Introduction
Primary gastric lymphoma is the most common form of
extranodal lymphoma, including gastric mucosa-associated lymphoid
tissue (gMALT) lymphoma and gastric diffuse large B-cell lymphoma
(gDLBCL) (1). Histological
transformation (HT) from gMALT lymphoma to gDLBCL has recently been
reported (2–4), and molecular mechanisms for transition
exists. Kaneko et al suggested the model for gMALT lymphoma
development and progression (4). The
pathogenesis of gDLBCL in that study, has two distinct pathways,
some gDLBCL may arise from indolent gMALT via the step of H.
pylori dependent as HT, and the residual gDLBCLs may develop
along antigen-independent pathways as de novo gDLBCL. Some
gDLBCL may arise from indolent gMALT via the step of H.
pylori-dependent HT, and the residual gDLBCLs may develop along
antigen-independent pathways as de novo gDLBCL. However,
information concerning patients with HT is very limited.
Thus, a retrospective analysis of patients with HT
was conducted by investigating their clinical features, treatment
and prognosis.
Materials and methods
Patients and staging procedures
We reviewed 71 patients with HT who were diagnosed
at the Tianjin Medical University Cancer Institute and Hospital and
the Xuzhou Central Hospital from January 2001 to June 2013. The
diagnostic criteria for HT were based on the World Health
Organization (WHO) classification system for hematologic
malignancies (1) that manifest as
lymphomatous foci containing DLBCL as well as MALT components. For
those diagnosed with HT, we analyzed the tissues for
immunoreactivity towards CD10, Bcl-6, or MUM1 according to a
previous study (5). Helicobacter
pylori (H. pylori) infection status was determined if
either the histologic examinations or urease breath tests was
positive. All the original histological slides were reviewed
independently by three histopathologists.
Clinical information was obtained from
the medical records
The staging procedures included routine laboratory
tests, whole body computed tomography, endoscopy of the upper
gastrointestinal tract, bone marrow aspiration, and optional
positron emission tomography. The Lugano staging system, which is a
modified version of the Ann Arbor criteria for gastrointestinal
non-Hodgkin's lymphoma, was used for staging.
Treatment modalities and response
assessment
Four therapeutic modalities were used: H.
pylori eradication (HPE); chemotherapy (CT); or radiotherapy
(RT); surgery (ST), or any combination. Chemotherapy included
anthracycline-based or non-anthracycline regimens. The HPE regimen
included proton pump inhibitors and a combination of antibiotics.
Response to treatment was assessed according to the revised
response criteria for malignant lymphomas (6).
Statistical analysis
Overall survival (OS) and progression-free survival
(PFS) were the primary endpoints of the survival analysis. OS was
considered as the time from the data of diagnosis to the date of
death from any cause. PFS was determined as the time from the start
of treatment to the date of treatment failure, relapse, or death
from any cause. Survival curves were estimated by the Kaplan-Meier
method. The life table method was used for survival estimations.
Survival curves were compared using the log-rank test. Univariate
analysis was performed for all variables. The Cox regression model
was fitted with some variables that influenced the prognosis
(p<0.05) in the univariate analysis to determine independent
prognostic factors for survival. P-values ≤0.05 were considered to
be statistically significant. All reported P-values were
two-tailed.
Results
Patient characteristics
Of the 71 patients with HT, 40 were male and 31 were
female [male/female (M/F) ratio, 1.3:1], with a median age of 56
years (age range, 18–86 years) (Table
I). Their clinical course was often insidious, lacking specific
clinical presentation with the most common complaints including
abdominal pain or discomfort, weight loss, poor appetite, nausea
and vomiting. The majority of patients presented with good
performance status (PS, 0–1 for 81%). Routine laboratory tests,
including serum LDH and β2-MG, were usually within normal limits.
Macroscopically, we found that the antrum was the most commonly
involved site (48/71, 67.6%), followed by the corpus (39/71,
54.9%), and fundus (21/71, 29.5%). According to the Lugano staging
system, a larger proportion of patients had stage IE disease (45%).
Among patients who presented with stage II (35%), 18 were diagnosed
with local lymph node involvement (stage II1) and 7 had distant
abdominal nodal extension (stage II2). Stages IIE and IV accounted
for 12 and 8%, respectively.
 | Table I.Characteristics of 71 patients with
HT. |
Table I.
Characteristics of 71 patients with
HT.
| Characteristics | No. of assessable
patients |
|---|
| Sex |
| Male | 38 (53%) |
|
Female | 33 (47%) |
|
Male/female ratio | 1.2 |
| Age (years) |
| ≤60 | 50 (70%) |
|
>60 | 21 (30%) |
| Mean
(range) | 55 (19–81) |
| Lugano staging |
|
I–II2 | 56 (68%) |
|
IIE-IV | 15 (32%) |
| Serum LDH level |
|
Normal | 51 (72%) |
|
Abnormal | 20 (28%) |
| β2-MG |
|
Normal | 58 (81%) |
|
Abnormal | 13 (19%) |
| m-IPIa |
| 0–1 | 54 (76%) |
| ≥2 | 17 (16%) |
| Infection with H.
pylori |
|
Positive | 45 (63%) |
|
Negative | 26 (37%) |
| Immunophenotype
classification |
| GCB
subtype | 30 (42%) |
| Non-GCB
subtype | 41 (58%) |
| Tumor mass (cm) |
|
<10 | 49 (69%) |
| ≥10 | 22 (31%) |
| Performance
status |
|
<2 | 58 (81%) |
| ≥2 | 13 (19%) |
| Anemia |
|
Present | 47 (66%) |
|
Absent | 24 (34%) |
Survival analysis
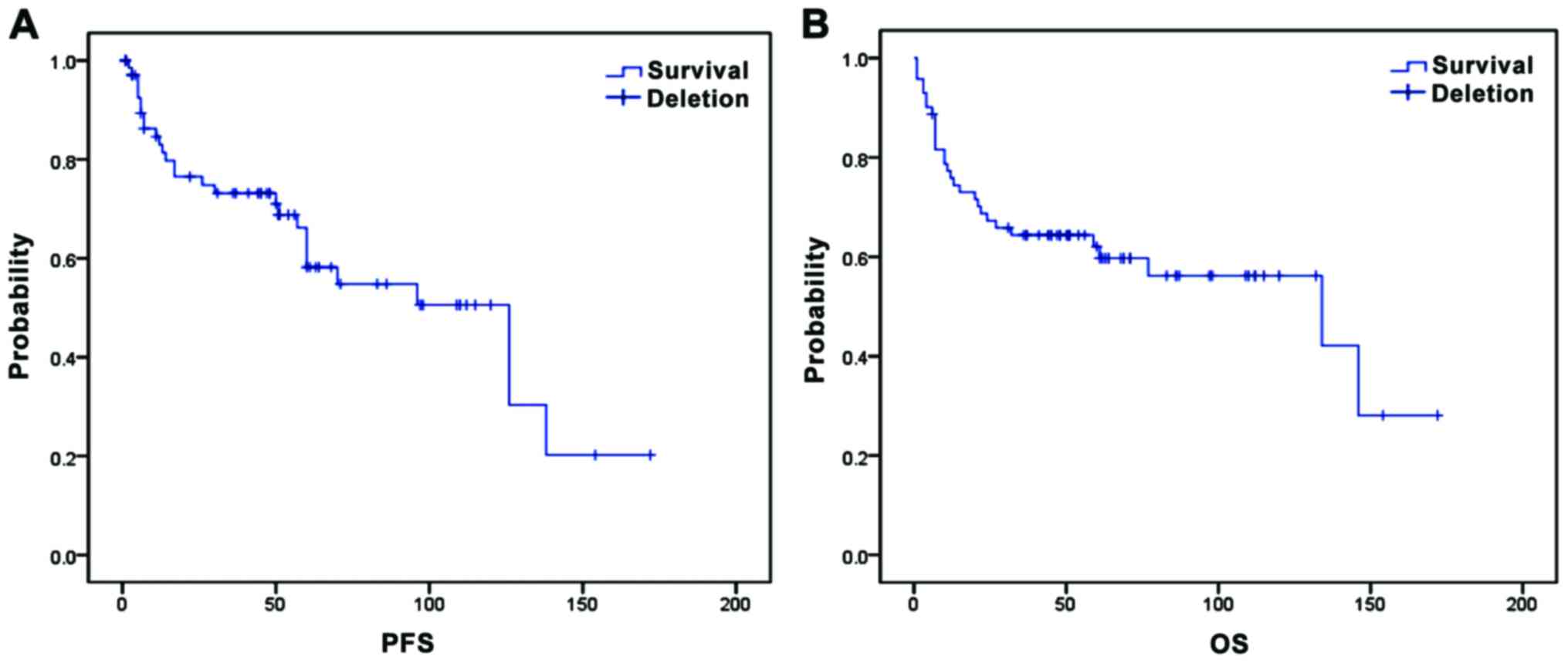
The median time of follow-up was 52.5.9 months
(range, 1–172 months). The median time to progression was 49.5
months (range, 1–138 months), and the median OS was 59.5 months
(range, 2–172 months). The PFS and OS rates after 5 years were 50
and 56%, respectively (Fig. 1).
Treatment outcomes
For all 71 patients, the overall response rate (ORR)
was achieved at 85%. Thirty-six (52%), 23 (33), six (9%), and four
(6) patients attained complete
remission (CR), partial remission (PR), stable disease (SD), and
progressive disease (PD), respectively.
Four therapeutic modalities (ST, CT, RT and HPE)
were used in the present study. Specifically, 13 patients (18%)
were treated by ST with (n=8) or without (n=5) CT, 42 patients
(59%) were treated by CT with (n=25) or without (n=17) HPE, and 16
patients (23%) were given RT to treat residual disease after CT
with or without HPE.
HPE was recommended as standard treatment for gMALT,
while the role of H. pylori in HT has been controversial
(7–9).
Three patients in our study were treated with HPE as initial
therapy. H. pylori was eradicated in all of them.
Histological PR was achieved in 2 of them after HPE, but each
experienced local relapse and then received other treatments. Among
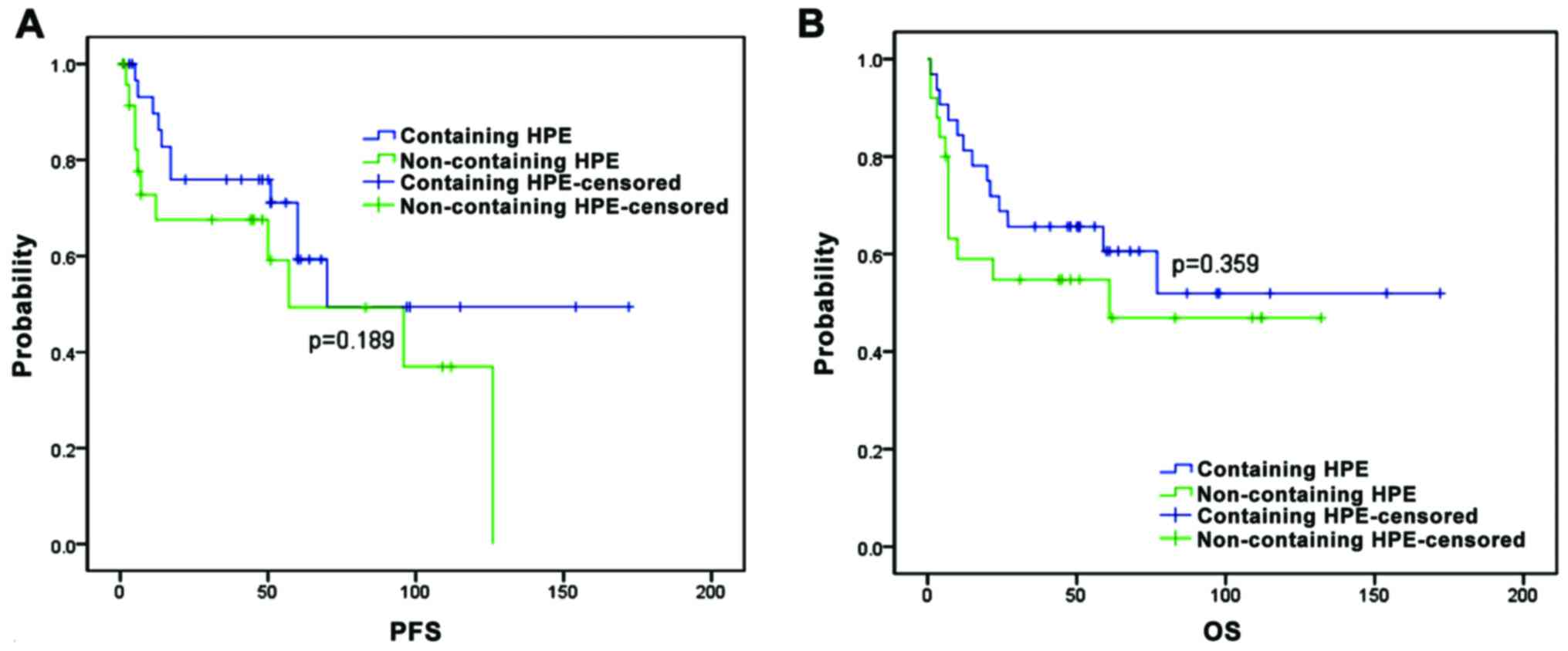
patients receiving no-surgical treatment, five-year PFS and OS
estimates were compared between patients receiving HPE treatment
and those receiving non-HPE treatment. We found that the 5-year PFS
estimates were similar for patients receiving HPE treatment (59%)
and for those receiving non-HPE treatment (49%) (p=0.189) (Fig. 2A). The 5-year OS estimates were
similar for patients receiving HPE treatment (61%) and for those
receiving non-HPE treatment (55%) (p=0.359, Fig. 2B).
The influence of various prognostic factors was
examined by univariate analysis. Among these, advanced stages,
serum LDH, β2-MG, m-IPI, and tumor mass were found to adversely
affected PFS and OS, while PS adversely affected PFS (Table II). Upon the Cox regression model,
advanced stages were the only independent prognostic factors
associated with shorter PFS, and m-IPI retained the prognostic
significance for shorter PFS and OS (Table III).
 | Table II.Univariate analysis of prognostic
factors for PFS and OS. |
Table II.
Univariate analysis of prognostic
factors for PFS and OS.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Prognostic
factors | χ2 | P-value | χ2 | P-value |
|---|
| Sex | 1.715 | 0.190 | 2.115 | 0.146 |
| Age (years) | 2.967 | 0.085 | 1.158 | 0.282 |
| Advanced stages | 13.516 | <0.001 | 5.726 | 0.017 |
| Serum LDH >245
U/l | 11.558 | 0.001 | 7.067 | 0.008 |
| β2-MG >2.2
mg/l | 10.590 | 0.001 | 5.050 | 0.025 |
| m-IPI ≥2 | 5.931 | 0.015 | 4.993 | 0.025 |
| Infection with
H. pylori | 0.805 | 0.370 | 1.264 | 0.261 |
| Non-GCB
subtype | 1.086 | 0.257 | 0.124 | 0.725 |
| Tumor mass ≥10
cm | 6.060 | 0.014 | 3.949 | 0.047 |
| PS ≥2 | 1.448 | 0.229 | 4.037 | 0.045 |
| Hemoglobin <120
g/l | 0.439 | 0.507 | 0.509 | 0.476 |
 | Table III.Factors retaining prognostic
significance for PFS and OS with multivariate and Cox proportional
hazards analysis. |
Table III.
Factors retaining prognostic
significance for PFS and OS with multivariate and Cox proportional
hazards analysis.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Prognostic
factors | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| LDH >245
U/l | 2.785 | 0.550–14.093 | 0.216 | 0.689 | 0.166–2.852 | 0.607 |
| Advanced
stages | 3.055 | 0.955–9.774 | 0.060 | 3.261 | 1.071–9.931 | 0.038 |
| Non-GCB
subtype | 0.590 | 0.177–1.964 | 0.389 | 3.133 | 0.809–12.130 | 0.108 |
| m-IPI ≥2 | 9.138 | 2.743–30.444 | 0.000 | 0.219 | 0.060–0.802 | 0.022 |
| PS ≥2 | 0.569 | 0.096–3.379 | 0.535 | 0.864 | 0.170–4.402 | 0.860 |
| Hemoglobin <120
g/l | 1.905 | 0.651–5.581 | 0.240 | 2.764 | 0.829–9.211 | 0.098 |
| β2-MG >2.2
mg/l | 1.592 | 0.402–6.308 | 0.508 | 2.923 | 0.734–11.639 | 0.128 |
| Tumor mass ≥10
cm | 0.579 | 0.117–2.872 | 0.503 | 0.624 | 0.149–2.610 | 0.519 |
Discussion
gDLBCL is the most common form of gastrointestinal
lymphoma (10,11). The pathogenesis of gDLBCL may have two
distinct pathways (4): some gDLBCL
may arise from indolent gMALT via the step of H.
pylori-dependent HT, and the residual gDLBCLs may develop along
antigen-independent pathways as de novo gDLBCL. Information
and data concerning patients with HT are very limited. In our
study, there was no specific clinical presentation for them with
the most common complaints such as abdominal pain or discomfort,
weight loss, poor appetite, nausea and vomiting. Their clinical
course was indolent, duration of symptoms before diagnosis often
ranged from a few weeks to several years. H. pylori
infections occurred more frequently in patients with HT, compared
with de novo gDLBCL in our previous study (12). It seems reasonable as HT is generally
considered to arise from H. pylori-dependent gMALT lymphoma
(13). Moreover, literature indicates
HPE is effective in treating gDLBCL (14,15). Our
data suggested that HPE treatment tend to improve the prognosis of
patients with HT, although there was no significant difference in
five-year PFS/OS between the patients treated with HPE treatment
and non-HPE treatment. Our results seem to further strengthen the
current hypothesis (4) that the
pathogenesis of gDLBCL has two distinct pathways.
Currently, there is still controversy regarding the
most effective treatment strategy for gastric lymphoma (7,8,16,17). Most
cases of gMALT lymphoma are cured with HPE, while intensive CT is
the mainstay of treatment for gDLBCL. As a transitional phase from
gMALT lymphoma to gDLBCL, major guidelines (18) recommend the use of intensive CT with
rituximab as the initial therapy for patients with HT.
Nevertheless, recent studies (16,17) have
demonstrated the efficacy of eradication therapy for patients with
HT. Morgner et al (19)
reported that HPE achieved CR in six of eight patients with HT. A
Taiwanese prospective study (14)
also demonstrated that HPE led to CR in 10 of 16 patients with HT
and none experienced recurrence during a long-term follow-up. In
our study, among three patients treated with HPE as first-line
therapy, histological PR was achieved in 2 of them after
antibiotics. Moreover, patients receiving HPE treatment tend to
have a good prognosis, although there were no statistically
different on five-year PFS/OS between patients receiving HPE
treatment and those receiving non-HPE treatment (49%). This finding
is consistent with recent studies. Besides, HPE is far less toxic
and cheaper than chemotherapy, thus it may be a promising
therapeutic approach for patients with HT. However, in order to
obtain accurate benefits of HPE for patients with HT, conducting a
large-scale prospective randomized clinical trial is ideal.
Prognostic factors for patients with HT have not
been reported previously. In the present study, m-IPI retained
their prognostic significance for shorter PFS and OS upon the Cox
regression model. Previous studies (20,21) have
indicated that m-IPI, in gDLBCL, is an effective prognostic factor,
although it has not been reported that m-IPI at HT were adverse
factors for PFS/OS. In a previous study, patients in the low-risk
group (m-IPI ≤1) had significantly longer survival than
intermediate/high-risk (m-IPI ≥2) patients. In our study, advanced
stages were demonstrated to be an independent prognostic factor
associated with shorter PFS. Findings have also shown that the
difference in stages may have been associated with the difference
in prognosis for gDLBCL (22,23). Medina-Franco et al (23) showed that patients with early stages
had a significantly better survival than patients with advanced
stages. As HT and gDLBCL share many of the same biological
characteristics, we believe that m-IPI or advanced stages were
effective predictors for prognosis of HT.
References
|
1
|
Tomonaga M: Outline and direction of
revised WHO classification of tumors of haematopoietic and lymphoid
tissues. Rinsho Ketsueki. 50:1401–1406. 2009.(In Japanese).
PubMed/NCBI
|
|
2
|
Nakamura S, Ye H, Bacon CM, Goatly A, Liu
H, Kerr L, Banham AH, Streubel B, Yao T, Tsuneyoshi M, et al:
Translocations involving the immunoglobulin heavy chain gene locus
predict better survival in gastric diffuse large B-cell lymphoma.
Clin Cancer Res. 14:3002–3010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshino T, Omonishi K, Kobayashi K,
Mannami T, Okada H, Mizuno M, Yamadori I, Kondo E and Akagi T:
Clinicopathological features of gastric mucosa associated lymphoid
tissue (MALT) lymphomas: high grade transformation and comparison
with diffuse large B cell lymphomas without MALT lymphoma features.
J Clin Pathol. 53:187–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaneko Y, Sakurai S, Hironaka M, Sato S,
Oguni S, Sakuma Y, Sato K, Sugano K and Saito K: Distinct
methylated profiles in Helicobacter pylori dependent and
independent gastric MALT lymphomas. Gut. 52:641–646. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, et al: NCI Sponsored International Working Group:
Report of an international workshop to standardize response
criteria for non-Hodgkin's lymphomas. J Clin Oncol. 17:12441999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo SH, Yeh KH, Wu MS, Lin CW, Hsu PN,
Wang HP, Chen LT and Cheng AL: Helicobacter pylori eradication
therapy is effective in the treatment of early-stage H
pylori-positive gastric diffuse large B-cell lymphomas. Blood.
119:4838–4844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreri AJ, Govi S, Raderer M, Mulè A,
Andriani A, Caracciolo D, Devizzi L, Ilariucci F, Luminari S, Viale
E, et al: Helicobacter pylori eradication as exclusive treatment
for limited-stage gastric diffuse large B-cell lymphoma: results of
a multicenter phase 2 trial. Blood. 120:3858–3860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hussell T, Isaacson PG, Crabtree JE and
Spencer J: The response of cells from low-grade B-cell gastric
lymphomas of mucosa-associated lymphoid tissue to Helicobacter
pylori. Lancet. 342:571–574. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghimire P, Wu GY and Zhu L: Primary
gastrointestinal lymphoma. World J Gastroenterol. 17:697–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arora N, Manipadam MT, Pulimood A,
Ramakrishna BS, Chacko A, Kurian SS and Nair S: Gastrointestinal
lymphomas: pattern of distribution and histological subtypes: 10
years experience in a tertiary centre in South India. Indian J
Pathol Microbiol. 54:712–719. 2011.PubMed/NCBI
|
|
12
|
Li X, Xia B, Guo S, Zhan Z, Zhang L, Zhao
D, Wu X and Zhang Y: A retrospective analysis of primary gastric
diffuse large B-cell lymphoma with or without concomitant
mucosa-associated lymphoid tissue (MALT) lymphoma components. Ann
Hematol. 92:807–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiyama T, Haruma K, Kitadai Y, Ito M,
Masuda H, Miyamoto M, Tanaka S, Yoshihara M, Sumii K, Shimamoto F,
et al: Helicobacter pylori eradication therapy for high-grade
mucosa- associated lymphoid tissue lymphomas of the stomach with
analysis of p53 and K-ras alteration and microsatellite
instability. Int J Oncol. 18:1207–1212. 2001.PubMed/NCBI
|
|
14
|
Chen LT, Lin JT, Shyu RY, Jan CM, Chen CL,
Chiang IP, Liu SM, Su IJ and Cheng AL: Prospective study of
Helicobacter pylori eradication therapy in stage I(E) high-grade
mucosa-associated lymphoid tissue lymphoma of the stomach. J Clin
Oncol. 19:4245–4251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura S, Matsumoto T, Suekane H,
Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M and
Fujishima M: Predictive value of endoscopic ultrasonography for
regression of gastric low grade and high grade MALT lymphomas after
eradication of Helicobacter pylori. Gut. 48:454–460. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wotherspoon AC, Doglioni C, Diss TC, Pan
L, Moschini A, de Boni M and Isaacson PG: Regression of primary
low-grade B-cell gastric lymphoma of mucosa-associated lymphoid
tissue type after eradication of Helicobacter pylori. Lancet.
342:575–577. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zucca E and Dreyling M; ESMO Guidelines
Working Group, : Gastric marginal zone lymphoma of MALT type: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21 Suppl 5:v175–v176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shannon EM, MacQueen IT, Miller JM and
Maggard-Gibbons M: Management of primary gastrointestinal
non-Hodgkin lymphomas: a population-based survival analysis. J
Gastrointest Surg. 20:1141–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgner A, Miehlke S, Fischbach W, Schmitt
W, Müller- Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer
A, Stolte M, et al: Complete remission of primary high-grade B-cell
gastric lymphoma after cure of Helicobacter pylori infection. J
Clin Oncol. 19:2041–2048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ibrahim EM, Ezzat AA, Raja MA, Rahal MM,
Ajarim DS, Mann B, Baloush A, Stuart RK and Bazarbashi SN: Primary
gastric non-Hodgkin's lymphoma: clinical features, management, and
prognosis of 185 patients with diffuse large B-cell lymphoma. Ann
Oncol. 10:1441–1449. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cortelazzo S, Rossi A, Roggero F, Oldani
E, Zucca E, Tondini C, Ambrosetti A, Pasini F, Pinotti G, Bertini
M, et al: International Extranodal Lymphoma Study Group (IELSG):
Stage-modified international prognostic index effectively predicts
clinical outcome of localized primary gastric diffuse large B-cell
lymphoma. Ann Oncol. 10:1433–1440. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Psyrri A, Papageorgiou S and Economopoulos
T: Primary extranodal lymphomas of stomach: Clinical presentation,
diagnostic pitfalls and management. Ann Oncol. 19:1992–1999. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medina-Franco H, Germes SS and Maldonado
CL: Prognostic factors in primary gastric lymphoma. Ann Surg Oncol.
14:2239–2245. 2007. View Article : Google Scholar : PubMed/NCBI
|