Introduction
Thymic carcinoma (TC) is a rare mediastinal
malignancy with an annual incidence of 0.13 cases/100,000
population (1) and accounts for ~5%
of all thymic epithelial tumors (TETs) (2). TC has a propensity to invade the
surrounding tissues and metastasize, and ~2/3 of all patients with
TC are diagnosed with locally advanced or systemic disease
(3). These aggressive features result
in poor prognoses in the inoperable patients within Japan, with the
5-year survival rate of 24% (4).
Although systemic chemotherapy is considered the
standard of care for patients with advanced thymic carcinoma (ATC),
an optimal regimen has not yet been established due to the disease
rarity. Based on the results of a few small phase II trials and
retrospective studies, combination chemotherapy, such as
carboplatin/paclitaxel (3,5), cisplatin/etoposide (6) and
doxorubicin/cisplatin/vincristine/cyclophosphamide (ADOC) (7,8), is a
treatment option for patients with ATC in clinical practice. These
regimens yield modest efficacy, however the response to
chemotherapy and outcome vary considerably between patients.
Therefore, biomarkers are required that predict the efficacy of
chemotherapy and prognosis in patients with ATC receiving
combination chemotherapy.
Taxane is a microtubule-stabilizing agent used in
the treatment of several malignant tumors. Tubulin heterodimers
consisting of α- and β-tubulin are the basic structural components
of microtubules (9). Despite the
existence of various α- and β-tubulin isotypes, several studies in
different types of tumor have highlighted the association of class
III β-tubulin (TUBB3) expression with resistance to taxane
chemotherapy and poor prognosis (10–22).
However, it remains unknown whether TUBB3 expression correlates
with clinical outcome in patients with ATC.
Etoposide and anthracycline function by targeting
topoisomerase II (topo-II), which serves an essential role during
mitosis by generating transient DNA double-strand breaks and
changing DNA topology and by controlling decatenation checkpoints
and regulating sister chromosome segregation (23). Although previous studies have
demonstrated the implication of topo-II expression in
chemoresistance and poor prognoses in several malignancies
(24–28), the clinical significance of topo-II
expression in patients with ATC remains unknown. Therefore,
immunohistochemical analysis of TUBB3 and topo-II expression was
performed to elucidate whether the level of expression of these
markers correlates with chemoresistance and clinical outcomes in
patients with ATC.
Materials and methods
Patients and clinical outcome
A total of 40 patients with advanced or recurrent TC
receiving combination chemotherapy at three Japanese institutions
(Gunma Prefectural Cancer Center, Gunma University Hospital and
National Hospital Organization Nishigunma Hospital, Gunma, Japan)
were enrolled between April 1998 and April 2014. There were six
patients excluded from the analysis as patient information or tumor
specimen was not available for three, and the other three patients
did not receive combination chemotherapy. As a result, 34 patients
were eligible for the final analysis.
Baseline patient characteristics, data on antitumor
effect of chemotherapy and survival data were retrospectively
collected from the medical records of the enrolled patients. The
clinical stage of each TC case was determined according to the
Masaoka-Koga classification (29).
The histological type was assessed according to the 2004 World
Health Organization histological classification (30). Progression-free survival (PFS) was
calculated from the beginning of the treatment for advanced or
recurrent disease to the date of disease progression or mortality
due to any cause. Similarly, overall survival (OS) was calculated
until the date of mortality or the last follow-up consultation. The
protocol was approved by the institutional review board of each
institution (Gunma Prefectural Cancer Center, Gunma University
Hospital and National Hospital Organization Nishigunma Hospital)
and complied with the Declaration of Helsinki.
Immunohistochemical staining
Tumor specimens were obtained by surgical excision
or biopsy. Immunohistochemical staining procedure has been
previously described (31,32). Antibodies used in the present study
were as follows: TUBB3 mouse monoclonal antibody (cat. no.,
MMS-435P; dilution, 1:100; Covance, Inc., Princeton, NJ, USA),
topo-II rabbit polyclonal antibody (cat. no., ab180393; dilution,
1:100; Abcam, Tokyo, Japan), and MIB-1 mouse monoclonal antibody,
specific for human nuclear antigen Ki-67 (cat. no., M7240; dilution
1:40; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Cells were considered positive for
TUBB3/topo-II/Ki-67 if the staining was present in the cytoplasm or
the nuclei. The proportion of TUBB3/topo-II-positive cells was
assessed by a semi-quantitative scoring method where samples were
assigned a score based on the percentage of positive cells: 1,
≤10%; 2, >10-≤25%; 3, >25-≤50%; 4, >50-≤75%; 5, >75%.
Samples with scores of 1 and 2 were considered to exhibit low
levels of expression, whereas those with scores of 3, 4 and 5 were
considered to exhibit high levels of expression. Expression of
Ki-67 was evaluated using Ki-67 labeling index (KI), which was
defined as the proportion of positive cells among ~1,000 tumor
cells in each sample. As the samples used in the analysis also
included biopsy specimens, only the presence of the staining, but
not the intensity, was considered. Sections were examined by light
microscopy in a blinded fashion by at least two investigators. In
case of discrepancies, the two investigators simultaneously
evaluated the slides until a consensus was reached. The
investigators were blinded to patient outcomes.
Statistical analysis
P<0.05 was considered to indicate a statistically
significant difference. The association between immunohistochemical
staining and patient characteristics was examined using Fisher's
exact test. The correlation between different variables was
analysed using the nonparametric Spearman's rank test. The
Kaplan-Meier method was used to estimate survival, and the survival
differences were analysed by the log-rank test. Multivariate
analyses were performed using the Cox proportional hazards model to
identify independent prognostic factors. Statistical analysis was
performed using GraphPad Prism 6 software (Graph Pad Software,
Inc., La Jolla, CA, USA) and EZR (Saitama Medical Center, Jichi
Medical University, Saitama, Japan) for Windows.
Results
Patient characteristics
Patient characteristics are summarized in Table I. The study included 21 males (68%)
and 13 females (32%) with a median age of 62 years, range, 36–75
years. Prior to the enrollment, 10 patients (29%) experienced
recurrence subsequent to curative resection of the primary tumor.
According to the Masaoka-Koga staging, 5 patients (15%) were stage
III, 11 (32%) were stage IVa, and 18 (53%) were stage IVb. The main
histological types were squamous cell carcinoma (SqCC), 19 patients
(56%) undifferentiated carcinoma, 6 patients (18%) and carcinoid
tumor, 4 patients (12%). A total of 21 patients (62%) received
chemotherapy alone and 13 (38%) received chemoradiotherapy (CRT) as
the initial treatment. The main first-line chemotherapy regimens
included etoposide-based doublet, 14 patients (41%) taxane-based
doublet, 13 patients (38%) and ADOC, 4 patients (12%). Subsequent
to the failure of initial treatment, the two main subsequent
therapies were chemotherapy, 19 patients (56%) and palliative
radiation, 12 patients (35%).
 | Table I.Baseline characteristics of 34
patients with advanced thymic carcinoma treated with
chemotherapy. |
Table I.
Baseline characteristics of 34
patients with advanced thymic carcinoma treated with
chemotherapy.
| Characteristic | Number |
|---|
| Age, median years
(range) | 62 (36–75) |
| Gender,
male/female | 21/13 |
| Smoking,
yes/no | 17/17 |
| Post-operation
recurrence | 10 |
| Stage (Masaoka-Koga
classification), III/IVa/IVb | 5/11/18 |
| Long diameter of
primary | 70 mm |
| tumora, median (range) | (26–120 mm) |
| PS, 0/1/2 | 22/11/1 |
| Metastatic
site |
| Pleural
dissemination | 14 |
|
Pericardial dissemination | 8 |
|
Lung | 12 |
| Lymph
node | 8 |
|
Bone | 3 |
|
Liver | 3 |
|
Others | 4 |
| Histology |
|
Squamous cell carcinoma | 19 |
|
Carcinoid tumor | 4 |
| Poorly
differentiated neuroendocrine carcinoma | 2 |
|
Undifferentiated
carcinoma | 6 |
|
Others | 2 |
|
Unknown | 1 |
| Initial
treatment |
|
Chemoradiotherapy | 13 (seq. 9, conc.
4) |
|
Chemotherapy | 21 |
| First-line
chemotherapy regimen |
|
CBDCA+PTX | 11 |
|
CDDP/CBDCA+DOC | 2 |
|
CDDP/CBDCA+ETP | 14 |
|
ADOC | 4 |
|
Others | 3 |
| Post-progression
therapy |
| Single
agent chemotherapy | 7 |
|
Combination chemotherapy | 12 |
|
Surgery | 2 |
|
Palliative radiation | 12 |
|
Others | 2 |
| Best
supportive care alone | 6 |
Immunohistochemical analysis
Immunohistochemical analysis was performed on
specimens from 24 primary lesions and 10 metastatic lesions. The
expression levels of TUBB3, topo-II and Ki-67 were evaluable in 32,
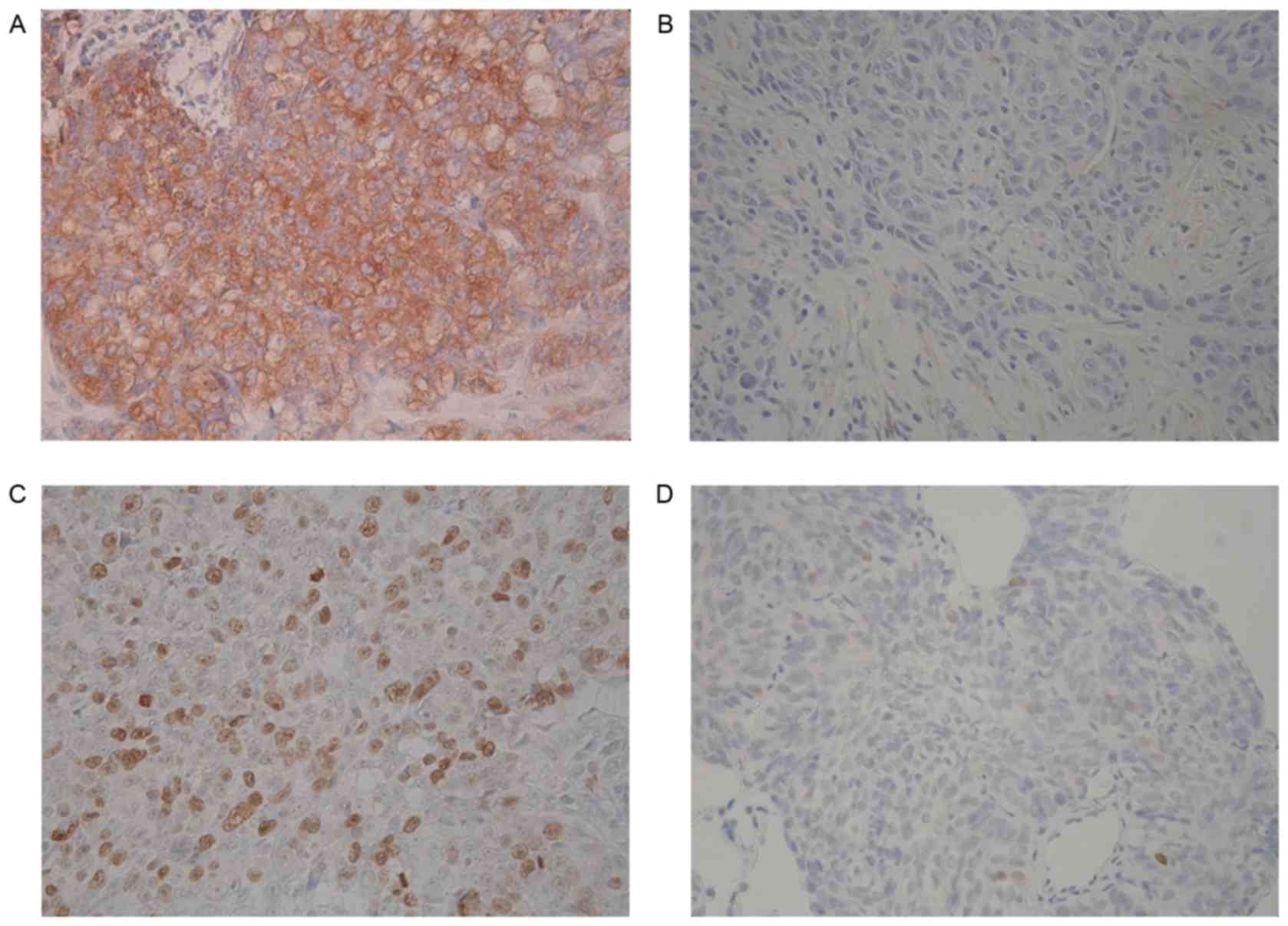
34 and 34 patients, respectively. Fig.
1 demonstrates representative images of TUBB3 and topo-II
staining. KI ranged from 0 to 68% with the median value of 20%, and
the cutoff value was set at 20%. High expression levels of TUBB3,
topo-II and Ki-67 were detected in 20 (62%), 18 (53%) and 17 tumors
(50%), respectively. The mean scores of TUBB3 and topo-II were
2.09±1.28 and 2.59±0.99, respectively.
Patient characteristics according to
the expression level of TUBB3/topo-II
Detailed data on patient characteristics according
to the expression level of TUBB3 and topo-II are summarized in
Table II. Although TUBB3 expression
and any clinicopathological factors were not significantly
associated, topo-II expression significantly correlated with age
and KI (P<0.01 and P=0.02, respectively). Additionally,
Spearman's rank test demonstrated that topo-II expression
positively correlated with age (r=0.57, P<0.01) and KI (r=0.42,
P=0.02).
 | Table II.Patient characteristics according to
biomarkers. |
Table II.
Patient characteristics according to
biomarkers.
|
| TUBB3b | Topo-II |
|---|
|
|
|
|
|---|
| Parameter | High (n=12) | Low (n=20) | P-value | High (n=18) | Low (n=16) | P-value |
|---|
| Age, years |
|
≤61 | 7 | 10 | 0.73 | 5 | 13 |
<0.01c |
|
≥62 | 5 | 10 |
| 13 | 3 |
|
| Gender |
|
Male | 6 | 14 | 0.29 | 13 | 8 | 0.29 |
|
Female | 6 | 6 |
| 5 | 8 |
|
| Smoking status |
|
Smoker | 7 | 10 | 0.73 | 10 | 7 | 0.73 |
|
Non-smoker | 5 | 10 |
| 8 | 9 |
|
| Stage
(Masaoka-Koga) |
|
III | 0 | 5 | 0.13 | 2 | 3 | 0.65 |
| IV | 12 | 15 |
| 16 | 13 |
|
| Histology |
|
Squamous | 4 | 14 | 0.07 | 11 | 8 | 0.73 |
|
Non-squamous | 8 | 6 |
| 7 | 8 |
|
| Tumor size |
| <70
mm | 7 | 11 | 1.00 | 10 | 9 | 1.00 |
| ≥70
mm | 5 | 9 |
| 8 | 7 |
|
| PS |
| 0 | 7 | 13 | 0.72 | 13 | 9 | 0.48 |
|
1/2 | 5 | 7 |
| 5 | 7 |
|
| Initial
treatment |
|
CRT | 3 | 9 | 0.45 | 7 | 6 | 1.00 |
|
Chemotherapy | 9 | 11 |
| 11 | 10 |
|
| Post-progression
chemotherapy |
|
Yes | 7 | 11 | 1.00 | 12 | 7 | 0.30 |
| No | 5 | 9 |
| 6 | 9 |
|
| Ki-67 labeling
index |
|
<20 | 6 | 10 | 1.00 | 5 | 12 | 0.02c |
|
≥20 | 6 | 10 |
| 13 | 4 |
|
| TUBB3a |
|
High | – | – | – | 9 | 3 | 0.08 |
|
Low | – | – |
| 8 | 12 |
|
| Topo-II |
|
High | 9 | 8 | 0.08 | – | – | – |
|
Low | 3 | 12 |
| – | – |
|
Response to first-line
chemotherapy
Overall response rate (ORR) in all 34 patients was
35%. ORRs in patients with high and low TUBB3 expression were 36%
(5 in 14 patients) and 35% (7 in 20 patients), respectively
(P=1.00). In patients with high and low topo-II expression, ORRs
were 39% (7 in 18 patients) and 31% (5 in 16 patients),
respectively (P=0.73). Amongst patients treated with taxanes, ORRs
were 25% (1 in 4 patients) and 29% (2 in 7 patients) in patients
with high and low TUBB3 expression, respectively (P=1.00). Finally,
amongst patients treated with topo-II inhibitors, ORRs in patients
with high and low topo-II expression levels were 56% (5 in 9
patients) and 33% (4 in 12 patients), respectively (P=0.40).
Survival analysis and
clinicopathological factors
During the median follow-up period of 27.5 months,
range, 1.3–119.7 months, 33 patients experienced disease
progression and there were 23 patient mortalities. The median PFS
was 7.4 and the median OS was 37.1 months. The two-five-year OS
rates were 62.5 and 25.3%, respectively. Patients with high TUBB3
expression exhibited a shorter median PFS compared with patients
with low TUBB3 expression, 6.4 vs. 10.5 months, respectively
(P<0.01; Fig. 2A), whilst no
significant difference was observed in the length of median PFS was
observed between patients with high and low topo-II expression, 6.6
vs. 7.7 months, respectively (P=0.31; Fig. 2B). Similarly, patients with high TUBB3
expression exhibited shorter median OS compared with patients with
low TUBB3 expression, 14.4 vs. 52.3 months, respectively (P=0.01;
Fig. 2C), and the median OS in
patients with high and low topo-ii expression was 23.9 and 58.9
months, respectively (P=0.01; Fig.
2D). Amongst patients with initial treatment consisting of
chemotherapy alone, subjects with TUBB3 overexpression tended to
achieve shorter OS compared with those with TUBB3 low expression,
although this difference did not appear statistically significant
(P=0.051), whereas high topo-II expression significantly correlated
with poor OS (P=0.02).
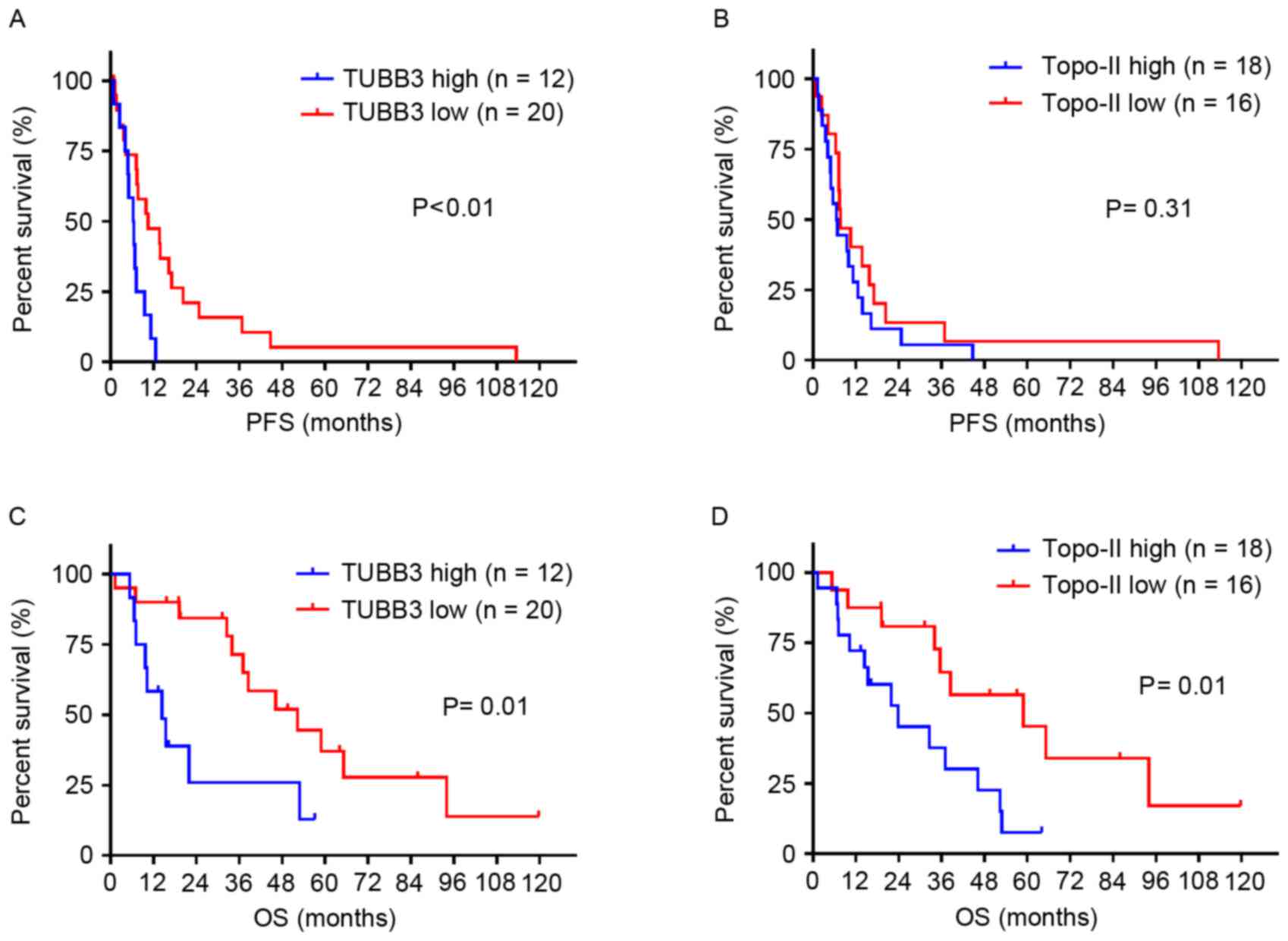 | Figure 2.Survival analysis using the
Kaplan-Meier method. (A) The median PFS for patients with high
TUBB3 expression was significantly shorter compared with patients
with low TUBB3 expression, median, 6.4 vs. 10.5 months,
respectively (HR, 2.44; P<0.01). (B) No significant difference
was observed in median PFS between patients with high and low
topo-II expression, median, 6.6 vs. 7.7 months, respectively (HR,
1.42; P=0.31). (C) Median OS for patients with high TUBB3
expression was significantly shorter compared with patients with
low TUBB3 expression, median, 14.4 vs. 52.3 months, respectively
(HR, 2.73; P=0.01). (D) Median OS for patients with high topo-II
expression was significantly shorter compared with patients with
low topo-II expression, median, 23.9 vs. 58.9 months, respectively
(HR, 2.62; P=0.01). Topo-II, topoisomerase-II; TUBB3, class III
β-tubulin, PFS, progression-free survival; OS, overall survival
rate. |
Univariate analysis demonstrated that older age,
histological type, SqCC vs. others, and high levels of TUBB3 and
topo-II expression were significant prognostic factors associated
with decreased OS (Table III).
Multivariate analysis demonstrated that age (HR, 2.95; P=0.04) and
TUBB3 expression (HR, 3.05; P=0.04) were independent factors for
predicting OS (Table III). High
topo-II expression tended to correlate with poor prognosis without
statistical significance (HR, 2.13; P=0.17; Table III).
 | Table III.Cox regression analysis of OS. |
Table III.
Cox regression analysis of OS.
| A, Univariate
analysis. |
|---|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| Age (≥62 vs. ≤61,
years) | 3.28 | 1.79–10.3 |
<0.01a |
| Gender (male vs.
female) | 0.92 | 0.40–2.15 | 0.85 |
| Smoking (yes vs.
no) | 0.78 | 0.34–1.77 | 0.55 |
| Stage (III vs.
IV) | 0.87 | 0.31–2.43 | 0.79 |
| PS (0 vs. 1/2) | 1.13 | 0.50–2.63 | 0.76 |
| Long diameter of
primary tumor (<70 vs. ≥70 mm) | 1.03 | 0.43–2.44 | 0.95 |
| Initial treatment
(CRT vs. chemotherapy) | 0.42 | 0.18–1.01 | 0.05 |
| Histology (Sq. vs.
others) | 0.38 | 0.14–0.80 | 0.02a |
| Ki-67 labeling
index (≥15 vs. ≤15) | 1.05 | 0.47–2.39 | 0.90 |
| TUBB3 (high vs.
low) | 2.73 | 1.40–11.2 | 0.01a |
| Topo-II (high vs.
low) | 2.62 | 1.31–7.09 | 0.01a |
|
| B, Multivariate
analysis. |
|
| Variables | HR | 95% CI | P-value |
|
| Age (≥62 vs. ≤61,
years) | 2.95 | 1.06–8.21 | 0.04a |
| TUBB3 (high vs.
low) | 3.05 | 1.04–8.90 | 0.04a |
| Topo-II (high vs.
low) | 2.13 | 0.72–6.28 | 0.17 |
Survival analysis according to
treatment and TUBB3/topo-II expression
A survival analysis was conducted in patients with
TUBB3 overexpression who received taxanes (n=12) or topo-II
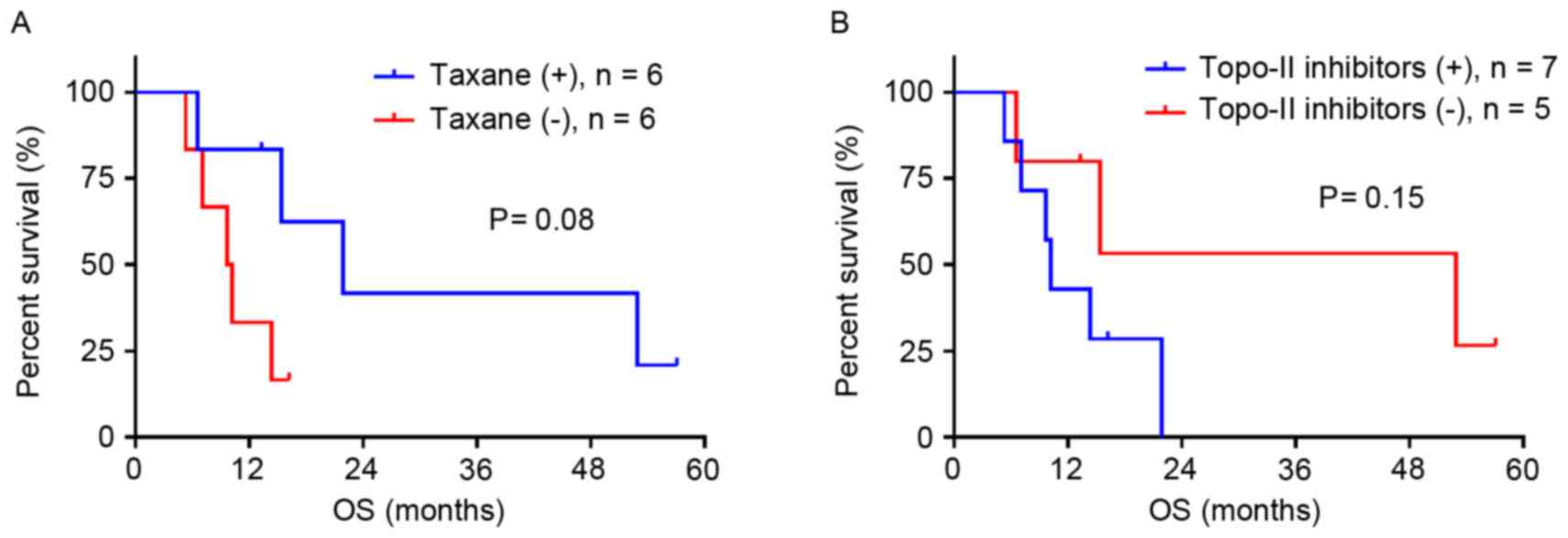
inhibitors (n=12) at any time during the treatment. Taxane
treatment demonstrated a tendency to prolong OS (P=0.08; Fig. 3A), whereas treatment with topo-II
inhibitors tended to shorten OS (P=0.15; Fig. 3B).
A survival analysis was also performed in patients
initially treated with CRT (n=13) or chemotherapy alone (n=21). A
trend toward prolonged OS was observed in all patients treated with
CRT in comparison with those treated with chemotherapy alone
(P=0.05; Fig. 4A). OS did not differ
significantly between patients treated with CRT or chemotherapy
alone with regard to TUBB3 status (low TUBB3, P=0.19; high TUBB3,
P=0.37; Fig. 4B and C, respectively).
In contrast, the survival benefit of CRT over chemotherapy alone
was statistically significant in patients with high topo-II
expression (P=0.01; Fig. 4E), whereas
in patients with low levels of topo-II, OS was equivalent in the
two treatment groups (P=0.32; Fig.
4D).
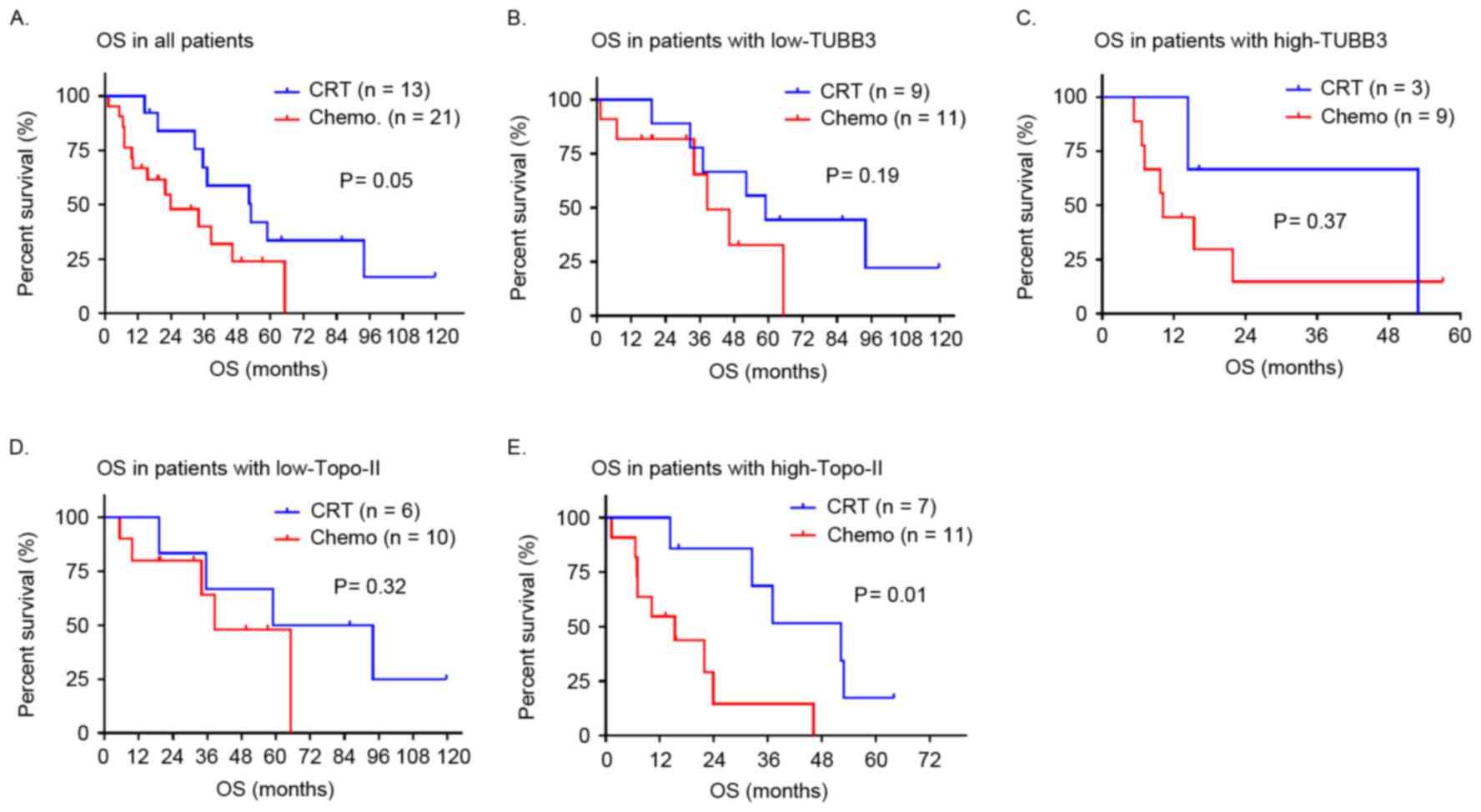 | Figure 4.Kaplan-Meier curves demonstrate OS
according to initial treatment. (A) OS in patients treated with CRT
tended to be longer compared with patients treated with
chemotherapy alone, median OS, 52.9 vs. 23.9 months, respectively
(HR, 0.42; P=0.05). (B and C) There was no significant difference
in OS according to initial treatment between patients with low
TUBB3 expression, median OS 58.9 vs. 38.5 months, respectively (HR,
0.42; P=0.19) and high TUBB3 expression, median OS, 52.9 vs. 10.2
months, respectively (HR, 0.53; P=0.37). (D and E) Among patients
with high topo-II expression, OS in patients treated with CRT was
significantly longer compared with patients treated with
chemotherapy alone, median OS, 52.3 vs. 15.4 months, respectively
(HR, 0.22; P=0.01), whereas no significant difference was observed
in OS according to initial treatment among patients with low
topo-II expression median OS, 76.4 vs. 39.8 months (HR, 0.48;
P=0.32). Topo-II, topoisomerase-II; TUBB3, class III β-tubulin; OS,
overall survival rate. |
Discussion
In the present study, it was revealed that a high
level of TUBB3 expression was a significant predictive marker for
shorter PFS and OS, and a high level of topo-ii expression was also
correlated with poor OS in patients with ATC receiving combination
chemotherapy including taxanes or topo-II inhibitors. Additionally,
it was noted that treatment with taxanes, but not topo-II
inhibitors, tended to prolong OS in patients with TUBB3
overexpression, and a survival advantage of CRT compared with
chemotherapy was detected in patients with topo-II overexpression.
To the best of our knowledge, this is the first study in patients
with ATC to demonstrate the prognostic significance of TUBB3 and
topo-II expression.
A significant association between TUBB3 expression
level and response to taxane-based regimen has been observed in
patients with NSCLC (10–13) and gastric cancer (21,22).
Similar results have been demonstrated in patients with TET
receiving carboplatin/paclitaxel chemotherapy (33). These results indicated that TUBB3
expression may be a potential candidate for a predictive marker of
response to taxane-based chemotherapy. Conversely, the significance
of TUBB3 expression for predicting response to chemotherapy was not
confirmed. Results consistent with those of the present study have
been demonstrated in patients with unresectable ovarian cancer
(14) and advanced NSCLC (15,16).
Therefore, whether TUBB3 expression predicts effectiveness of
taxanes remains debatable. Previous preclinical studies have
indicated that TUBB3 promotes tumorigenesis, metastases, and
anoikis resistance (34–36). Immunohistochemical analysis of TETs
has suggested that TUBB3 overexpression correlates with tumor
aggressiveness, regulation of cell cycle and angiogenesis (33). Thus, TUBB3 may contribute to the
intrinsic aggressiveness of ATC as opposed to the resistance of ATC
to chemotherapy. Validation of the prognostic significance of TUBB3
overexpression and additional investigations of biological
mechanisms underlying clinical effects of TUBB3 overexpression are
required.
The guidelines recommend less toxic
carboplatin/paclitaxel chemotherapy compared with
anthracycline-based regimens (37).
However, conventional anthracycline-based regimens remain
frequently administered as they appear more effective compared with
carboplatin/paclitaxel combinations (3,5,7,8). Notably,
the data of the present study suggest that taxanes, but not topo-II
inhibitors, may improve OS in patients with high TUBB3 expression.
Additionally, Galmarini et al (38) have demonstrated that patients with
advanced breast cancer with high TUBB3 expression exhibited an
improved response to docetaxel compared with to doxorubicin. Thus,
TUBB3 expression may be a useful marker that provides an indication
for taxane-based regimen in patients with ATC. However, as a direct
correlation between the type of treatment and tumor response was
not observed, these results must be interpreted with caution.
Previous in vitro studies (39,40) and
clinical retrospective studies (41–43) have
suggested that an increased level of topo-II may predict a better
response to topo-II inhibitors. In addition, Liu et al
(44) have demonstrated that topo-II
overexpression was significantly correlated with chemosensitivity
in 20 patients with TET receiving anthracycline-based chemotherapy.
In contrast, the data of the present study did not confirm the
significance of topo-II overexpression in predicting responses to
chemotherapy. This discrepancy may be attributed to the fact that,
contrary to the study by Liu et al (44), the present study was restricted to
patients with ATC and included various chemotherapy regimens. In
agreement with the data of the present study, no obvious
association between topo-II expression and response to chemotherapy
was detected in lung cancer (24–26,45).
Instead of predicting chemosensitivity, high levels of topo-II
expression may serve as a specific marker of cell proliferation,
invasive behavior and unfavorable prognosis. Topo-II has been
revealed to be involved in cell proliferation and tumorigenicity in
prostate cancer cells (28). Clinical
retrospective studies have demonstrated that elevated levels of
topo-II were correlated with increased levels of Ki-67 and
metastasis (42,46) and poor clinical outcomes (24–28) in
various types of malignancy. These data support the results of the
present study that topo-II overexpression was significantly
associated with Ki-67 overexpression and unfavorable prognosis. In
accordance with the data of a large-scale randomized trial in
breast cancer (27), the present
study also demonstrated a significant correlation between topo-II
overexpression and old age. This association is consistent with the
results of the previous preclinical studies suggesting that topo-II
may interact with several DNA metabolism proteins associated with
human aging (47). Notably, the
present study demonstrated that old age was also an independent
prognostic factor for shorter OS. These data indicate that topo-II
may confer the propensity to exhibit poor prognoses through a
mechanism involved in aging. However, as the present study did not
confirm this conjecture and did not exclude the possibility that
topo-II overexpression was only a confounding factor, the
prognostic value of topo-II and the association of topo-ii with
Ki-67 and age requires additional investigation in other
studies.
The clinical benefit of radiation in ATC has not
been evaluated in prospective trials, and the clinicopathological
backgrounds in which radiation confers higher effectiveness remain
unclear. The present study indicated that in patients with ATC,
topo-II overexpression may serve as a predictive marker for a
survival advantage of CRT compared with chemotherapy alone. In
agreement with this result, several preclinical studies have
revealed that elevated topo-II expression levels positively
correlate with radiation-induced chromatid breaks and increased
radiosensitivity in various types of cancer cell lines (48–50).
However, additional studies are required to elucidate the role of
topo-II in augmentation of radiosensitivity in ATC.
The present study has several limitations. Firstly,
it is retrospective and possesses a small sample size. However,
notably, the present study is the largest assessing biomarkers for
ATC as such evaluation is difficult in large-scale prospective
studies. Secondly, biopsy specimens, whenever sufficient and
adequate for evaluation, were also included in immunohistochemical
examination. Finally, the inclusion of patients who received CRT as
initial treatment may confound the interpretation of the results.
Therefore, the data from patients initially treated with
chemotherapy alone were separately analyzed. Results consistent
with previous studies were obtained, suggesting that the
overexpression of TUBB3/topo-II serves an important role in
predicting prognoses regardless of the type of initial treatment.
These data propose the prospect of personalized therapy according
to molecular markers, such as TUBB3 and topo-II in ATC. In
addition, the present study hypothesizes that the identification of
TUBB3 and topo-II as the novel prognostic factors may assist in the
development of therapeutic agents and treatment strategies for
patients with ATC possessing unfavorable prognosis.
In conclusion, a significant correlation between
overexpression of TUBB3/topo-II and poor clinical outcomes in
patients with ATC receiving combination chemotherapy including
taxanes or topo-ii inhibitors was demonstrated. Additionally, these
markers may be helpful in determining the optimal chemotherapy
regimen and in selecting patients with the indication for CRT.
Additional validation studies are required to verify the clinical
effect of these markers.
Acknowledgements
The authors would like to thank Dr Akira Mogi,
Department of General Surgical Science, Dr Kimihiro Shimizu,
Department of Thoracic Visceral Organ Surgery and Dr Masataka
Maeno, Department of Medicine and Biological Science, for data
collection. They would also like to express gratitude to all the
staff in the pathology departments of the Gunma University
Hospital, Gunma Prefectural Cancer Center and National Hospital
Organization Nishigunma Hospital for technical assistance in
immunohistochemical analysis. This work was supported in part by
grant no. 26461154 (awarded to Dr Kyoichi Kaira) from the Ministry
of Education, Culture, Sports, Science and Technology, Japan.
References
|
1
|
Kelly RJ, Petrini I, Rajan A, Wang Y and
Giaccone G: Thymic malignancies: From clinical management to
targeted therapies. J Clin Oncol. 29:4820–4827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engels EA and Pfeiffer RM: Malignant
thymoma in the United States: Demographic patterns in incidence and
associations with subsequent malignancies. Int J Cancer.
105:546–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemma GL, Lee JW, Aisner SC, Langer CJ,
Tester WJ, Johnson DH and Loehrer PJ Sr: Phase II study of
carboplatin and paclitaxel in advanced thymoma and thymic
carcinoma. J Clin Oncol. 29:2060–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo K and Monden Y: Therapy for thymic
epithelial tumors: A clinical study of 1,320 patients from Japan.
Ann Thorac Surg. 76:878–885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirai F, Yamanaka T, Taguchi K, Daga H,
Ono A, Tanaka K, Kogure Y, Shimizu J, Kimura T, Fukuoka J, et al: A
multicenter phase II study of carboplatin and paclitaxel for
advanced thymic carcinoma: WJOG4207L. Ann Oncol. 26:363–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giaccone G, Ardizzoni A, Kirkpatrick A,
Clerico M, Sahmoud T and van Zandwijk N: Cisplatin and etoposide
combination chemotherapy for locally advanced or metastatic
thymoma. A phase II study of the European organization for research
and treatment of cancer lung cancer cooperative group. J Clin
Oncol. 14:814–820. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fornasiero A, Daniele O, Ghiotto C, Piazza
M, Fiore-Donati L, Calabró F, Rea F and Fiorentino MV: Chemotherapy
for invasive thymoma. A 13-year experience. Cancer. 68:30–33. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agatsuma T, Koizumi T, Kanda S, Ito M,
Urushihata K, Yamamoto H, Hanaoka M and Kubo K: Combination
chemotherapy with doxorubicin, vincristine, cyclophosphamide, and
platinum compounds for advanced thymic carcinoma. J Thorac Oncol.
6:2130–2134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kavallaris M: Microtubules and resistance
to tubulin-binding agents. Nat Rev Cancer. 10:194–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levallet G, Bergot E, Antoine M, Creveuil
C, Santos AO, Beau-Faller M, de Fraipont F, Brambilla E, Levallet
J, Morin F, et al: High TUBB3 expression, an independent prognostic
marker in patients with early non-small cell lung cancer treated by
preoperative chemotherapy, is regulated by K-Ras signaling pathway.
Mol Cancer Ther. 11:1203–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang CL, Kadota K, Liu D, Ueno M,
Nakasima N, Ishikawa S, Gotoh M, Misaki N, Chang SS and Yokomise H:
Expression of ERCC1 and class III β-tubulin is associated with the
survival of resected stage III non-small cell lung cancer patients
treated with induction chemoradiotherapy using carboplatin-taxane.
Exp Ther Med. 1:445–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sève P and Dumontet C: Is class III
beta-tubulin a predictive factor in patients receiving
tubulin-binding agents? Lancet Oncol. 9:168–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaira K, Takahashi T, Murakami H, Shukuya
T, Kenmotsu H, Ono A, Naito T, Tsuya A, Nakamura Y, Endo M, et al:
The role of βIII-tubulin in non-small cell lung cancer patients
treated by taxane-based chemotherapy. Int J Clin Oncol. 18:371–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrandina G, Zannoni GF, Martinelli E,
Paglia A, Gallotta V, Mozzetti S, Scambia G and Ferlini C: Class
III beta-tubulin overexpression is a marker of poor clinical
outcome in advanced ovarian cancer patients. Clin Cancer Res.
12:2774–2779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vilmar AC, Santoni-Rugiu E and Sørensen
JB: Class III β-tubulin in advanced NSCLC of adenocarcinoma subtype
predicts superior outcome in a randomized trial. Clin Cancer Res.
17:5205–5214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edelman MJ, Schneider CP, Tsai CM, Kim HT,
Quoix E, Luft AV, Kaleta R, Mukhopadhyay P, Trifan OC, Whitaker L
and Reck M: Randomized phase II study of ixabepilone or paclitaxel
plus carboplatin in patients with non-small-cell lung cancer
prospectively stratified by beta-3 tubulin status. J Clin Oncol.
31:1990–1996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roque DM, Buza N, Glasgow M, Bellone S,
Bortolomai I, Gasparrini S, Cocco E, Ratner E, Silasi DA, Azodi M,
et al: Class III β-tubulin overexpression within the tumor
microenvironment is a prognostic biomarker for poor overall
survival in ovarian cancer patients treated with neoadjuvant
carboplatin/paclitaxel. Clin Exp Metastasis. 31:101–110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roque DM, Bellone S, English DP, Buza N,
Cocco E, Gasparrini S, Bortolomai I, Ratner E, Silasi DA, Azodi M,
et al: Tubulin-β-III overexpression by uterine serous carcinomas is
a marker for poor overall survival after platinum/taxane
chemotherapy and sensitivity to epothilones. Cancer. 119:2582–2592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasegawa S, Miyoshi Y, Egawa C, Ishitobi
M, Taguchi T, Tamaki Y, Monden M and Noguchi S: Prediction of
response to docetaxel by quantitative analysis of class I and III
beta-tubulin isotype mRNA expression in human breast cancers. Clin
Cancer Res. 9:2992–2997. 2003.PubMed/NCBI
|
|
20
|
Paradiso A, Mangia A, Chiriatti A, Tommasi
S, Zito A, Latorre A, Schittulli F and Lorusso V: Biomarkers
predictive for clinical efficacy of taxol-based chemotherapy in
advanced breast cancer. Ann Oncol. 16 Suppl 4:iv14–iv19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang JE, Hong JY, Kim K, Kim SH, Choi WY,
Kim MJ, Jung SH, Shim HJ, Bae WK, Hwang EC, et al: Class III
β-tubulin is a predictive marker for taxane-based chemotherapy in
recurrent and metastatic gastric cancer. BMC Cancer. 13:4312013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Gao J, Lu Z, Gong J, Li Y, Dong B,
Li Z, Zhang X and Shen L: Combination of microtubule associated
protein-tau and β-tubulin III predicts chemosensitivity of
paclitaxel in patients with advanced gastric cancer. Eur J Cancer.
50:2328–2335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nitiss JL: DNA topoisomerase II and its
growing repertoire of biological functions. Nat Rev Cancer.
9:327–337. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dingemans AM, Witlox MA, Stallaert RA, van
der Valk P, Postmus PE and Giaccone G: Expression of DNA
topoisomerase IIalpha and topoisomerase IIbeta genes predicts
survival and response to chemotherapy in patients with small cell
lung cancer. Clin Cancer Res. 5:2048–2058. 1999.PubMed/NCBI
|
|
25
|
Ceppi P, Longo M, Volante M, Novello S,
Cappia S, Bacillo E, Selvaggi G, Saviozzi S, Calogero R, Papotti M
and Scagliotti GV: Excision repair cross complementing-1 and
topoisomerase IIalpha gene expression in small-cell lung cancer
patients treated with platinum and etoposide: A retrospective
study. J Thorac Oncol. 3:583–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dingemans AC, van Ark-Otte J, Span S,
Scagliotti GV, van der Valk P, Postmus PE and Giaccone G:
Topoisomerase IIalpha and other drug resistance markers in advanced
non-small cell lung cancer. Lung Cancer. 32:117–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knoop AS, Knudsen H, Balslev E, Rasmussen
BB, Overgaard J, Nielsen KV, Schonau A, Gunnarsdóttir K, Olsen KE,
Mouridsen H, et al: Retrospective analysis of topoisomerase IIa
amplifications and deletions as predictive markers in primary
breast cancer patients randomly assigned to cyclophosphamide,
methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and
fluorouracil: Danish breast cancer cooperative group. J Clin Oncol.
23:7483–7490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Liu Y, Chen W, Fang Y, Xu H, Zhu HH,
Chu M, Li W, Zhuang G and Gao WQ: TOP2Ahigh is the phenotype of
recurrence and metastasis whereas TOP2Aneg cells represent cancer
stem cells in prostate cancer. Oncotarget. 5:9498–9513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masaoka A: Staging system of thymoma. J
Thorac Oncol. 5 10 Suppl 4:S304–S312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Travis W, Brambilla W, Müller-Hermelink H
and Harris C: Chapter 3. Tumors of the thymus. World health
organization classification of tumorsPathology and genetics of
tumors of the lung, pleura, thymus and heart. IARC press; 3rd.
Lyon: 2004
|
|
31
|
Shimizu K, Kaira K, Tomizawa Y, Sunaga N,
Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M,
et al: ASC amino-acid transporter 2 (ASCT2) as a novel prognostic
marker in non-small cell lung cancer. Br J Cancer. 110:2030–2039.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buck AC, Schirrmeister HH, Guhlmann CA,
Diederichs CG, Shen C, Buchmann I, Kotzerke J, Birk D, Mattfeldt T
and Reske SN: Ki-67 immunostaining in pancreatic cancer and chronic
active pancreatitis: Does in vivo FDG uptake correlate with
proliferative activity? J Nucl Med. 42:721–725. 2001.PubMed/NCBI
|
|
33
|
Kaira K, Serizawa M, Koh Y, Miura S, Kaira
R, Abe M, Nakagawa K, Ohde Y, Okumura T, Naito T, et al: Expression
of excision repair cross-complementation group 1, breast cancer
susceptibility 1, and β III-tubulin in thymic epithelial tumors. J
Thorac Oncol. 6:606–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCarroll JA, Gan PP, Liu M and Kavallaris
M: betaIII-tubulin is a multifunctional protein involved in drug
sensitivity and tumorigenesis in non-small cell lung cancer. Cancer
Res. 70:4995–5003. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCarroll JA, Gan PP, Erlich RB, Liu M,
Dwarte T, Sagnella SS, Akerfeldt MC, Yang L, Parker AL, Chang MH,
et al: TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis
to promote tumorigenesis and anoikis resistance in non-small cell
lung cancer. Cancer Res. 75:415–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCarroll JA, Sharbeen G, Liu J, Youkhana
J, Goldstein D, McCarthy N, Limbri LF, Dischl D, Ceyhan GO, Erkan
M, et al: βIII-tubulin: A novel mediator of chemoresistance and
metastases in pancreatic cancer. Oncotarget. 6:2235–2249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
National Comprehensive Cancer Network
(NCCN), . NCCN clinical practice guidelines in oncology. Thymomas
and thymic carcinomas version 1. 2015.https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
|
|
38
|
Galmarini CM, Treilleux I, Cardoso F,
Bernard-Marty C, Durbecq V, Gancberg D, Bissery MC, Paesmans M,
Larsimont D, Piccart MJ, et al: Class III beta-tubulin isotype
predicts response in advanced breast cancer patients randomly
treated either with single-agent doxorubicin or docetaxel. Clin
Cancer Res. 14:4511–4516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen T, Sun Y, Ji P, Kopetz S and Zhang W:
Topoisomerase IIα in chromosome instability and personalized cancer
therapy. Oncogene. 34:4019–4031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Burgess DJ, Doles J, Zender L, Xue W, Ma
B, McCombie WR, Hannon GJ, Lowe SW and Hemann MT: Topoisomerase
levels determine chemotherapy response in vitro and in vivo. Proc
Natl Acad Sci USA. 105:pp. 9053–9058. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Desmedt C, Di Leo A, de Azambuja E,
Larsimont D, Haibe-Kains B, Selleslags J, Delaloge S, Duhem C,
Kains JP, Carly B, et al: Multifactorial approach to predicting
resistance to anthracyclines. J Clin Oncol. 29:1578–1586. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tinari N, Lattanzio R, Natoli C,
Cianchetti E, Angelucci D, Ricevuto E, Ficorella C, Marchetti P,
Alberti S, Piantelli M and Iacobelli S: Changes of topoisomerase
IIalpha expression in breast tumors after neoadjuvant chemotherapy
predicts relapse-free survival. Clin Cancer Res. 12:1501–1506.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferrandina G, Petrillo M, Carbone A,
Zannoni G, Martinelli E, Prisco M, Pignata S, Breda E, Savarese A
and Scambia G: Prognostic role of topoisomerase-IIalpha in advanced
ovarian cancer patients. Br J Cancer. 98:1910–1915. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu JM, Wang LS, Huang MH, Hsu WH, Yen SH,
Shiau CY, Li AF, Tiu CM, Tseng SW and Huang BS: Topoisomerase
2alpha plays a pivotal role in the tumor biology of stage IV thymic
neoplasia. Cancer. 109:502–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsu C, Kuo SH, Hu FC, Cheng AL, Shih JY,
Yu CJ, Lin CC, Huang TC, Yang PC and Yang CH: Gemcitabine plus
conventional-dose epirubicin versus gemcitabine plus cisplatin as
first-line chemotherapy for stage IIIB/IV non-small cell lung
carcinoma-a randomized phase II trial. Lung Cancer. 62:334–343.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wong N, Yeo W, Wong WL, Wong NL, Chan KY,
Mo FK, Koh J, Chan SL, Chan AT, Lai PB, et al: TOP2A overexpression
in hepatocellular carcinoma correlates with early age onset,
shorter patients survival and chemoresistance. Int J Cancer.
124:644–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pichierri P, Franchitto A, Mosesso P, de
Santis L Proietti, Balajee AS and Palitti F: Werner's syndrome
lymphoblastoid cells are hypersensitive to topoisomerase II
inhibitors in the G2 phase of the cell cycle. Mutat Res.
459:123–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Terry SY, Riches AC and Bryant PE:
Suppression of topoisomerase IIalpha expression and function in
human cells decreases chromosomal radiosensitivity. Mutat Res.
663:40–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bryant PE, Riches AC, Shovman O, Dewar JA
and Adamson DJ: Topoisomerase IIα levels and G2 radiosensitivity in
T-lymphocytes of women presenting with breast cancer. Mutagenesis.
27:737–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim JS, Kim SY, Lee M, Kim SH, Kim SM and
Kim EJ: Radioresistance in a human laryngeal squamous cell
carcinoma cell line is associated with DNA methylation changes and
topoisomerase IIα. Cancer Biol Ther. 16:558–566. 2015. View Article : Google Scholar : PubMed/NCBI
|