Introduction
B7 homolog 6 (B7-H6), a novel member of the B7
family, was identified on tumor cell surfaces in 2009 (1). B7-H6 has sequence homology with other B7
molecules and similar to other members of the B7 family, B7-H6
contains two extracellular immunoglobulin (Ig) domains; however,
the receptor for B7-H6, located on natural killer (NK) cells, was
not consistent with other B7 family member receptors, which are
located on activated T cells. The receptor for B7-H6 is natural
cytotoxicity triggering receptor 3 (NKp30) (1–3). NK cells
are large granular lymphocytes that produce chemokines and
cytokines, and participate in the inflammatory and adaptive immune
response (4). NK cells are also an
important part of the innate immune system as they directly kill
transformed and virally infected cells (5).
B7 family members serve an essential role in
regulating the immune response against transformed cells through a
variety of mechanisms (6). As
specific niches of B7 family members continue to be dissected,
their diagnostic and therapeutic potential in tumors is becoming
more apparent (6,7), and this was highlighted in 2011 by the
US Food and Drug Administration approval of an antibody targeting
programmed death-ligand 1 (PD-L1) and cytotoxic
T-lymphocyte-associated protein 4 in cancer (8,9). The
ability to successfully target cell cycle checkpoint regulators has
since led to multiple clinical trials investigating antibodies
targeting the signaling pathway that the B7 family participate in
(10).
B7-H6 binds to NKp30, triggering antitumor NK cell
cytotoxicity and cytokine secretion, thus B7-H6 functions as a
tumor-induced self-molecule that alerts the innate immune system to
cellular transformation (11,12). Previous studies have investigated the
association between B7-H6 expression and numerous types of tumor,
including 65 cases of lung cancer, 60 cases of gastric cancer and
110 cases of human ovarian cancer (12–14). To
the best of our knowledge, no previous studies have investigated
the clinical significance of B7-H6 protein expression in patients
with breast cancer.
The present study aimed to investigate the
expression of B7-H6 protein in primary breast cancer by
immunohistochemistry (IHC), and to identify the association between
B7-H6 expression and the clinicopathological features and survival
time of patients with breast cancer. The present study will aid in
future studies examining the function of B7-H6 in the tumor immune
response. The results of the present study also suggest that B7-H6
may have prognostic and therapeutic value in breast cancer.
Materials and methods
Patients
Cancer tissues from 305 patients, who underwent
surgery for breast cancer between January 2009 and March 2014 at
the Department of General Surgery of The Fourth Hospital of Suzhou
(Suzhou, China) and Shanghai Renji Hospital (Shanghai, China), were
used in the present study. Patients who had received any
preoperative chemotherapy or radiotherapy prior to surgery were
excluded from the study. A total of 74 patients from this cohort
were treated at the Department of General Surgery of The Fourth
Hospital of Suzhou between January 2009 and December 2009. The
other 231 patients were treated at the Department of General
Surgery of Shanghai Renji Hospital between April 2010 and March
2014. The 305 tissue samples were stained with hematoxylin and
eosin and final pathological diagnoses were confirmed. The
patients' pathological reports were recorded, and their
clinicopathological characteristics are presented in Table I. Survival data were collected from
patient follow-ups. The present study was approved by the Ethics
Review Board of The Fourth Hospital of Suzhou and Shanghai Renji
Hospital. Written informed consent was obtained from all patients
prior to enrollment in the present study.
 | Table I.Association between B7-H6 protein
expression and the clinicopathological characteristics of patients
with breast cancer. |
Table I.
Association between B7-H6 protein
expression and the clinicopathological characteristics of patients
with breast cancer.
|
| Percentage of
patients |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | Low expression of
B7-H6 | High expression of
B7-H6 | P-value |
|---|
| Breast cancer
subtype |
|
| 0.264 |
| Lumin
A | 14 | 10 |
|
| Lumin
B | 61 | 34 |
|
|
HER2+ | 58 | 31 |
|
|
Basal-like | 12 | 1 |
|
|
Normal-like |
4 | 1 |
|
| Tumor location |
|
| 0.254 |
| Left
breast | 101 | 38 |
|
| Right
breast | 101 | 56 |
|
|
Bitemporal breast |
5 | 4 |
|
| Tumor size, cm |
|
| 0.8243 |
|
<2.5 | 110 | 45 |
|
| ≥2.5 | 97 | 42 |
|
| Lymph node
metastasis |
|
| 0.003a |
|
Absent | 87 | 25 |
|
|
Present | 65 | 45 |
|
| HER2 expression
status |
|
| 0.0001a |
|
Negative | 74 | 22 |
|
| Weak | 42 | 12 |
|
|
Medium | 43 | 14 |
|
|
Strong | 36 | 36 |
|
| ER expression
status |
|
| 0.159 |
|
Negative | 88 | 30 |
|
|
Positive | 115 | 57 |
|
| PR expression
status |
|
| 0.0392a |
|
Negative | 99 | 32 |
|
|
Positive | 79 | 45 |
|
Construction of the tissue microarray
(TMA)
A TMA containing a total of 305 tissue samples was
prepared, with core tissue sections (diameter, 1.6 mm) obtained
from individual paraffin-embedded breast tumor samples (donor
blocks) using a trephine. Sections (4-µm-thick) were cut from each
tissue sample, deparaffinized and dehydrated for IHC staining using
the procedure described by Chen et al (15).
IHC staining
Rabbit anti-human B7-H6 polyclonal antibody was
purchased from Abcam (Cambridge, UK; cat. no. Ab121794) and was
diluted 1:40 to produce a working concentration of 7.5 µg/ml. Mouse
anti-human cluster of differentiation (CD) 56, a marker of NK
cells, antibody was purchased from Fuzhou Maixin Biotech Co., Ltd.
(Fuzhou, China; cat. no. Kit-0028). The horseradish peroxidase
(HRP)-conjugated anti-mouse/rabbit secondary antibodies kit was
also purchased from Fuzhou Maixin Biotech Co., Ltd. (cat. no.
kit-5010) and the IHC staining for B7-H6 and CD56 was performed
according to the manufacturer's protocol. Breast cancer TMA
sections were incubated with Mayer's hematoxylin solution, and
incubated at 100°C for 30 min in citrate solutions. The tissue
sections were incubated at 4°C for 16 h with anti-B7-H6 and
anti-CD56 antibodies and further incubated at room temperature for
15 min with the HRP-conjugated secondary antibodies. Unrelated
isotype-matched rabbit IgG Ab-1 antibody (cat. no. NC-100, Fuzhou
Maixin Biotech. Co., Ltd.) and mouse IgG Ab-1 antibody (cat. no.
NC-748, Fuzhou Maxin Biotech. Co., Ltd.) were used as the control
for nonspecific staining.
Evaluation of IHC staining
Two independent observers who were blinded to the
clinicopathological characteristics of the patients examined the
immunostained sections. B7-H6 expression level analysis was limited
to tumors with a sufficient amount of tissue at a certain stage of
cancer development for IHC evaluation. A semiquantitative scoring
system based on the amount of positively stained tumor cells and
the staining intensity was used to evaluate the expression level of
B7-H6 protein. An estimate of the proportion of positively stained
cells of 0–10% scored 1, 11–30% scored 2, 31–60% scored 3, and
61–100% scored 4. An intensity factor ranged from 1 (weak positive,
staining intensity marginally exceeding the background) to 4
(strong positive, dark brown staining clearly visible on
macroscopic inspection of the slide). The immunostaining scoring
was conducted by multiplying the scores for the proportion and the
intensity of positive-staining cells. Scores between 1 and 8 were
defined as the low expression group, and scores between 9 and 16
were defined as the high expression group.
Statistical analysis
SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA)
was used for statistic analysis. All data are presented as the mean
± standard deviation. Statistical analysis was performed using the
Student's t-test and analysis of one-way analysis of variance.
Correlations were evaluated with Pearson's correlation coefficient.
P<0.05 was considered to indicate a statistically significant
difference. Kaplan-Meier estimator survival plots were generated,
and comparisons between the survival curves were performed using a
log-rank test.
Results
B7-H6 protein expression in breast
cancer tissues
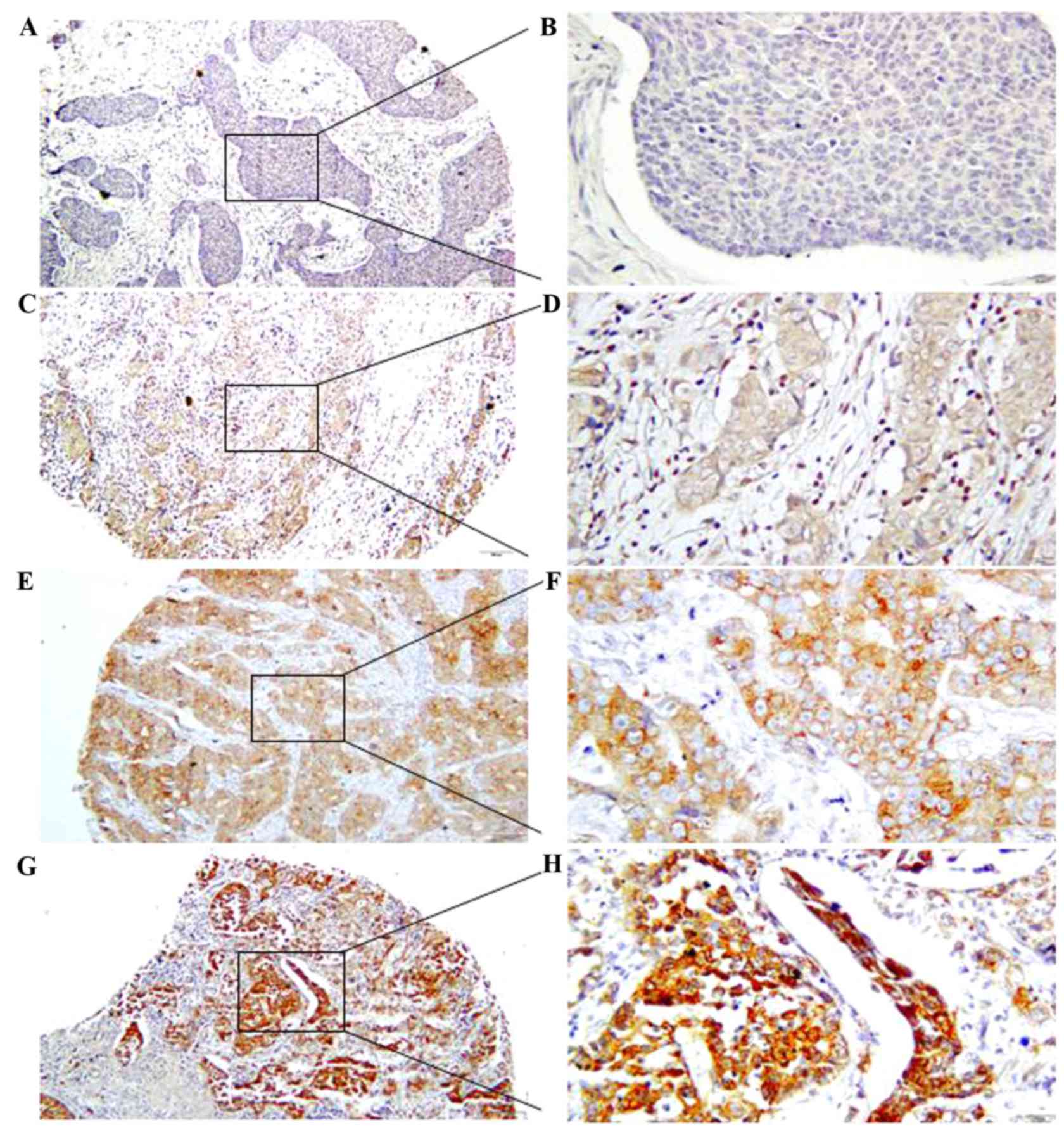
In order to determine the expression levels of B7-H6
protein in breast cancer tissues, IHC analysis was performed
(Fig. 1). This revealed that B7-H6
was present in 216/305 (70.82%) of the breast cancer tissues. B7-H6
was predominantly localized in the membrane and cytoplasm of
breasts tumor cells. In order to investigate the association
between the clinicopathological characteristics of breast cancer
and B7-H6 protein expression, the 305 patients were divided into
two major subgroups according to their amount of positively stained
cells and intensity of B7-H6 staining as follows: Low B7-H6
expression (n=206); and high B7-H6 expression (n=99).
Association between B7-H6 expression
and the clinicopathological characteristics of patients with breast
cancer
The association between the clinicopathological
characteristics of patients with breast cancer and B7-H6 expression
are presented in Table I. This
revealed that B7-H6 expression was significantly correlated with
nodal metastasis (P=0.003); however, it was not associated with the
other clinicopathological parameters, including age, tumor location
and tumor size. Furthermore, B7-H6 expression was significantly
positively correlated with human epidermal growth factor receptor 2
(HER2) expression status (P<0.0001), a marker of metastasis and
predictor of poor prognosis in breast cancer. B7-H6 expression was
significantly negatively correlated with progesterone receptor (PR)
expression status (P=0.0392), a marker of good prognosis in breast
cancer. These results suggest that patients with breast cancer who
have higher B7-H6 expression levels have a higher risk of
metastases and a poorer prognosis.
Association between B7-H6 expression
and post-operative prognosis in patients with breast cancer
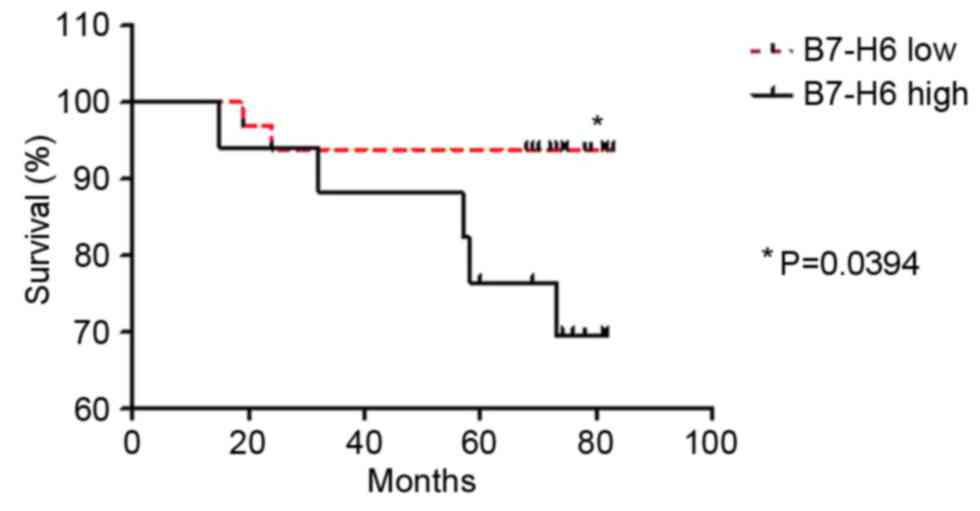
In order to further investigate the prognostic value
of B7-H6 expression in human breast cancer, a log-rank test was
performed. A total of 51 patient overall survival rates and
cumulative survival rate were determined using the Kaplan-Meier
estimator method and analyzed using a log-rank test. The survival
analysis demonstrated that the overall survival rate of the patient
subgroup with low B7-H6 expression was significantly higher
compared with the subgroup with high B7-H6 expression (P=0.0394;
hazard ratio, 0.1941, 95% confidence interval, 0.04076–0.9238;
Fig. 2).
Discussion
The B7 family, and their CD28 receptor family of
costimulatory and coinhibitory molecules, have previously been
demonstrated to serve a potential role in the immune response, and
these molecules have been revealed to be effective diagnostic
markers and therapeutic targets for tumors (16,17). This
was highlighted by the approval of the drug ipilimumab and ongoing
clinical trials of treatments targeting the PD-L1 signaling pathway
(18). B7-H6, a novel B7 family
member, acts as a co-stimulatory ligand that delivers a stimulatory
signal to NK cells through its receptor NKp30 (1). The present study aimed to investigate
the prognostic value of B7-H6 expression in breast cancer and its
potential therapeutic role in tumor adoptive immunotherapy, using
TMAs and IHC.
It has previously been reported that B7-H6 mRNA was
undetectable in a tissue array of 65 normal adult tissues (1), consistent with the absence of the
protein on circulating cells isolated from healthy individuals
(19). In contrast, B7-H6 cell
surface expression has been observed in cell lines from various
tumor types, including lymphoma, leukemia and melanoma (3,14,20). In addition, B7-H6 expression has been
detected in tumor tissues, such as gastrointestinal, lung and
ovarian (1,12–14,21). The
results of the present study demonstrated that B7-H6 was
predominantly localized to the membrane of breast cancer cells in
216/305 (70.82%) of the patients. The pattern of B7-H6 expression,
which appears to be limited to tumor cells, is frequently regarded
as an example of stress-induced self-recognition by NK cells.
B7-H6 was revealed to be a ligand of NKp30 on NK
cells, which delivers an activating signal into NK cells (1). NKp30 consists of a single IgV domain in
its extracellular region, a unique structural feature of the CD28
family (22). Another ligand of NKp30
is HLA-B associated transcript, which is a nuclear protein involved
in the tumor protein p53 signaling pathway and apoptosis induction
following DNA damage-induced cellular stress (23,24). NKp30
was previously revealed to mediate antitumor effects in
gastrointestinal stromal tumors and lymphoid leukemia (25,26).
Numerous previous studies have indicated that the B7-H6-NKp30
signaling pathway is implicated in antitumor activity by
stimulating primary NK cells to produce interleukin-2 and
interferon-γ (IFN-γ), thus enhancing cytotoxic activity (1,21).
The present study investigated the theory that B7-H6
is able to facilitate the elimination of tumor cells by interacting
with its receptor NKp30, thus serving a role in antitumor immunity.
Previous in vitro studies have also suggested that B7-H6 may
be useful for treatment to target tumor cells expressing B7-H6
(3,19,27).
Incubation of lymphoma cells with a fusion protein containing B7-H6
and 7D8, an antibody that recognizes CD20, enhanced NK cell
activation and cytotoxicity in vitro (28). In the present study, the analysis of
305 tissue samples from patients with breast cancer did not suggest
that B7-H6 has an antitumor function. However, the significant
positive correlation identified between B7-H6 expression and lymph
node metastasis indicates that B7-H6 may participate in tumor
progression and development. Furthermore, a significant positive
correlation was identified between B7-H6 protein expression and
HER2 expression status, an important maker of poor breast cancer
prognosis, recurrence and metastasis.
Similarly to other B7 family members, B7-H6 may
exist as a surface/cytosolic molecule in tumor cells and as a
soluble molecule in the peritoneal fluid (21). The soluble from of B7-H6 has been
detected in the serum of patients with malignant melanoma,
neuroblastoma and ovarian carcinoma (3,27,29). Previous studies have identified
elevated levels of soluble B7-H6 in the blood sera of patients with
gram-negative sepsis compared with healthy control individuals
(23,24). Furthermore, the serum concentration of
soluble B7-H6, which was detected by disintegrin and
metalloprotease (ADAM)-10 and ADAM-17, was associated with the
downregulation of NKp30 and inhibited NK cell functions in
vitro by impairing IFN-γ production (3,29). This
indicates that high expression levels of B7-H6 in breast cancer
tissues may contribute to cancer cell immunoevasion.
The present study, to the best of our knowledge,
demonstrated for the first time the clinical significance of B7-H6
expression in breast cancer, and revealed that B7-H6 protein
expression was positively associated with breast cancer metastasis,
supporting the hypothesis that B7-H6 expression is involved in the
progression of human breast cancer. The present study revealed the
possibility that B7-H6 serves a role in tumor immunoevasion and may
be a potential target for tumor therapy in the future.
Acknowledgements
The present study was supported by the Jiangsu
Province University Outstanding Science and Technology Innovation
Team (grant no. 2015023) and the Qinglan Project (2013).
References
|
1
|
Brandt CS, Baratin M, Yi EC, Kennedy J,
Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et
al: The B7 family member B7-H6 is a tumor cell ligand for the
activating natural killer cell receptor NKp30 in humans. J Exp Med.
206:1495–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu X, Narni-Mancinelli E, Cantoni C, Li Y,
Guia S, Gauthier L, Chen Q, Moretta A, Vély F, Eisenstein E, et al:
Structural insights into the inhibitory mechanism of an Antibody
against B7-H6, a stress-induced cellular ligand for the natural
killer cell receptor NKp30. J Mol Biol. 428:4457–4466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pesce S, Tabellini G, Cantoni C, Patrizi
O, Coltrini D, Rampinelli F, Matta J, Vivier E, Moretta A, Parolini
S and Marcenaro E: B7-H6-mediated downregulation of NKp30 in NK
cells contributes to ovarian carcinoma immune escape.
Oncoimmunology. 4:e10012242015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bretscher P and Cohn M: A theory of
self-nonself discrimination. Science. 169:1042–1049. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hurwitz AA, Kwon ED and van Elsas A:
Costimulatory wars: The tumor menace. Curr Opin Immunol.
12:589–596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahoney KM and Atkins MB: Prognostic and
predictive markers for the new immunotherapies. Oncology (Williston
Park). 28 Suppl 3:S39–S48. 2016.
|
|
9
|
Mahoney KM, Freeman GJ and McDermott DF:
The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in
melanoma. Clin Ther. 37:764–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ceeraz S, Nowak EC and Noelle RJ: B7
family checkpoint regulators in immune regulation and disease.
Trends Immunol. 34:556–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matta J, Baratin M, Chiche L, Forel JM,
Cognet C, Thomas G, Farnarier C, Piperoglou C, Papazian L,
Chaussabel D, et al: Induction of B7-H6, a ligand for the natural
killer cell-activating receptor NKp30, in inflammatory conditions.
Blood. 122:394–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Zhang G, Qin Y, Bai R and Huang
J: B7-H6 expression in non-small cell lung cancers. Int J Clin Exp
Pathol. 7:6936–6942. 2014.PubMed/NCBI
|
|
13
|
Chen XJ, Shen J, Zhang GB and Chen WC:
B7-H6 protein expression has no prognostic significance in human
gastric carcinoma. Pathol Oncol Res. 20:203–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Xu Y, Chen L, Xu B, Wu C and Jiang
J: B7-H6 expression correlates with cancer progression and
patient's survival in human ovarian cancer. Int J Clin Exp Pathol.
8:9428–9433. 2015.PubMed/NCBI
|
|
15
|
Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang
J and Wu C: B7-H1 expression associates with tumor invasion and
predicts patient's survival in human esophageal cancer. Int J Clin
Exp Pathol. 7:6015–6023. 2014.PubMed/NCBI
|
|
16
|
Seliger B and Quandt D: The expression,
function, and clinical relevance of B7 family members in cancer.
Cancer Immunol Immunother. 61:1327–1341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J
and Zhang X: T-cell-mediated tumor immune surveillance and
expression of B7 co-inhibitory molecules in cancers of the upper
gastrointestinal tract. Immunol Res. 50:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity and immune correlates
of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaifu T, Escalière B, Gastinel LN, Vivier
E and Baratin M: B7-H6/NKp30 interaction: A mechanism of alerting
NK cells against tumors. Cell Mol Life Sci. 68:3531–3539. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo JG, Guo CC, He ZQ, Liu ZG, Wang Y and
Mou YG: Clinical significance of B7-H6 protein expression in
astrocytoma. Onco Targets Ther. 9:3291–3297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiegler N, Textor S, Arnold A, Rölle A,
Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M and Cerwenka A:
Downregulation of the activating NKp30 ligand B7-H6 by HDAC
inhibitors impairs tumor cell recognition by NK cells. Blood.
122:684–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joyce MG, Tran P, Zhuravleva MA, Jaw J,
Colonna M and Sun PD: Crystal structure of human natural
cytotoxicity receptor NKp30 and identification of its ligand
binding site. Proc Natl Acad Sci USA. 108:pp. 6223–6228. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Strandmann E Pogge, Simhadri VR, von
Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Böll B,
Simhadri VL, Borchmann P, et al: Human leukocyte
antigen-B-associated transcript 3 is released from tumor cells and
engages the NKp30 receptor on natural killer cells. Immunity.
27:965–974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasaki T, Gan EC, Wakeham A, Kornbluth S,
Mak TW and Okada H: HLA-B-associated transcript 3 (Bat3)/Scythe is
essential for p300-mediated acetylation of p53. Genes Dev.
21:848–861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Correia DV, Fogli M, Hudspeth K, da Silva
MG, Mavilio D and Silva-Santos B: Differentiation of human
peripheral blood Vδ11+ T cells expressing the natural cytotoxicity
receptor NKp30 for recognition of lymphoid leukemia cells. Blood.
118:992–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delahaye NF, Rusakiewicz S, Martins I,
Ménard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro
M, et al: Alternatively spliced NKp30 isoforms affect the prognosis
of gastrointestinal stromal tumors. Nat Med. 17:700–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlecker E, Fiegler N, Arnold A, Altevogt
P, Rose-John S, Moldenhauer G, Sucker A, Paschen A, von Strandmann
EP, Textor S and Cerwenka A: Metalloprotease-mediated tumor cell
shedding of B7-H6, the ligand of the natural killer cell-activating
receptor NKp30. Cancer Res. 74:3429–3440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kellner C, Maurer T, Hallack D, Repp R,
van de Winkel JG, Parren PW, Valerius T, Humpe A, Gramatzki M and
Peipp M: Mimicking an induced self phenotype by coating lymphomas
with the NKp30 ligand B7-H6 promotes NK cell cytotoxicity. J
Immunol. 189:5037–5046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Semeraro M, Rusakiewicz S, Minard-Colin V,
Delahaye NF, Enot D, Vély F, Marabelle A, Papoular B, Piperoglou C,
Ponzoni M, et al: Clinical impact of the NKp30/B7-H6 axis in
high-risk neuroblastoma patients. Sci Transl Med. 7:283ra2552015.
View Article : Google Scholar
|