of 2.5×104 CF41. Mg
cells were seeded into the upper chamber and incubated for 48 h in
the presence of 0.25 µg/ml meloxicam and no FBS as previously
described (20) against a gradient of
5% FBS in the lower chamber. Non-invading cells on the upper side
were wiped away with a cotton swab and the membrane was fixed with
cold methanol (−20°C) for 20 min. DAPI was used to stain the
invading cells, which were examined by epifluorescence microscopy,
with 5 microscopic fields examined per insert.
Gelatin zymography
MMP-2 and −9 release was detected using gelatin
zymography. After cells were exposed to meloxicam, the culture
medium was removed, mixed with sample buffer (0.125 M Tris-HCl, pH
6.8, 10% SDS, 8% sucrose and 0.05% bromophenol blue) for 30 min at
25°C. An electrophoretic run was then performed in a polyacrylamide
gel copolymerized with 0.1% gelatin. Subsequently, the gels were
washed with 2.5% Triton X-100 and incubated for 18 h at 37°C in a
buffer containing 50 mM Tris pH 7.4, 5 mM CaCl2 and 0.5
mM NaN3. Finally, the gels were stained with Coomassie
blue and examined.
ELISA detection of
PGE2
CF41.Mg cells (2×104/well) were incubated in 24-well
plates at 37°C until 70% of confluence was reached. The culture
medium was then changed and 0.25 µg/ml meloxicam was added. After
24 and 48 h incubation, supernatants were removed and centrifuged
at 1,000 × g for 10 min. The amount of PGE2 was determined using
the Prostaglandin E2 ELISA kit-Monoclonal from Cayman
Chemical Company (Ann Arbor, MI, USA) according the manufacturer's
protocol as previously described (31).
Statistical analysis
One way analysis of the variance for the cell
viability assays, a Student's t test for the cell invasion,
western blotting and annexin assays, and the Mann-Whitney U test
for the scrath plate assays were used to determine statistical
significance between samples and their respective controls.
P<0.05 was considered to indicate a statistically significant
difference. Data were analyzed with SPSS software (version 22; IBM
SPSS, Armonk, NY, USA).
Results
COX-2 is expressed in MDCK and CF41.Mg
cells
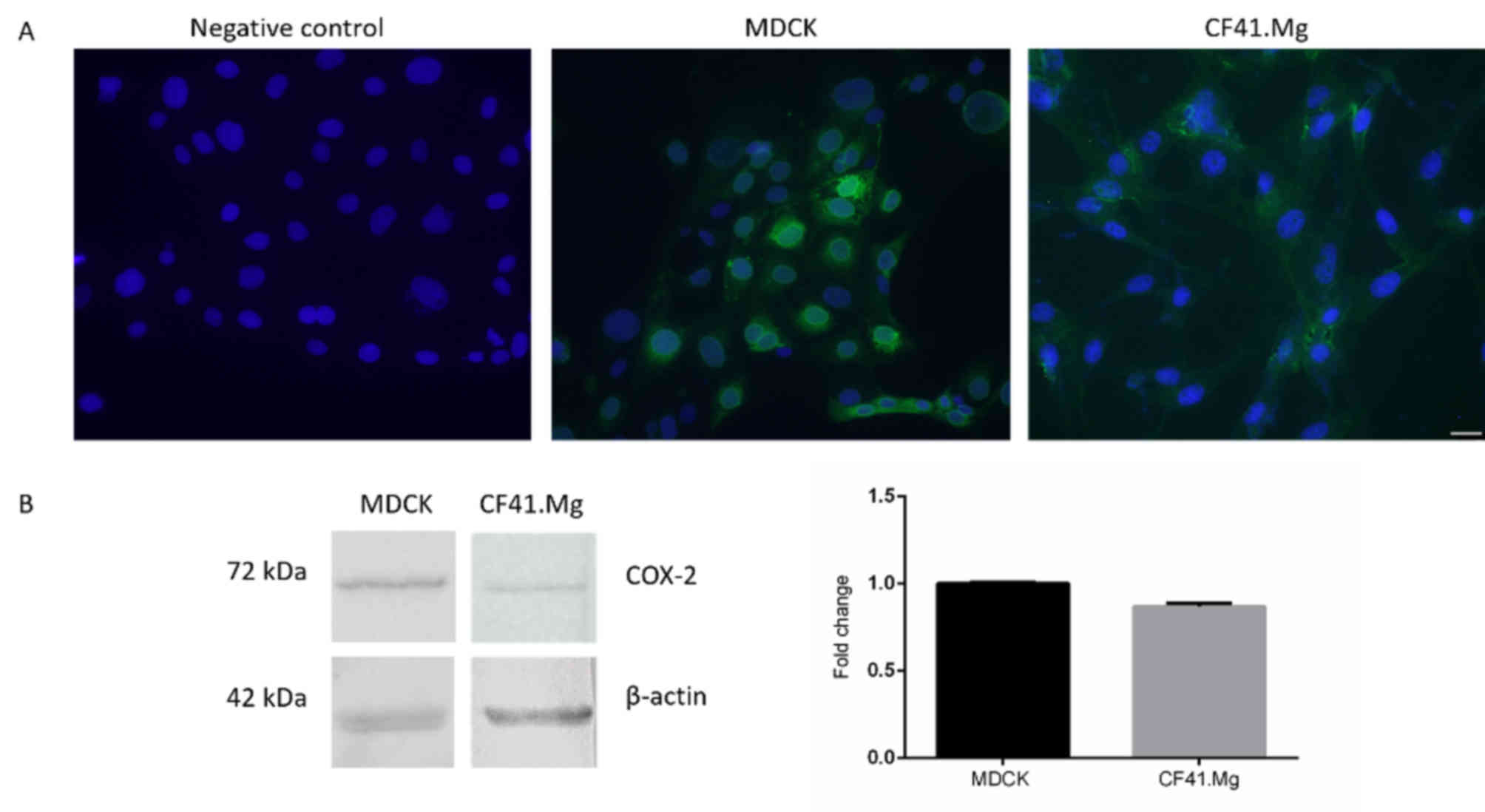
MDCK and CF41.Mg cells were identified to exhibit
COX-2 expression through immunofluorescence (Fig. 1A) and western blotting (Fig. 1B). COX-2 was localized in the
cytoplasm of CF41.Mg cells and cytoplasm and perinucleus in MDCK
cells (Fig. 1A). Western blot
confirmed these results by revealing a band indicating COX-2 at 72
kDa. No notable differences in the relative expression of COX-2
between MDCK and CF41.Mg cells were observed (Fig. 1B). The growth kinetics of MDCK and
CF41.Mg cells without drug treatment (exhibited an increase in
total cell number of ~3-fold over a 48 h period (data not
shown).
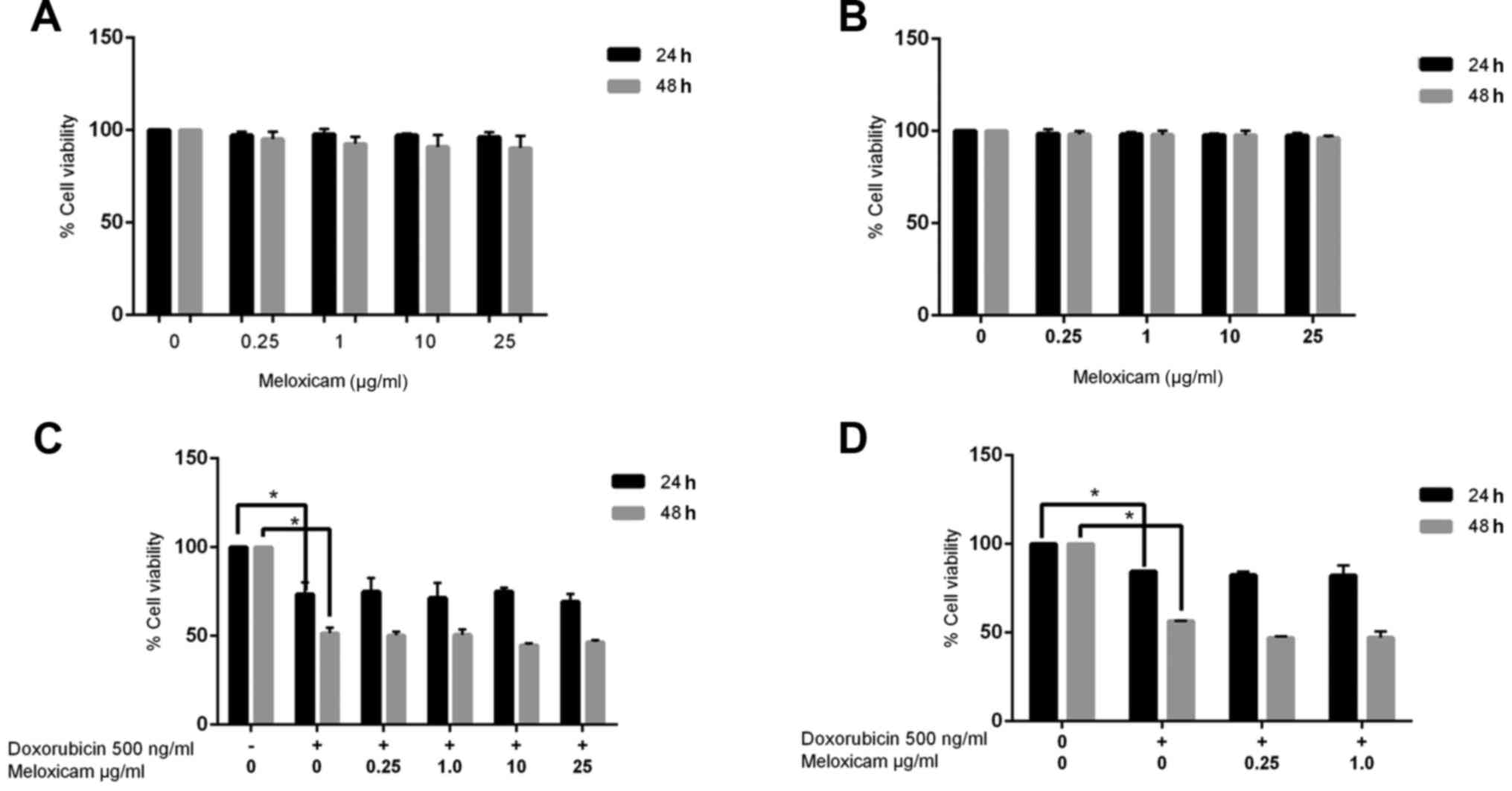
Meloxicam does not inhibit CF41.Mg
cell viability
Since meloxicam is known to inhibit COX+ tumor
cells, its potential antineoplastic effect on CF41.Mg cells was
examined using a cell viability assay. Meloxicam (0.25–25 µg/ml)
did not decrease cell viability after 24 (100 vs. 96.33% viability
in the control and 25 µg/ml meloxicam-treated cells, respectively)
or 48 h (100 vs. 90.29% viability in the control and 25 µg/ml
meloxicam-treated cells, respectively) of exposure (Fig. 2A). In order to evaluate the potential
cytotoxic effects of meloxicam on COX+ epithelial untransformed
cells, the same assays were performed on MDCK cells. Similarly to
in the tumor cells, meloxicam did not inhibit viability in MDCK
cells (Fig. 2B).
Meloxicam does not have a synergistic
effect with doxorubicin in CF41.Mg cells
To determine whether meloxicam could exert a
synergistic effect with doxorubicin, the viability of CF41.Mg cells
cultured in the presence of meloxicam and doxorubicin was examined.
The viability of cells treated with meloxicam and doxorubicin was
not significantly different compared with cells treated with
doxorubicin alone (73.44 vs. 51.60% viability in cells exposed to
doxorubicin alone at 24 and 48 h, respectively; 69.18 vs. 46.56%
viability in cells exposed to doxorubicin and 25 µg/ml meloxicam at
24 and 48 h, respectively; Fig.
2C).
Meloxicam is not associated with
chemosensitization in CF41.Mg cells
To determine whether chemosensitization was
associated with meloxicam, meloxicam was administered 24 h prior to
doxorubicin. No significant difference in cell viability was found
using this method (84.45 vs. 56.53% viability in cells exposed to
doxorubicin alone at 24 and 48 h, respectively; 82.13 and 47.15%
viability in cells exposed to doxorubicin and 25 µg/ml meloxicam at
24 and 48 h, respectively; Fig. 2D).
The following experiments were performed using the lower meloxicam
concentration (0.25 µg/ml), in order to mimic the plasma
concentration typically observed in dogs receiving oral meloxicam
at therapeutic doses (26).
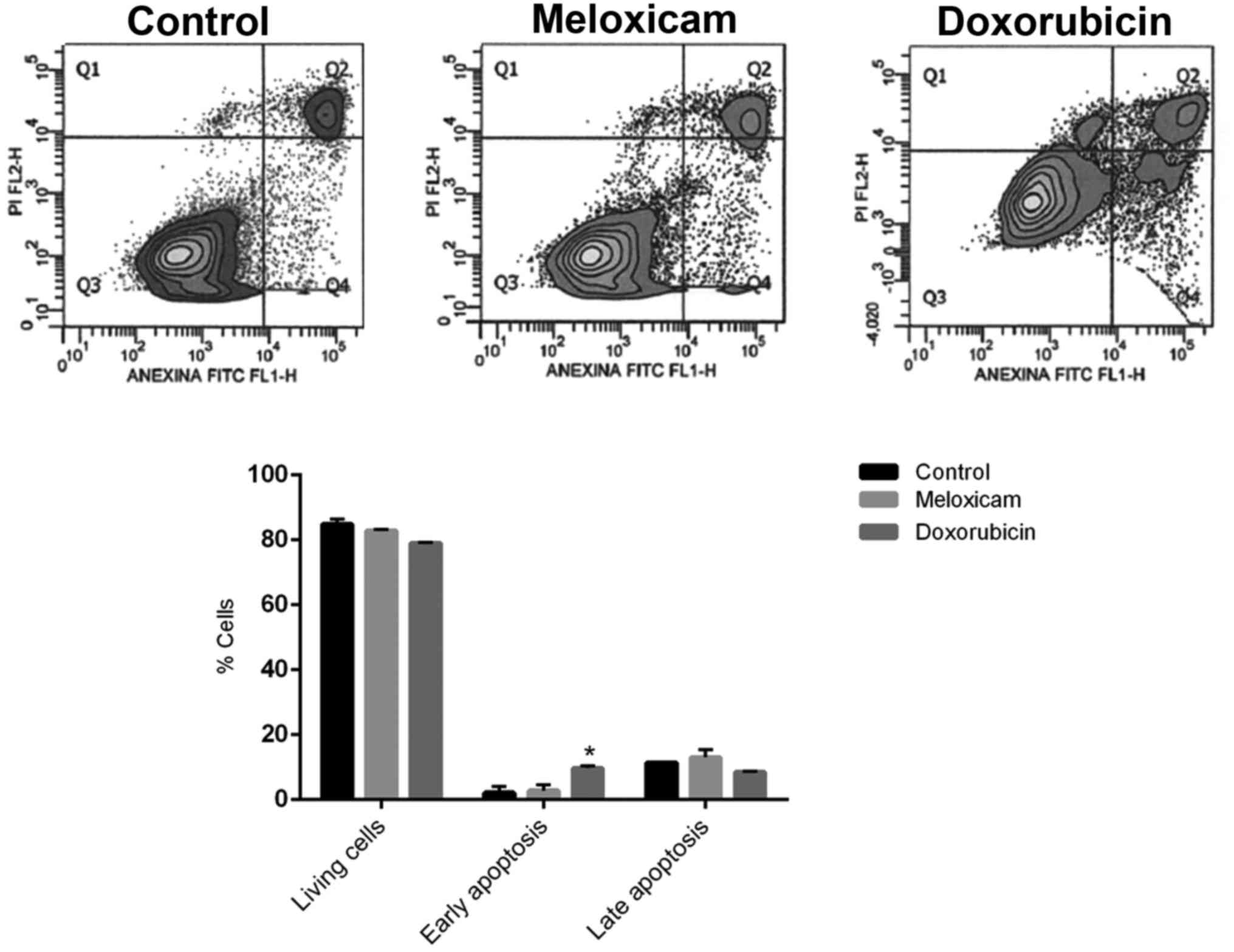
Meloxicam does not affect CF41.Mg cell
apoptosis
The incubation of CF41.Mg cells in the presence of
0.25 µg/ml meloxicam did significantly not affect early and late
apoptotic cell numbers after 24 h compared with the control group
(Fig. 3).
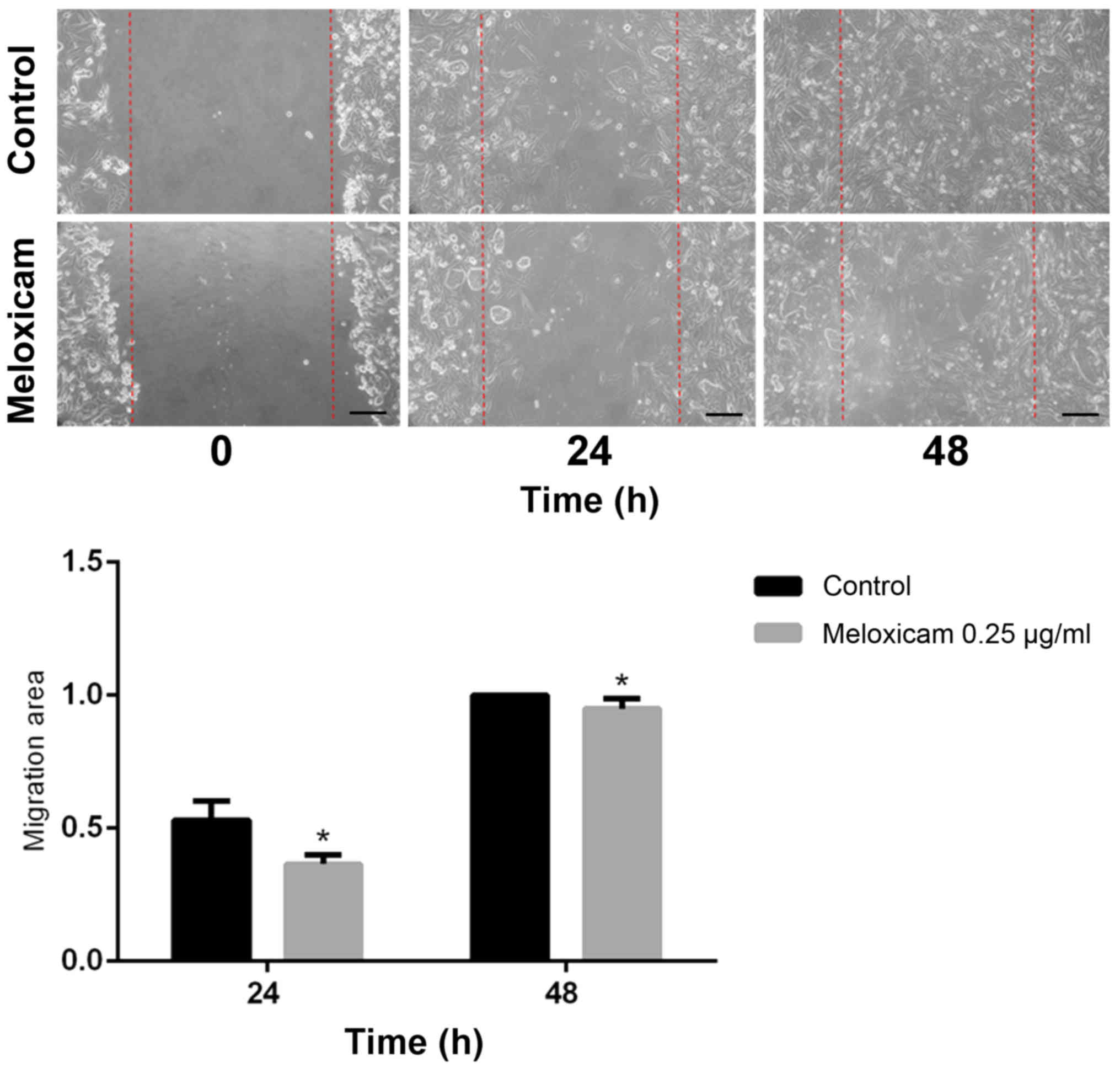
Meloxicam decreases CF41.Mg cell
migration and invasion
The migratory ability of CF41.Mg cells was analyzed
following exposure to meloxicam. Cells exposed to 0.25 µg/ml
meloxicam were significantly less migratory compared with the
control cells at 24 (P=0.001) and 48 (P=0.002) h in the wound
healing assay, as indicated by a higher migration area (Fig. 4). Exposure to 0.25 µg/ml meloxicam
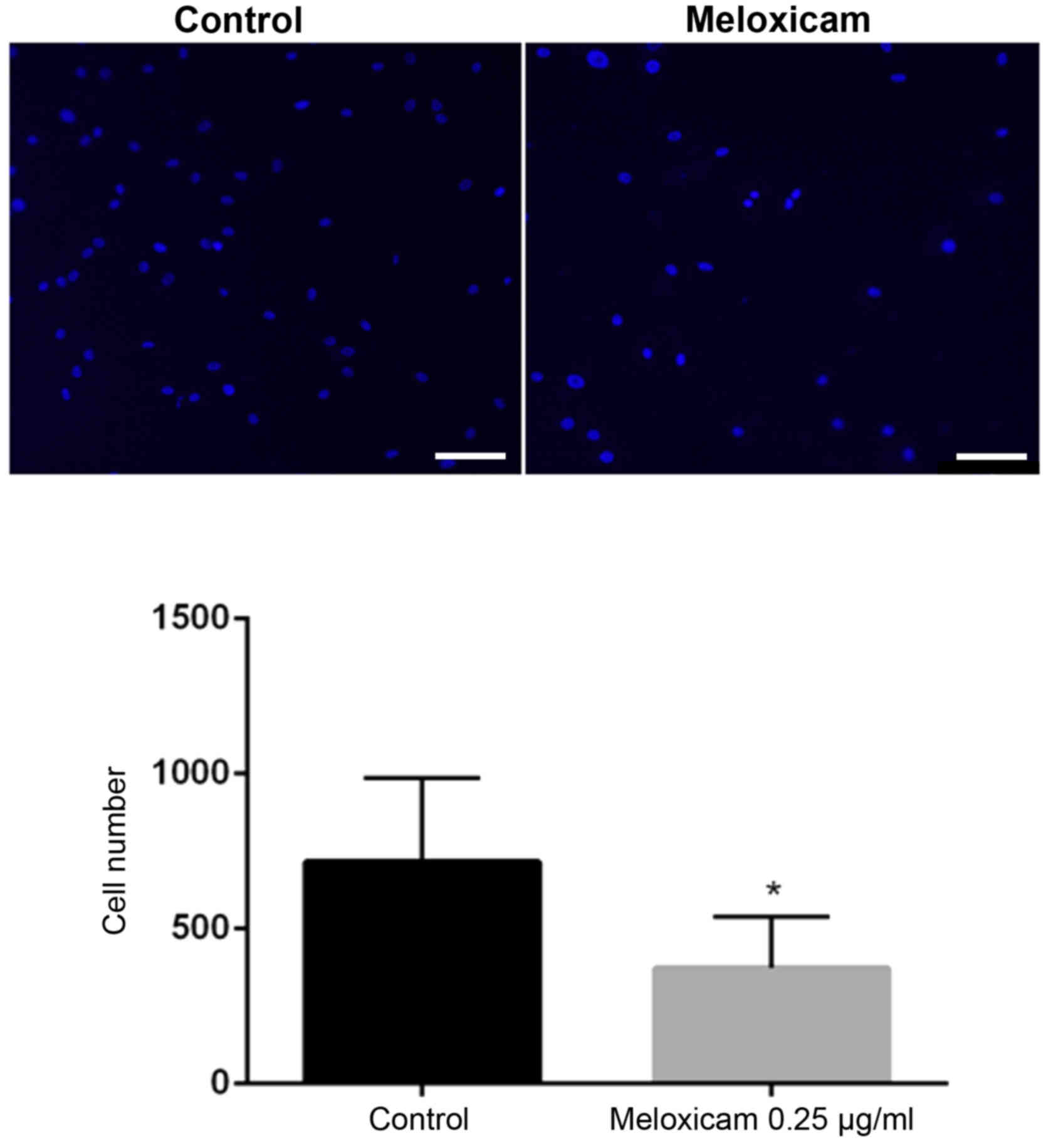
also impaired the invasiveness of CF41.Mg cells in a Matrigel
invasion assay. The number of invasive meloxicam-treated (0.25
µg/ml) cells was significantly lower compared with that of the
control cells (P=0.025; Fig. 5).
Meloxicam decreases MMP-2 expression
in CF41.Mg cells
Based on the observed reduction in cell migration
and invasion induced by meloxicam, the expression of
invasion-promoting molecules in response to meloxicam was
investigated. The release of MMP-2 and −9 into the culture medium
was detected through their gelatinase activity by gelatin
zymography. This was detected in the presence and absence of
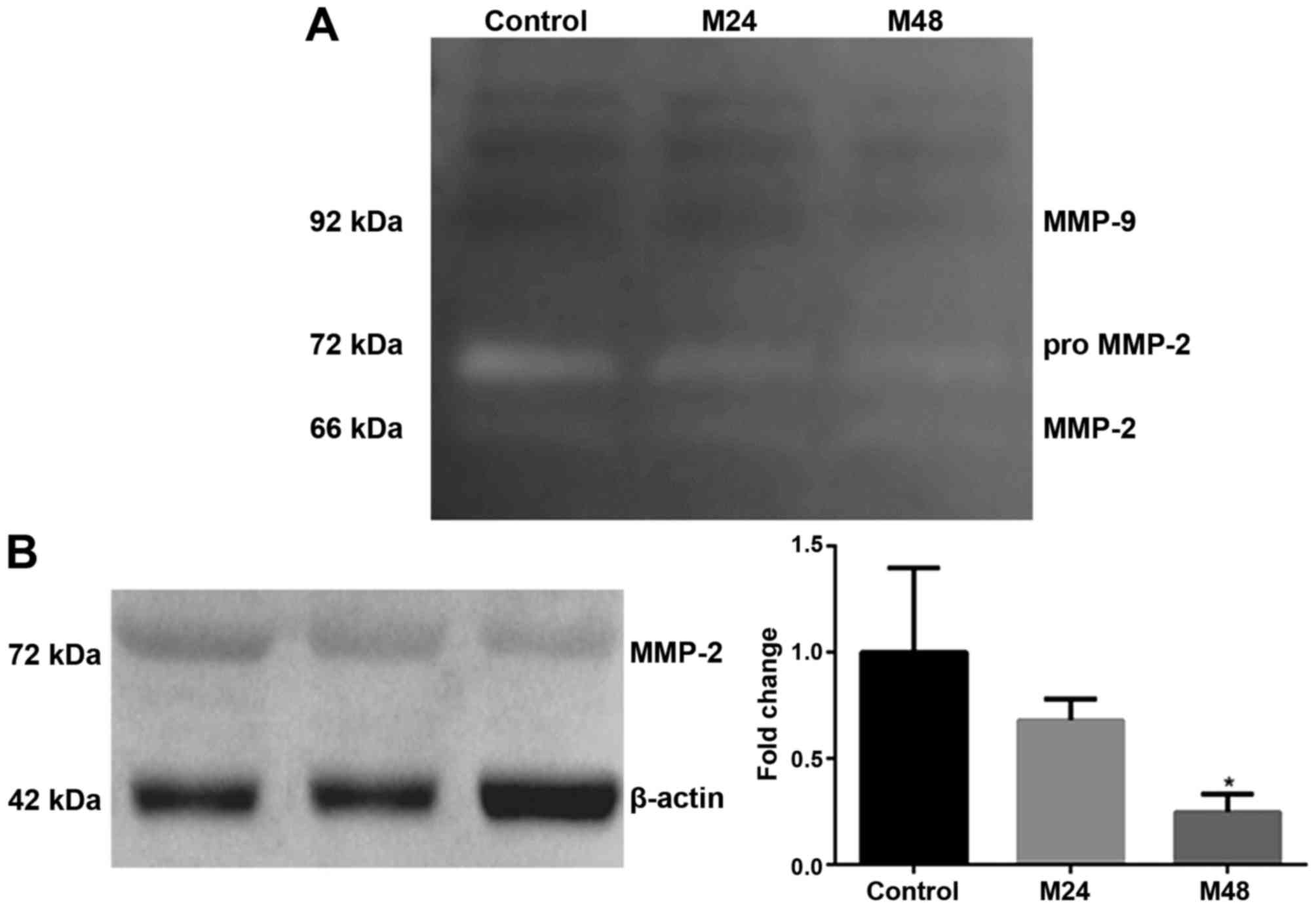
meloxicam. As illustrated in Fig. 6A,
only MMP-2 activity was detected in the supernatants studied.
Meloxicam reduced MMP-2 gelatinase activity at 24 and 48 h, where a
stronger intensity of gelatinolytic band was observed in absence of
meloxicam. Because this measurement of activity cannot be
objectively quantifiable, these results were confirmed through
western blotting, where meloxicam induced a significant decrease in
the expression of MMP-2 at 48 h (P=0.013; Fig. 6B).
Meloxicam increases β-catenin
phophorylation in CF41.Mg cells
The association between meloxicam and the COX-2
signaling pathway was investigated. A functional interaction
between COX-2 and β-catenin has been suggested in certain types of
cancer, where MMPs could act as mediators (6,17).
Therefore, the levels of total and p forms of β-catenin in response
to meloxicam were analyzed. A significantly lower expression of
total β-catenin was observed in CF41.Mg cells incubated with
meloxicam for 24 and 48 h compared with the control group
(P<0.0001 and P=0.014, respectively; Fig. 7A). In addition, an increased
expression of p-β-catenin was detected in CF41.Mg cells incubated
with meloxicam for 24 h (P=0.074), which was significant at 48 h
(P=0.009; Fig. 7B).
Meloxicam does not affect COX-2
expression in CF41.Mg cells
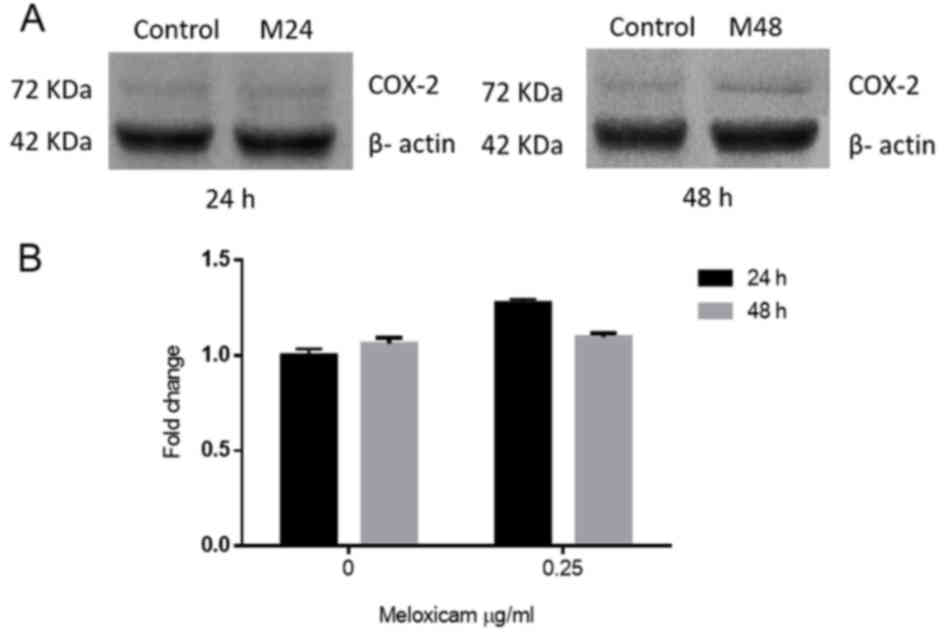
To evaluate whether meloxicam affects COX-2
expression in CF41.Mg cells, COX-2 protein levels in the absence
and presence of 0.25 µg/ml of meloxicam, at 24 and 48 h, were
compared. No significant difference in the expression of COX-2 was
observed between any of the groups (Fig.
8). The amount of PGE2 in supernatants could not be determined,
since all values were out of the curve provided by the ELISA kit,
being too low to be detected by this method.
Discussion
Several previous studies have proposed that NSAIDs
could be used as an anticancer drug or as part of a chemopreventive
therapy. Zhong et al (32)
recently suggested that the long-term use of aspirin may reduce the
risk of breast cancer in humans. In veterinary medicine, a number
of previous studies have evaluated the in vitro and in
vivo effects of different NSAIDs on animal tumors, including
canine mammary tumors. For example, etodolac, meloxicam and
celecoxib have been identified to suppress canine mammary tumor
cell growth in vitro (24). By
contrast, piroxicam, a non-selective NSAID, has been demonstrated
to trigger clinical partial remissions in dogs with mammary
carcinoma (33,34). Meloxicam is licensed for veterinary
use and is widely utilized in the management of pain and
inflammatory diseases; it is a potent NSAID of the enolic acid
class of oxicam derivatives, which has exhibited a preference for
COX-2 compared with COX-1 inhibition at therapeutic concentrations
(21,36). Due to its preferential COX-2
inhibitory capacity, meloxicam has a better gastric and renal
safety profile compared with non-selective NSAIDs (21,35,36).
Since COX-2 is associated with poor cancer
prognosis, the antitumor effect of meloxicam has been studied in
different canine tumor cells. Several previous studies have
demonstrated that meloxicam induces a reduction in cell
proliferation and/or increase in apoptosis at high concentrations,
exceeding the physiological maximum serum levels (23,24). For
example, in canine mammary tumor cells, 100 µM meloxicam was
demonstrated to inhibit cell proliferation, but not induce
apoptosis (24). By contrast,
Knottenbelt et al (23)
revealed that meloxicam (10–160 µg/ml) induced inhibition of cell
proliferation and an increase in apoptosis in mammary carcinoma
cells in a dose-dependent manner, with maximum inhibition observed
at a dose of 160 µg/ml.
Metastatic cancer cells exhibit high motility,
typically observed when cell-cell adhesions are lost and resulting
in increased invasiveness (4). Since
CF41.Mg cells exhibit a mesenchymal phenotype with characteristic
low expression of E-cadherin (25),
they were used as an in vitro model to study the invasive
potential of canine mammary carcinoma cells in the present study.
In this the present study, meloxicam had no effect on proliferation
or apoptosis. However, a lower dose of meloxicam was used in the
present study in order to mimic the plasma concentration observed
in dogs receiving the routinely therapeutic dose of 0.1 mg/kg
(35). In addition, meloxicam was not
demonstrated to augment the cytotoxic effect of doxorubicin, a
chemotherapy drug frequently used as an adjuvant treatment in dogs
with mammary carcinoma.
Notably, a low concentration of meloxicam (0.25
µg/ml) was identified to significantly inhibit CF41.Mg cell
migration and invasion in the present study. These results may be
mechanistically associated with the reduced expression/activity of
MMP-2 observed. In accordance with these results, Larkins et
al (37) demonstrated that
various COX-2 inhibitors reduce motility, invasion and MMP
expression in breast cancer cells. This group also reported a
decreased MMP-2 expression with undetectable levels of pro- and
active MMP-9 under basal conditions, similar to the observations of
the present study. These results suggest that MMP-9 does not effect
on the invasiveness of CF41.Mg cells, while MMP-2 does.
In the current study, exposure to meloxicam reduced
total β-catenin expression while increasing its phosphorylation.
These effects may induce the destabilization and degradation of
β-catenin via the proteasome, resulting in less β-catenin available
for nuclear translocation and transcription of target genes, as
described by Hugo et al (6).
Thus, it can be suggested that the anti-invasive effect of
meloxicam on CF41.Mg cells is associated with enhanced β-catenin
degradation.
No significant differences in the levels of COX-2 in
response to 0.25 µg/ml meloxicam treated were observed in the
present study, which is concordant with the results of previous
studies (24,38). This indicates that meloxicam does not
alter COX-2 expression; however, it does not exclude its
participation in regulating the activity of COX-2.
It was expected that PGE2, a product of COX-2
activity, would be reduced in a dose-dependent manner following
meloxicam treatment. However, PGE2 was not detected under the
experimental conditions of the present study. A previous report of
COX-2 expression and activity in CF41.Mg cells identified no
production of PGE2, which is consistent with our results (39). In this regard, it has been suggested
that the rapid metabolic inactivation of PGE2 in cancer cells may
limit its detection (40), explaining
these outcomes. Unfortunately, these findings prevent the
determination of whether the effects of meloxicam are dependent on
COX-2 activity.
The PG signaling pathway is not the only mechanism
of detecting inhibitory activity associated with meloxicam and
other NSAIDs, as the antiproliferative effects were observed also
in COX-negative cells lines (41).
Several inhibitors of COX-2 have been identified to inhibit
carbonic anhydrase (42), which is
associated with tumor malignancy in breast cancer (43). This potential association should be
explored in future studies to elucidate the COX-2-independent
anti-invasive effects of meloxicam on CF41.Mg cells. There are
numerous COX inhibitors available and it is necessary to extend
these observations by analyzing their potential effect on the
metastatic behavior of canine mammary tumor cells at in vivo
concentrations.
In conclusion, the results of the present study
demonstrate that meloxicam has no effect on the proliferation or
apoptosis of CF41.Mg cells. In addition, the present study
identified that meloxicam while significantly reduces CF41.Mg cell
invasion, at least in part by decreasing MMP-2 secretion and
enhancing the degradation of β-catenin. Thus, meloxicam at low
concentration of 0.25 µg/ml, has an anti-invasive effect in canine
mammary carcinoma cells, suggesting that meloxicam has a potential
adjunctive therapeutic application, which could be useful in
controlling the invasion and metastasis of canine mammary
carcinomas.
Acknowledgements
The present study was supported by the Universidad
Andres Bello (grant no. DI-425-13/I). The authors would like to
thank Ms. Valeska Simon, Dr Christian Hidalgo, Dr Caroll Stoore and
Dr María Pía García for their technical assistance.
References
|
1
|
Arenas C, Peña L, Granados-Soler JL and
Pérez-Alenza MD: Adjuvant therapy for highly malignant canine
mammary tumours: Cox-2 inhibitor versus chemotherapy: A
case-control prospective study. Vet Rec. 179:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torres CG, Pino AM and Sierralta WD: A
cyclized peptide derived from alpha fetoprotein inhibits the
proliferation of ER-positive canine mammary cancer cells. Oncol
Rep. 21:1397–1404. 2009.PubMed/NCBI
|
|
3
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hugo HJ, Saunders C, Ramsay RG and
Thompson EW: New insights on COX-2 in chronic inflammation driving
breast cancer growth and metastasis. J Mammary Gland Biol
Neoplasia. 20:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
B, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan KN, Knapp DW, Denicola DB and Harris
RK: Expression of cyclooxygenase-2 in transitional cell carcinoma
of the urinary bladder in dogs. Am J Vet Res. 61:478–481. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lavalle GE, De Campos CB, Bertagnolli AC
and Cassali GD: Canine malignant mammary gland neoplasms with
advanced clinical staging treated with carboplatin and
cyclooxygenase inhibitors. In Vivo. 26:375–379. 2012.PubMed/NCBI
|
|
10
|
Doré M, Lanthier I and Sirois J:
Cyclooxygenase-2 expression in canine mammary tumors. Vet Pathol.
40:207–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Queiroga FL, Alves A, Pires I and Lopes C:
Expression of COX-1 and COX-2 in canine mammary tumors. J Comp
Pathol. 136:177–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rozic JG, Chakraborty C and Lala PK:
Cyclooxygenase inhibitors retard murine mammary tumor progression
by reducing tumor cell migration, invasiveness and angiogenesis.
Int J Cancer. 93:497–506. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KY, Kim YJ, Yoo H, Lee SH, Park JB and
Kim HJ: Human brain endothelial cell-derived COX-2 facilitates
extravasation of breast cancer cells across the blood-brain
barrier. Anticancer Res. 31:4307–4313. 2011.PubMed/NCBI
|
|
14
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roarty K and Rosen JM: Wnt and mammary
stem cells: Hormones cannot fly wingless. Curr Opin Pharmacol.
10:643–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang LY and Argyle D: Cancer stem cells
and telomerase as potential biomarkers in veterinary oncology. Vet
J. 185:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dempke W, Rice C, Grothey A and Schmoll
HJ: Cyclooxygenase-2: A novel target for cancer chemotherapy? J
Cancer Res Clin Oncol. 127:411–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaheen NJ, Straus WL and Sandler RS:
Chemoprevention of gastrointestinal malignancies with nonsteroidal
antiinflammatory drugs. Cancer. 94:950–963. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sonzogni-Desautels K, Knapp DW, Sartin E
and Doré M: Effect of cyclooxygenase inhibitors in a xenograft
model of canine mammary tumours. Vet Comp Oncol. 9:161–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pang LY, Argyle SA, Kamida A, Morrison KO
and Argyle DJ: The long-acting COX-2 inhibitor mavacoxib
(Trocoxil™) has anti-proliferative and pro-apoptotic effects on
canine cancer cell lines and cancer stem cells in vitro. BMC Vet
Res. 10:1842014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Streppa HK, Jones CJ and Budsberg SC:
Cyclooxygenase selectivity of nonesteroidal anti-inflammatory drugs
in canine blood. Am J Vet Res. 63:91–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luna SP, Basilio AC, Steagall PV, Machado
LP, Mountinho FQ, Takahira RK and Brandão CV: Evaluation of adverse
effects of long-term oral administration of carprofen, etodolac,
flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet
Res. 68:258–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knottenbelt C, Chambers G, Gault E and
Argyle DJ: The in vitro effects of piroxicam and meloxicam on
canine cell lines. J Small Anim Pract. 47:14–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito T, Tamura D and Asano R: Usefulness
of selective COX-2 inhibitors as therapeutic agents against canine
mammary tumors. Oncol Rep. 31:1637–1644. 2014.PubMed/NCBI
|
|
25
|
Saito T, Dai T and Asano R: The hyaluronan
synthesis inhibitor 4-methylumbelliferone exhibits antitumor
effects against mesenchymal-like canine mammary tumor cells. Oncol
Let. 5:1068–1074. 2013.
|
|
26
|
Busch U, Schmid J, Heinzel G, Schmaus H,
Baierl J, Huber C and Roth W: Pharmacokinetics of meloxicam in
animals and the relevance to humans. Drug Metab Dispos. 26:576–584.
1998.PubMed/NCBI
|
|
27
|
Selting KA, Ogilvie GK, Gustafson DL, Long
ME, Lana SE, Walton JA, Hansen RA, Turner AS, Laible I and Fettman
MJ: Evaluation of the effects of dietary n-3 fatty acid
supplementation on the pharmacokinetics of doxorubicin in dogs with
lymphoma. Am J Vet Res. 67:145–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lavalle GE, Bertagnolli AC, Tavares WL and
Cassali GD: COX-2 expression in canine mammary carcinomas:
Correlation with angiogenesis and overall survival. Vet Pathol.
46:1275–1280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prada J, Queiroga FL, Gregório H and Pires
I: Evaluation of cyclooxygenase-2 expression in canine mast cell
tumours. J Comp Pathol. 147:31–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aubry J, Blaecke A, Lecoanet-Henchoz S,
Jeannin P, Herbault N, Caron G, Moine V and Bonnefory JY: Annexin V
used for measuring apoptosis in the early events of cellular
cytotoxicity. Cytometry. 37:197–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tamura D, Saito T, Murata K, Kawashima M
and Asano R: Celecoxib exerts antitumor effects in canine mammary
tumor cells via COX-2-independent mechanisms. Int J Oncol.
46:1393–1404. 2015.PubMed/NCBI
|
|
32
|
Zhong S, Zhang X, Chen L, Ma T, Tang J and
Zhao J: Association between aspirin use and mortality in breast
cancer patients: A meta-analysis of observational studies. Breast
Cancer Res Treat. 150:199–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knapp DW, Richardson RC, Bottoms GD,
Teclaw R and Chan TC: Phase I trial of piroxicam in 62 dogs bearing
naturally occurring tumours. Cancer Chemother Pharmacol.
29:214–218. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De M, Souza CH, Toledo-Piza E, Amorin R,
Barboza A and Tobias KM: Inflammatory mammary carcinoma in 12 dogs:
Clinical features, cyclooxygenase-2 expression, and response to
piroxicam treatment. Can Vet J. 50:506–510. 2009.PubMed/NCBI
|
|
35
|
Montoya L, Ambros L, Kreil V, Bonafine R,
Albarellos G, Hallu R and Soraci A: A pharmacokinetic comparison of
meloxicam and ketoprofen following oral administration to healthy
dogs. Vet Res Commun. 28:415–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kay-Mugford P, Benn S, LaMarre J and
Conlon P: In vitro effects of nonsteroidal anti-inflammatory drugs
on cyclooxygenase activity in dogs. Am J Vet Res. 61:802–810. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Larkins TL, Nowell M, Singh S and Sanford
GL: Inhibition of cyclooxygenase-2 decreases breast cancer cell
motility, invasion and matrix metalloproteinase expression. BMC
Cancer. 6:1812006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wolfesberger B, Hoelzl C, Walter I, Reider
GA, Fertl G, Thalhammer JG, Skalicky M and Egerbacher M: In vitro
effects of meloxicam with or without doxorubicin on canine
osteosarcoma cells. J Vet Pharmacol Ther. 29:15–23. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brunelle M, Sartin EA, Wolfe LG, Sirois J
and Doré M: Cyclooxygenase-2 expression in normal and neoplastic
canine mammary cell lines. Vet Pathol. 43:656–666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schrey MP and Patel KV: Prostaglandin E2
production and metabolism in human breast cancer cells and breast
fibroblasts. Regulation by inflammatory mediators. Br J Cancer.
72:1412–1419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waskewich C, Blumenthal RD, Li H, Stein R,
Goldenberg DM and Burton J: Celecoxib exhibits the greatest potency
amongst cyclooxygenase (COX) inhibitors for growth inhibition of
COX-2 negative hematopoietic and epithelial cell lines. Cancer Res.
62:2029–2033. 2002.PubMed/NCBI
|
|
42
|
De Monte C, Carradori S, Gentili A,
Mollica A, Trisciuoglio D and Supuran CT: Dual cyclooxygenase and
carbonic anhydrase inhibition by nonsteroidal anti-inflammatory
drugs for the treatment of cancer. Curr Med Chem. 22:2812–2818.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chu CY, Jin YT, Zhang W, Yu J, Yang HP,
Wang HY, Zhang ZJ, Liu XP and Zou Q: CA IX is upregulated in
CoCl2-induced hypoxia and associated with cell invasive potential
and a poor prognosis of breast cancer. Int J Oncol. 48:271–280.
2016.PubMed/NCBI
|