Introduction
Estrogen receptor (ER) and/or progesterone receptor
(PR) expression characterizes ~70% of all cases of breast cancer
(BC) (1,2). Thus, the majority of patients with BC
are affected by the estrogen signaling pathway, which serves an
important role in the development and progression of BC (1,2).
Therefore, endocrine therapy is an important treatment strategy for
all stages of hormone-dependent BC. Various therapeutic agents,
including selective ER modulators, gonadotropin-releasing hormone
agonists and aromatase inhibitors, are used in the endocrine
therapy of hormone-dependent BC and have demonstrated substantial
clinical benefits, including improved disease control and survival
outcomes (3). However, not all
patients with hormone-receptor positive BC respond to endocrine
therapy, termed de novo-resistance, and a substantial number
of patients who initially respond to endocrine therapy later suffer
from recurrence or disease progression, termed acquired resistance
(2). Potential reasons for endocrine
resistance include the following: Loss or modification of ERα
expression; ERα mutation; the altered regulation of signaling
pathways (phosphoinositide 3-kinase/protein kinase B/mechanistic
target of rapamycin and cyclin-dependent kinase 4/6 signaling
pathways); cross talk between the ER and growth factor receptor
signaling pathways [human epidermal growth factor receptor 2
(HER2)]; altered expression of specific microRNAs; and interactions
between the tumor microenvironment and host immune response.
Although significant progress has been made in understanding the
underlying molecular mechanisms of ligand-independent activation of
the ER, the mechanisms of endocrine resistance remain unclear
(4,5).
The functional versatility of the matricellular
protein cysteine-rich angiogenic inducer 61 (Cyr61) is recognized
by a growing number of studies, which identified that Cyr61 serves
a multitude of regulatory functions and has a range of potential
binding partners, including integrins (6–9). Cyr61 is
associated with essential signaling pathways in physiological
processes, including angiogenesis (7,10);
however, the alteration of Cyr61 expression has been demonstrated
to be associated with a number of pathologies (11–13),
including malignant neoplasms (14,15).
In previous studies by our group, Cyr61 expression
profiling in BC and its functional expression regulation was
examined (16,17) demonstrating a stage-dependent
induction of Cyr61 in BC tumorigenesis and hypoxia-induced
alternative splicing of Cyr61 in tumor cells. The results of
previous in vitro (18,19) and
in vivo (20–23) investigations have highlighted the
importance of Cyr61 in BC and the potential application of this
knowledge for the management of cancer. In combination with
Y-box-binding protein 1, Cyr61 acts on the urokinase plasminogen
activator surface receptor to stimulate the progression of
triple-negative BC (TNBC), while the expression of Cyr61 correlates
with increased malignancy and poorer patient survival (20). In addition, high grade ductal
carcinoma in situ may be characterized by Cyr61 expression,
independent of the ER expression status, suggesting that Cyr61
serves a role in the development of intraepithelial carcinoma
(23). In TNBC Cyr61 is described as
a mediator of the proto-oncogene tyrosine-protein kinase Src
signaling pathway, which modulates the metastatic potential of
malignant cells (18).
The functional role of Cyr61 in overcoming ER
dependency, which was suggested by Tsai et al (24), is of clinical importance. Using MCF7
cells and a mouse model, Tsai et al (24) demonstrated that Cyr61 was sufficient
to induce estrogen independence and antiestrogen resistance. In
addition, Jia et al (21)
reported that patients with Cyr61-expressing hormone-dependent BC
exhibited a poor response to letrozole treatment. The potential of
Cyr61 as a therapeutic target in BC management was also
demonstrated by another in vivo study (22).
The present study aimed to evaluate the potential
effects and clinical relevance of Cyr61 expression in patients with
primary non-metastatic BC. Therefore Cyr61 expression levels were
correlated to tumor characteristics and survival data.
Materials and methods
Patients and treatment
Tumor specimens were obtained from 67 patients with
histologically diagnosed primary non-metastatic invasive BC who
received treatment at the Department of Obstetrics and Gynecology
of the Medical Center of the University of Freiburg (Freiburg,
Germany) between April 2000 and September 2001, and for whom Cyr61
measurement data was available. The present study was approved by
the Ethics Committee of the University of Freiburg (approval no.
313/2001) and all patients provided written informed consent.
Clinicopathological characteristics of the patients,
including age, histological tumor type, tumor grade, ER, PR and
HER2 expression statuses and molecular BC subtype were evaluated.
The median age of the patients was 58 years at the time of
diagnosis (range, 33–87 years). The characteristics of the study
population are summarized in Table
I.
 | Table I.Clinicopathological characteristics of
patients included in the present study (n=67). |
Table I.
Clinicopathological characteristics of
patients included in the present study (n=67).
| Characteristics | Number of
patients | Percentage of
patients |
|---|
| Age, years |
|
<50 | 15 | 22.4 |
|
>50 | 52 | 77.6 |
| Histological tumor
type |
| Invasive
ductal | 43 | 64.2 |
| Invasive
lobular | 12 | 17.9 |
|
Other | 12 | 17.9 |
| Lymph node metastasis
status |
|
Present | 27 | 40.3 |
|
Absent | 40 | 59.7 |
| Tumor grade |
| G1 | 2 | 3.0 |
| G2 | 29 | 43.3 |
| G3 | 36 | 53.7 |
| ER/PR expression
status |
|
Positive | 49 | 73.1 |
|
Negative | 18 | 26.9 |
| HER2 expression
status |
| Score
0 | 13 | 19.4 |
| Score
1 | 19 | 28.4 |
| Score
2 | 9 | 13.4 |
| Score
3 | 26 | 38.8 |
| Molecular tumor
subtype |
| Luminal
like | 30 | 44.8 |
|
HER2/luminal | 19 | 28.4 |
|
HER2-like | 8 | 11.9 |
| Triple
negative | 10 | 14.9 |
The histological type of all tumors was categorized
according to the World Health Organization Histological Typing of
Breast Tumors (25). Tumor grade was
defined according to Black's nuclear grading system with
modification of numbers. ER, PR and HER2 expression statuses were
determined by immunohistochemistry (IHC). Molecular BC subtypes
were defined by IHC assessment as follows: Luminal-like (HER2
negative, ER/PR positive); HER2/luminal (HER2/ER/PR positive);
HER2-like (HER2-positive, ER/PR negative); and triple-negative
(HER2/ER/PR-negative).
All patients were treated according to national
guidelines (26) with surgery
(breast-conserving surgery/mastectomy with sentinel node biopsy
and/or axillary dissection), which was followed by adjuvant
chemotherapy, radiation and antihormonal therapy depending on the
tumor stage and characteristics. Follow-up data [disease-free
survival (DFS) and overall survival (OS)] were obtained from the
local cancer registry, clinical records and from the general
practitioners of the patients. Patients were censored at the time
they were last observed alive.
IHC
Formalin-fixed paraffin-embedded BC tissue
specimens, which were taken during surgery, were sectioned
(3-µm-thick) and stained for Cyr61, human transformer-2 protein
homolog β (hTRA2β) and cluster of differentiation (CD) 44 proteins.
After removing the paraffin with xylene and a descending series of
alcohol, antigen retrieval was performed for 10 min in a microwave
oven at 600 W using High pH Target Retrieval Solution (cat. no.
K8004, Dako; Agilent Technologies GmbH, Waldbronn, Germany).
Endogenous peroxidase activity was blocked using EnVision™ Flex
Peroxidase Blocking Reagent (Dako; Agilent Technologies GmbH) for
10 min at room temperature. The sections were then incubated at
room temperature overnight with the following primary antibodies:
Cyr61 (clone H-78; cat. no. sc-13100; dilution, 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); hTRA2ß [provided by
Professor Stefan Stamm (27);
dilution, 1:5,000]; HER4 (clone C-18, cat. no. sc-283, dilution
1:1500, Santa Cruz), CD44 (clone F10-44-2; dilution, 1:4,000;
Abcam, cat. no. ab6124, Cambridge, UK); CD44v5 (clone VFF-8;
dilution, 1:1,200; Abcam, cat. no. ab34235) and CD44v6 (clone
VFF-7; dilution, 1:1,200; Abcam, cat. no 36). Subsequently, the
primary antibodies were detected using the ImmPRESS™ HRP Universal
Antibody (Anti-Mouse IgG/Anti-Rabbit IgG, Peroxidase) Polymer
Detection kit (cat. no. MP-7500; Vector Laboratories, Ltd.,
Peterborough, U.K.), according to the manufacturer's protocol.
Protein bands were then visualized using the ImmPACT DAB Peroxidase
(HRP) Substrate (cat. no. SK-4105, Vector Laboratories, Ltd.) for
10 min at room temperature. Following 2 washing steps of 5 min each
with PBS, sections were counterstained at room temperature for 10
min with Mayer's Hemalaun Solution (cat. no. 109249; Merck KGaA,
Darmstadt, Germany) and dehydrated in an ascending series of
alcohol concentrations. Coverslips were mounted with Entellan® New
Rapid Mounting Medium (cat. no. 10796; Merck KGaA).
Assessment of Cyr61, hTRA2ß, CD44 and
HER4 expression
IHC assessment of Cyr61, hTRA2ß, CD44 (std, v5 and
v6) and HER4 expression was reviewed with a Zeiss Axioplan 2
microscope (Zeiss AG, Oberkochen, Germany; magnification, ×100) by
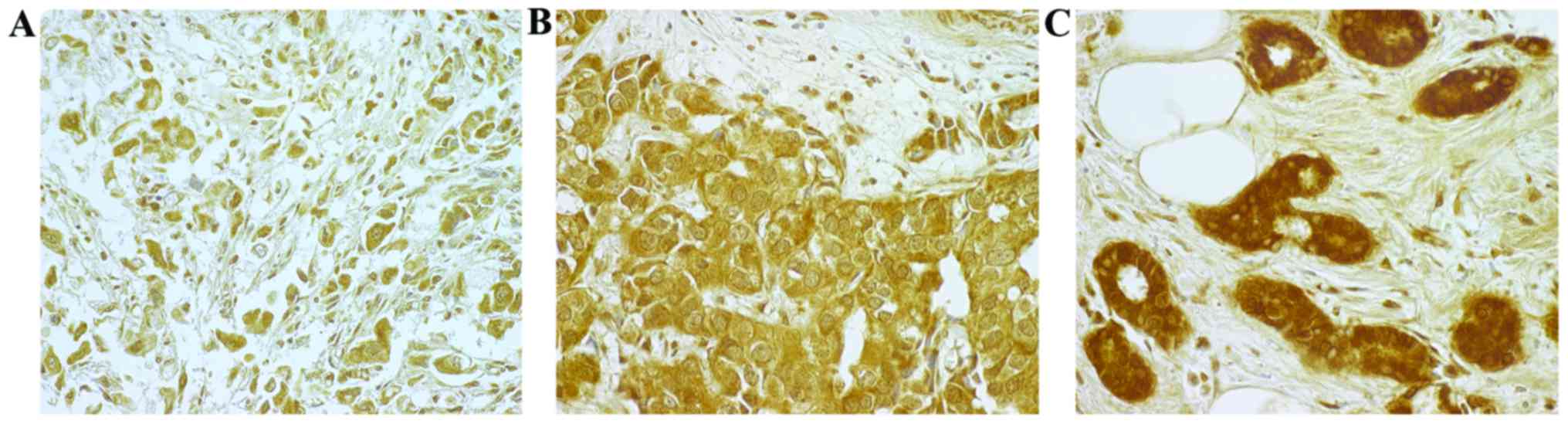
an experienced pathologist. IHC staining was considered positive if
a protein signal was observed in the cytoplasm or nucleus of the BC
cells. The intensity of IHC staining was classified as follows:
Absent, 0; weak, 1+; moderate, 2+; and strong, 3+. A strong
intensity of staining (3+) was considered to indicate
overexpression (Fig. 1).
Statistical analysis
Pearson's correlation coefficients between Cyr61
expression and clinical factors, including hTRA2ß, HER2, HER4,
CD44std, CD44v5 and CD44v6 expression, were calculated.
Multivariate logistic regression analyses were performed according
to Akaike's information criterion (AIC) (28), and the model with a minimum AIC
including ER/PR, CD44std and HER4 score 2 and 3 was selected. For
the analysis of OS and DFS, the Kaplan-Meier estimator and Cox
regression model were used. P<0.05 was considered to indicate a
statistically significant difference. All analyses were conducted
using the statistical software environment R, version 3.2.3
(29).
Results
Univariate analysis
Univariate analysis demonstrated a significant
positive association between Cyr61 overexpression and the molecular
tumor subtype of BC (P=0.039; data not shown). Cyr61 overexpression
was more frequently observed in luminal-like (66.67%) and
HER2/luminal (57.89%) molecular subtypes compared with HER2-like
(12.50%) and triple negative (40.00%) molecular subtypes.
Additionally, Cyr61 overexpression was identified to be
significantly positively associated with ER/PR expression status
(P=0.013; data not shown). Cyr61 overexpression was not observed to
be significantly associated with any of the other clinical factors
studied.
Multivariate logistic regression
analysis
The final regression model with a minimum AIC
(86.678) included ER/PR expression status, CD44std, HER4 score 2
and HER4 score 3. This multivariate logistic regression analysis
confirmed the significant positive association between Cyr61
overexpression and ER/PR expression status (P=0.016; Table II). In addition, a notable negative
association was identified between Cyr61 overexpression and CD44std
expression was observed (P=0.074; Table
II).
 | Table II.Multivariate regression analysis for
cysteine-rich angiogenic inducer 61 with the optimal Akaike's
information criterion score (86.678). |
Table II.
Multivariate regression analysis for
cysteine-rich angiogenic inducer 61 with the optimal Akaike's
information criterion score (86.678).
| Variable | Regression
coefficient | 95% CI | P-value |
|---|
| Intercept |
0.793 | −1.323–2.908 | 0.463 |
| ER/PR |
1.598 |
0.303–2.893 | 0.016 |
| CD44std | −0.003 | −0.007–0.000 | 0.074 |
| HER4 score 2 |
0.029 | −1.115–1.174 | 0.960 |
| HER4 score 3 |
1.728 | −0.743–4.199 | 0.171 |
Survival analysis
Overall survival (OS)
The median follow-up time was 115 months (range,
0–131 months; data not shown). During follow-up, 18 recurrences and
22 mortalities were observed (data not shown). At 60 months, 10
patients had succumbed, resulting in a 5-year OS-rate of 84.8%
(Table III). At 120 months, 21
mortalities had occurred, resulting in a 10-year OS rate of 66.8%
and 45 patients were censored (Table
III).
 | Table III.OS of patients with breast cancer
stratified by Cyr61 expression. |
Table III.
OS of patients with breast cancer
stratified by Cyr61 expression.
| A, All patients
with BC (n=67) |
|---|
|
|---|
| Time point | Cyr61 expression
level | Number of
patients | Events
(Mortality) | OS (%) |
|---|
| Baseline | All | 66 | 0 | 100.0 |
|
| Scores 1 + 2 | 31 | 0 | 100.0 |
|
| Score 3 | 35 | 0 | 100.0 |
| 60 months | All | 57 | 10 |
84.8 |
|
| Scores 1 + 2 | 28 | 4 |
87.1 |
|
| Score 3 | 30 | 6 |
82.9 |
| 108 months | All | 47 | 20 |
69.7 |
|
| Scores 1 + 2 | 25 | 7 |
77.4 |
|
| Score 3 | 23 | 13 |
62.9 |
| 120 months | All | 24 | 21 |
66.8 |
|
| Scores 1 + 2 | 10 | 8 |
69.7 |
|
| Score 3 | 23 | 13 |
62.9 |
|
|---|
| B, Patients with
HR-positive BC (n=49) |
|---|
|
|---|
| Time point | Cyr61 expression
level | Number of
patients | Events | OS (%) |
|
|---|
| Baseline | All | 48 | 0 | 100.0 |
|
| Scores 1 + 2 | 18 | 0 | 100.0 |
|
| Score 3 | 30 | 0 | 100.0 |
| 60 months | All | 42 | 7 |
85.4 |
|
| Scores 1 + 2 | 17 | 2 |
88.9 |
|
| Score 3 | 26 | 5 |
83.3 |
| 108 months | All | 33 | 16 |
66.7 |
|
| Scores 1 + 2 | 15 | 4 |
77.8 |
|
| Score 3 | 19 | 12 |
60.0 |
| 120 months | All | 17 | 17 |
62.7 |
|
| Scores 1 + 2 | 5 | 5 |
62.2 |
|
| Score 3 | 19 | 12 |
60.0 |
OS stratified for Cyr61
expression
A subgroup analysis according to Cyr61 expression
level was performed (Table III).
Kaplan-Meier-survival analysis demonstrated a lower OS rate in
patients with Cyr61 overexpression (n=35) at 60 months (5-year OS,
82.9 vs. 87.1%) and 120 months (10-year OS, 62.9 vs. 69.7%)
compared with patients without Cyr61 overexpression (n=31). The
most distinct difference between the two groups was observed at 108
months (9 years) with an OS rate of 62.9% in patients with
Cyr61-overexpression and 77.4% in patients without Cyr61
overexpression (Table III).
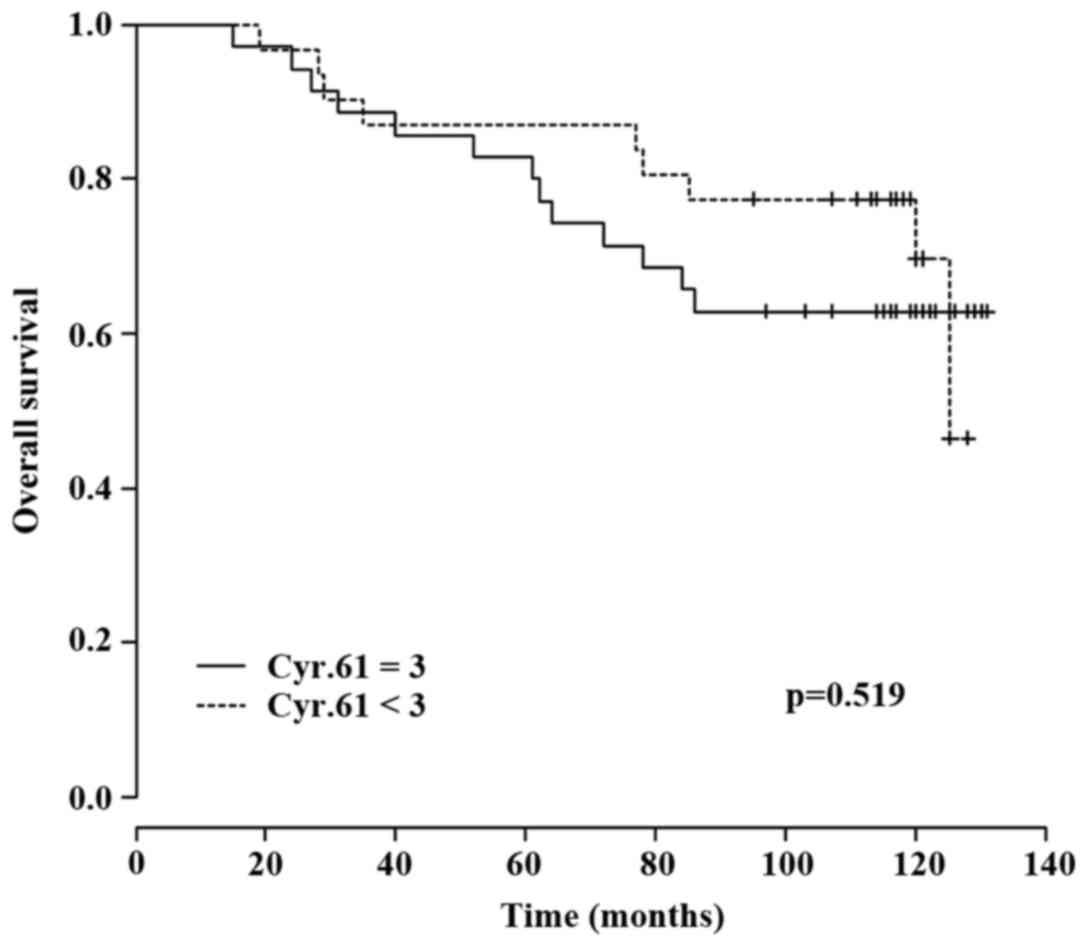
However, using the Cox regression model, a significant association
between Cyr61 overexpression and OS was not detected (P=0.519;
Fig. 2).
OS stratified for HR status and Cyr61
expression
The OS subgroup analysis for patients with
HR-positive BC (n=48) demonstrated a 5-year OS rate of 85.4%, a
9-year OS rate of 66.7% and a 10-year OS rate of 62.7% (Table III). Stratifying this subgroup
according to Cyr61 expression also revealed a lower OS rates for
patients with Cyr61 overexpression compared with patients without
Cyr61 overexpression at 60 months (83.3 vs. 88.9%) and at 108
months (60.0 vs. 77.8%), but not at 120 months (60.0 vs. 62.2%)
(Table III). However, a significant
difference between these two groups was not observed based on the
Cox regression model (P=0.397; data not shown).
Progression free survival (PFS)
During follow-up, 17 recurrences were observed (0–60
months, 14 recurrences; 61–131 months, 3 recurrences) resulting in
a recurrence rate of 25.4% (data not shown). A total of 12/17
(70.6%) patients with a recurrence succumbed during this time (data
not shown). During follow-up for PFS, a total of 27 events (e.g.
mortalities plus recurrences) were observed and 39 patients were
censored (Table IV).
 | Table IV.PFS of patients with breast cancer
stratified by Cyr61 expression. |
Table IV.
PFS of patients with breast cancer
stratified by Cyr61 expression.
| A, All patients
with BC (n=67) |
|---|
|
|---|
| Time point | Cyr61 expression
level | Number of
patients | Events (Mortalities
and recurrence) | PFS (%) |
|---|
| Baseline | All | 66 | 0 | 100.0 |
|
| Score 1 + 2 | 31 | 0 | 100.0 |
|
| Score 3 | 35 | 0 | 100.0 |
| 60 months | All | 48 | 20 |
69.7 |
|
| Score 1 + 2 | 25 | 8 |
74.2 |
|
| Score 3 | 24 | 12 |
65.7 |
| 108 months | All | 40 | 25 |
62.0 |
|
| Score 1 + 2 | 20 | 11 |
64.4 |
|
| Score 3 | 22 | 14 |
60.0 |
| 120 months | All | 23 | 27 |
57.0 |
|
| Score 1 + 2 | 9 | 12 |
57.2 |
|
| Score 3 | 15 | 15 |
56.0 |
|
|---|
| B, Patients with
HR-positive BC (n=49) |
|
|---|
| Time point | Cyr61 expression
level | Number of
patients | Events | PFS (%) |
|
|---|
| Baseline | All | 48 | 0 | 100.0 |
|
| Scores 1 + 2 | 18 | 0 | 100.0 |
|
| Score 3 | 30 | 0 | 100.0 |
| 60 months | All | 36 | 14 |
70.8 |
|
| Scores 1 + 2 | 16 | 4 |
77.8 |
|
| Score 3 | 21 | 10 |
66.7 |
| 108 months | All | 29 | 18 |
62.4 |
|
| Scores 1 + 2 | 12 | 6 |
66.2 |
|
| Score 3 | 19 | 12 |
60.0 |
| 120 months | All | 17 | 20 |
55.6 |
|
| Scores 1 + 2 | 5 | 7 |
53.0 |
|
| Score 3 | 19 | 12 |
60.0 |
PFS stratified for Cyr61
expression
Compared with patients without Cyr61 overexpression,
patients with Cyr61 overexpression were more likely to experience a
recurrence (31.4 vs. 19.4%) and exhibited a higher risk of
mortality subsequent to recurrence (81.8 vs. 50.0%) (data not
shown). Kaplan-Meier estimator analysis demonstrated a lower PFS
for patients with Cyr61 overexpression compared with those without
Cyr61 overexpression at 60 months (65.7 vs. 74.2%), whereas at 120
months (56.0 vs. 57.2%) no notable difference in PFS was observed
between the two groups (Table IV).
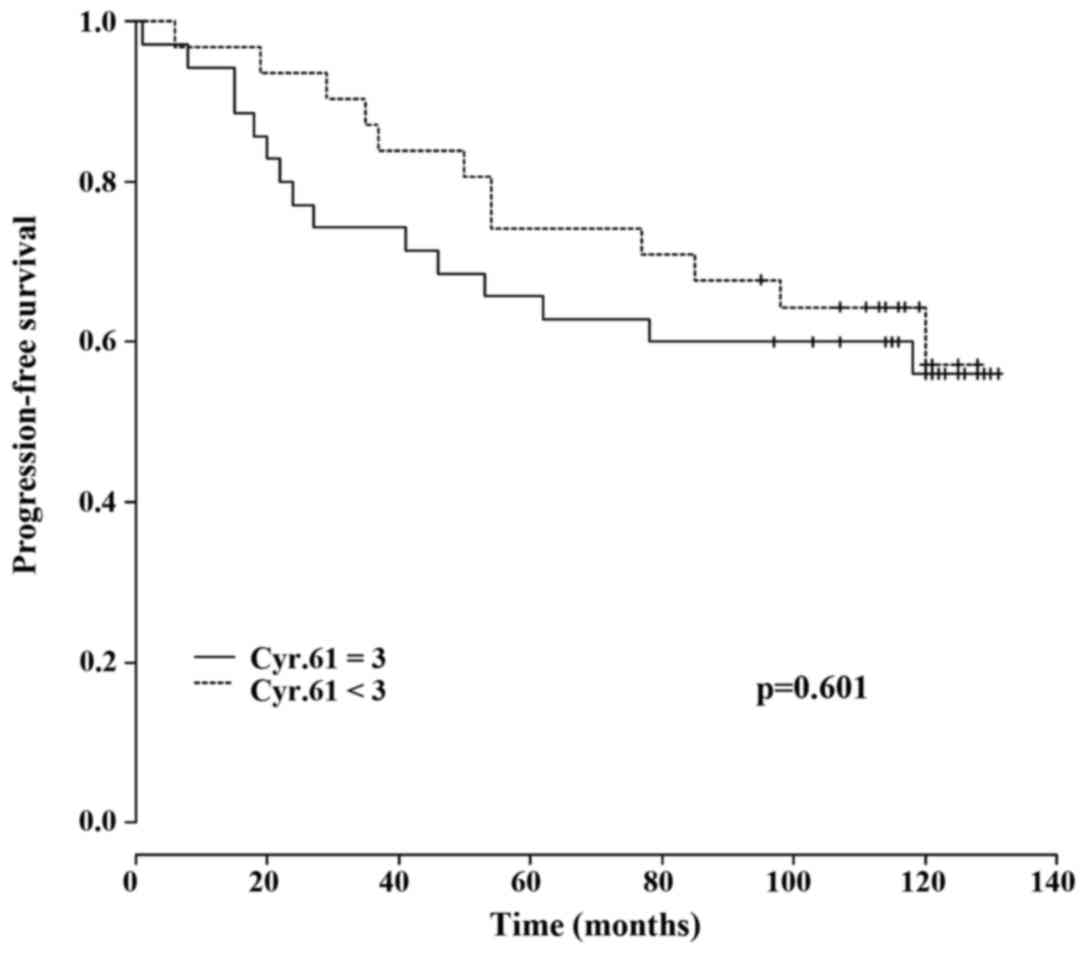
Therefore, no significant association between Cyr61 overexpression
and PFS was detected (P=0.601; Fig.
3).
PFS stratified for HR status and Cyr61
expression
Subgroup analysis for PFS in regard to HR status
also revealed a higher recurrence rate for patients with Cyr61
overexpression compared with patients without Cyr61 overexpression
(33.3 vs. 16.7%), in addition to a higher mortality rate (90.0 vs.
33.3%) (data not shown). Kaplan-Meier estimator analysis in regard
to HR status (HR positive vs. HR negative expression) demonstrated
a lower PFS for HR positive patients with Cyr61 overexpression
compared with patients without Cyr61 overexpression at 60 months
(66.7 vs. 77.8%), whereas at 120 months (55.4 vs. 53.0%) no marked
difference was observed between the two groups (Table IV). Thus, no significant association
between Cyr61 overexpression and PFS in HR positive patients was
demonstrated (P=0.638; data not shown).
Discussion
In the present study, based on 67 patients with
primary BC, a positive association between Cyr61 expression and
ER/PR expression status was observed. This data was consistent in
univariate and multivariate analyses. In addition, an association
between Cyr61 overexpression and the BC molecular subtype was
observed, with increased Cyr61 overexpression rates in luminal-like
and HER2/luminal tumors. In multivariate logistic regression
analysis, a notable negative association between Cyr61 and CD44std
expression was observed.
These data are supported by the patient outcome
data, which revealed increased recurrence rates and decreased OS
rates in patients with Cyr61 overexpression compared with those
without Cyr61 overexpression. In particular, long-term OS was
impaired in patients with Cyr61 overexpression. Similar results
were obtained in patients with ER/PR positive BC. However, the
observed OS trend did not reach statistical significance. Potential
reasons for this include the relatively small patient cohort and
the high number of censored patients at the end of the
observation.
Resistance to endocrine therapy is a major challenge
in the treatment of patients with BC. Although a range of signaling
pathways have been identified to serve a role in this treatment
resistance, the highly complex and heterogeneous underlying
molecular mechanisms are not completely understood. The results of
the present study support the findings of a previous study by Tsai
et al (24), which reported an
association between Cyr61 and carcinogenesis, tumor invasiveness,
and the induction of estrogen-independence and anti-estrogen
resistance. These data are in accordance with the data of a study
by Jia et al (21), which
reported that Cyr61 contributes to the poor response to letrozole
treatment in patients with ER positive BC.
The results of these previous studies and the
present study indicate that Cyr61 serves an important role in the
development of endocrine treatment resistance in BC. Although the
results for the association between survival and Cyr61
overexpression did not reach a statistically significant level in
the present study, they do suggest that patients with BC
overexpressing Cyr61 exhibit a decreased long-term survival. In
order to confirm the clinical significance of Cyr61 as a marker for
OS and PFS, additional long-term survival analyses with larger
patient populations are required.
In conclusion, the results of the present study
indicate that Cyr61 overexpression serves a role in the development
of endocrine treatment resistance in patients with BC. In addition,
the results of the present study identify Cyr61 as a potential
therapeutic target to overcome endocrine therapy resistance.
References
|
1
|
Johnston SR and Dowsett M: Aromatase
inhibitors for breast cancer: Lessons from the laboratory. Nat Rev
Cancer. 3:821–831. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massarweh S and Schiff R: Resistance to
endocrine therapy in breast cancer: Exploiting estrogen
receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 13
Suppl 1:S15–S24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), ; Davies C, Godwin J, Gray R, Clarke
M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, et al: Relevance
of breast cancer hormone receptors and other factors to the
efficacy of adjuvant tamoxifen: Patient-level meta-analysis of
randomised trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
García-Becerra R, Santos N, Díaz L and
Camacho J: Mechanisms of resistance to endocrine therapy in breast
cancer: Focus on signaling pathways, miRNAs and genetically based
resistance. Int J Mol Sci. 14:108–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rugo HS, Vidula N and Ma C: Improving
response to hormone therapy in breast cancer: New targets, new
therapeutic options. Am Soc Clin Oncol Educ Book. 35:e40–e54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau LF: CCN1/CYR61: The very model of a
modern matricellular protein. Cell Mol Life Sci. 68:3149–3163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y and Du XY: Functional properties
and intracellular signaling of CCN1/Cyr61. J Cell Biochem.
100:1337–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsube K, Sakamoto K, Tamamura Y and
Yamaguchi A: Role of CCN, a vertebrate specific gene family, in
development. Dev Growth Differ. 51:55–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leask A and Abraham DJ: All in the CCN
family: Essential matricellular signaling modulators emerge from
the bunker. J Cell Sci. 119:4803–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lau LF and Lam SC: The CCN family of
angiogenic regulators: The integrin connection. Exp Cell Res.
248:44–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emre Y and Imhof BA: Matricellular protein
CCN1/CYR61: A new player in inflammation and leukocyte trafficking.
Semin Immunopathol. 36:253–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weiskirchen R and Tacke F: Liver Fibrosis:
From pathogenesis to novel therapies. Dig Dis. 34:410–422. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu T, He YH, Wang MQ, Yao HW, Ni MM, Zhang
L, Meng XM, Huang C, Ge YX and Li J: Therapeutic potential of
cysteine-rich protein 61 in rheumatoid arthritis. Gene.
592:179–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Ye L, Owen S, Weeks HP, Zhang Z and
Jiang WG: Emerging role of CCN family proteins in tumorigenesis and
cancer metastasis (Review). Int J Mol Med. 36:1451–1463.
2015.PubMed/NCBI
|
|
15
|
Yeger H and Perbal B: CCN family of
proteins: Critical modulators of the tumor cell microenvironment. J
Cell Commun Signal. 10:229–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirschfeld M, Jaeger M, Buratti E, Stuani
C, Grueneisen J, Gitsch G and Stickeler E: Expression of
tumor-promoting Cyr61 is regulated by hTRA2-β1 and acidosis. Hum
Mol Genet. 20:2356–2365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirschfeld M, zur Hausen A, Bettendorf H,
Jäger M and Stickeler E: Alternative splicing of Cyr61 is regulated
by hypoxia and significantly changed in breast cancer. Cancer Res.
69:2082–2090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sánchez-Bailón MP, Calcabrini A,
Mayoral-Varo V, Molinari A, Wagner KU, Losada JP, Ciordia S, Albar
JP and Martín-Pérez J: Cyr61 as mediator of Src signaling in triple
negative breast cancer cells. Oncotarget. 6:13520–13538. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sarkissyan S, Sarkissyan M, Wu Y, Cardenas
J, Koeffler HP and Vadgama JV: IGF-1 regulates Cyr61 induced breast
cancer cell proliferation and invasion. PloS One. 9:e1035342014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huber MC, Falkenberg N, Hauck SM, Priller
M, Braselmann H, Feuchtinger A, Walch A, Schmitt M and Aubele M:
Cyr61 and YB-1 are novel interacting partners of uPAR and elevate
the malignancy of triple-negative breast cancer. Oncotarget.
7:44062–44075. 2016.PubMed/NCBI
|
|
21
|
Jia X, Liu G, Cheng J, Shen Z and Shao Z:
CYR61 contributes to poor response to letrozole in ER positive
breast carcinoma. Curr Cancer Drug Targets. 2016.
|
|
22
|
Lin J, Huo R, Wang L, Zhou Z, Sun Y, Shen
B, Wang R and Li N: A novel anti-Cyr61 antibody inhibits breast
cancer growth and metastasis in vivo. Cancer Immunol Immunother.
61:677–687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saglam O, Dai F, Husain S, Zhan Y, Toruner
G and Haines GK III: Matricellular protein CCN1 (CYR61) expression
is associated with high-grade ductal carcinoma in situ. Hum Pathol.
45:1269–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai MS, Bogart DF, Castañeda JM, Li P and
Lupu R: Cyr61 promotes breast tumorigenesis and cancer progression.
Oncogene. 21:8178–8185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
The world Health Organization Histological
Typing of Breast Tumors-Second Edition. The World Organization. Am
J Clin Pathol. 78:806–816. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harbeck N and Rody A: Diagnostik und
Therapie primärer metastasierter Mammakarzinome. http://www.ago-online.de/fileadmin/downloads/leitlinien/mamma/2017-03/AGO_deutsch/PDF_Einzeldateien_deutsch/2017D%2005_Prognostische%20und%20praediktive%20Faktoren.pdfAGO
in e.V. DGGG e.V., DKG e.V.2017.
|
|
27
|
Stoilov P, Daoud R, Nayler O and Stamm S:
Human tra2-beta1 autoregulates its protein concentration by
influencing alternative splicing of its pre-mRNA. Hum Mol Genet.
13:509–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwarz G: Estimating the dimensions of a
model. Ann Stat. 6:461–464. 1978. View Article : Google Scholar
|
|
29
|
R Core Team, . 2016, R: A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. https://www.R-project.org/May 25–2016
|