Introduction
Patinopecten yessoensis, a species of
scallop, is a marine bivalve mollusk from the pectinidae
family. It is a cold-tolerant species that predominately inhabits
coastal waters of the northern Korean peninsula and islands of
Japan. In traditional East Asian medicine, the flesh of scallops
was used as a drug for the treatment of diabetes, pollakisuria, and
indigestion (1,2).
Breast cancer is an escalating global public health
concern associated with a high mortality rate (3). Breast cancer was predicted to account
for 232,670 (29%) of all new cancer cases and cause 40,000 (15%) of
all cancer-related mortalities of women in the US during 2014
(4).
Chemoprevention has received increasing attention as
an approach for breast cancer prevention. It entails the use of
natural or synthetic antioxidants to prevent or delay cancer
progression (5). The G1 phase of the
cell cycle is regulated by a balance of factors, including the
critical regulatory components, cyclin D, cyclin E,
cyclin-dependent kinases (Cdks) and cyclin-Cdk inhibitor proteins
(6). Cell cycle progression and Cdk
activity are inhibited by Cdk inhibitors (CKIs), including p21 and
p18, which inactivate Cdk-cyclin complexes to inhibit cell
proliferation (7,8). The p53 tumor suppressor protein is a
major regulator of cell cycle progression during G1 phase, as its
activation results in the upregulation of p21 (9–11).
Apoptosis is the process of programmed cell death,
which is critical for the homeostasis of multicellular organisms
(12). In addition, it may eliminate
malignant tumor cells without eliciting damage to normal cells
(13). A variety of diseases,
including cancer, may be triggered by abnormalities in apoptosis.
Apoptosis may be induced via two major pathways: The extrinsic
(death receptor) pathway and the intrinsic (mitochondrial) pathway
(14,15).
Mollusks are a rich reservoir of natural bioactive
compounds, which may possess antitumor, antioxidant, and
immunomodulatory activities (16);
shellfish proteins are considered a major potential resource for
the development of antitumor drugs (17). In particular, Sasaki et al
(18) identified that the
glycoprotein fraction from Patinopecten yessoensis extracts
exhibited an antitumor activity in mice. However, the action and
mechanism of scallop flesh extract (SE) on MCF-7 human breast
cancer cells have yet to be elucidated. Therefore, the present
study examined the antiproliferative effects of SE on MCF-7 cells.
The results demonstrated that SE inhibited cell proliferation by
cell cycle arrest at the G0/G1 phase, leading to apoptosis.
Materials and methods
Chemicals and antibodies
Antibodies to Bcl-2 and p53 were purchased from EMD
Millipore (Billerica, MA, USA). An anti-p21 antibody was obtained
from BD Biosciences (San Jose, CA, USA). Antibodies directed
against Actin (cat. no. sc-58673), Bcl-2 associated X (Bax) (cat.
no. sc-7480), Cdk2 (cat. no. sc-70829), and Cdk4 (cat. no.
sc-136241) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The antibodies for cleaved caspase-8 (cat. no.
8592) and −9 (cat. no. 7237), procaspase-3 (cat. no. 12742), poly
(ADP-ribose)-polymerase (PARP) (cat. no. 9532), cleaved-PARP (cat.
no. 5625), cyclin D1 (cat. no. 2922), cyclin E1 (cat. no. 20808),
cytochrome c (cat. no. 4272), and Fas-associated via death domain
(FADD) (cat. no. 2782) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-51625) was
obtained from Santa Cruz Biotechnology, Inc. DAPI, propidium iodide
(PI), MTT, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA),
and all other chemicals were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany).
Preparation of SE
Mature scallops were captured from the sea near the
East Sea Fisheries Research Institute (Gangneung, South Korea).
Extraction was performed using a standard extraction process:
Briefly, 50 g of scallop flesh was immersed in 1:l methanol,
sonicated for 30 min and allowed to stand for 48 h. The obtained
extract was filtered through No. 20 Whatman filter paper (GE
Healthcare Life Sciences, Chalfont, UK), evaporated under reduced
pressure using a vacuum evaporator (Eyela; Tokyo Rikakikai Co.,
Ltd., Tokyo, Japan) and lyophilized using a freeze dryer (Labconco,
Kansas City, MO, USA). Finally, 2.31 g of lyophilized powder was
obtained (yield, 4.62%). A sample of the lyophilized powder was
deposited at the Division of Pharmacology, School of Korean
Medicine, Pusan National University, Korea (deposition no.
MH2013-006).
Gas chromatographic analysis of fatty
acids in scallop flesh
A set of standards containing 37 mixtures of fatty
acid methyl esters from Supelco (Sigma-Aldrich; Merck KGaA,) were
prepared for analysis by dissolving in isooctane to a concentration
of 100 mg/ml. The total lipid in scallop flesh (10 g) was extracted
with a Soxhlet extractor and 200 ml of ether. The extracted lipid
(25 mg) was saponified with 2 ml of methanolic NaOH (0.5 M)
solution by refluxing 5 min at 100°C. Once cooled to room
temperature, 2 ml of 14% boron trifluoride-methanol solution was
added and the sample was boiled for 2 min. The sample was again
cooled to room temperature and 1 ml of isooctane was added. Sodium
sulfate (1 g) was added and the mixture was agitated to eliminate
residual water. The upper isooctane layer was used for gas
chromatography analysis. EPA and DHA concentrations were determined
by gas chromatography (GC-2010, Shimadzu Corporation, Kyoto, Japan)
equipped with a flame ionization detector (FID) and a SP-2560
capillary column (100 m length, 0.25 mm inner diameter, 0.22 µm
film thickness; Sigma-Aldrich, Merck KGaA). The operating
conditions were as follows: The initial temperature was 100°C,
which was increased by 3°C per min until the temperature was 240°C.
The detector and injector temperatures were 250 and 210°C,
respectively. DHA and EPA were identified by comparing their
retention time with a mixture of standard fatty acids.
Cell culture
MCF-7 human breast cancer cells were purchased from
the Korean Cell Line Bank (Seoul, Korea). The cells were cultured
in Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.), and maintained in a humidified incubator with 5%
CO2 at 37°C.
MTT assay
In order to determine cytotoxic concentrations of
SE, MCF-7 human breast cancer cells were plated in a 96-well plate
at a density of 2.5×104 cells per well. The cells were
incubated for 24 h, followed by treatment with 0.5, 1 or 2 mg/ml of
SE. The cells were then incubated for the next 24 h at 37°C in an
incubator with 5% CO2. Following the incubation of the
cells, viable cells were stained with 0.5 mg/ml MTT for 4 h at
37°C. The medium was removed from the cells, and formazan crystals
produced in the wells were dissolved with the addition of 200 µl
dimethylsulfoxide. Absorbance was measured at 595 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell viability was defined as relative to untreated control
cells.
DAPI staining
MCF-7 human breast cancer cells were grown on
96-well plates for 24 h, then treated with SE (0.5, 1 or 2 mg/ml)
for 24 h as described by Zhang et al (19) with minor modifications. The treated
cells were washed with PBS and fixed with 1% paraformaldehyde
solution for 20 min at room temperature. The solution was
eliminated and the fixed cells were washed with PBS, followed by
incubation with DAPI solution for 10 min at room temperature.
Fluorescence images were observed in a dark room using a confocal
microscope (LSM700; Carl Zeiss AG, Oberkochen, Germany).
Annexin V and PI staining
Detection of apoptotic cells was performed using a
Muse™ Annexin V and Dead Cell kit (EMD Millipore).
Briefly, the plated MCF-7 human breast cancer cells were treated
with SE as previously described. Suspended cells were collected and
adherent cells were trypsinized. The cells were harvested through
centrifugation at 500 × g for 5 min at 4°C. After washing with PBS,
the cells were resuspended in the binding buffer and stained with
Annexin V and PI. The proportion of apoptotic cells was quantified
using FACS (FACSCanto II, BD Biosciences, Franklin Lakes, NJ,
USA).
Flow cytometry analysis of the cell cycle. MCF-7
human breast cancer cells were seeded in 100 mm dishes. Following
24 h of SE exposure, the cells were trypsinized, harvested through
centrifugation (500 × g, 5 min, 4°C) and fixed in 70% ethanol. The
cells were resuspended and incubated with RNase (250 µg/ml, final
concentration) for 30 min and stained with PI (10 µg/ml, final
concentration) for 1 h. Flow cytometry was performed with FACS, and
analysis was performed with the associated CellQuestPro software
(BD Biosciences).
Western blot analysis
SE-treated MCF-7 human breast cancer cells were
collected by centrifugation (500 × g, 5 min, 4°C) and washed once
with PBS. Washed cell pellets were resuspended in extraction lysis
buffer [50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(pH 7.0), 250 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40, 1 mM
phenylmethane sulfonyl fluoride, 0.5 mM dithiothreitol, 5 mM NaF
and 0.5 mM sodium orthovanadate] containing 5 µg/ml each of
leupeptin and aprotinin and incubated for 20 min at 4°C.
Microcentrifugation (16,000 × g, 10 min, 4°C) was performed for
removal of cell debris, followed by rapid freezing of the
supernatants. A protein assay reagent (Bio-Rad Protein Assay kit
II; catalog no. 5000002; Bio-Rad Laboratories, Inc.) was used to
determine protein concentration, according to the manufacturer's
protocol. Total cellular protein from treated or untreated cell
extracts (30 µg) was separated with 10% SDS-PAGE and electroblotted
onto nitrocellulose membranes, followed by incubation overnight
with blocking solution (5% skimmed milk) at 4°C, and then with a
primary antibody (1:1,000 dilution) for 2 h. Blots were then washed
three times with Tween 20/Tris-buffered saline (TTBS), incubated
with a 1:1,000 dilution of horseradish peroxidase-conjugated
secondary antibody for 1 h at room temperature and washed three
times with TTBS again. Enhanced chemiluminescence (ECL) western
blotting detection reagents (GE Healthcare Life Sciences, Chalfont,
UK) were used for the development of blots. The bands were
visualized by WesternBright ECL HRP substrate (Advansta, Inc. Menlo
Park, CA, USA) and developed by Kodak film (Kodak, Rochester, NY,
USA). Quantification of band densities was performed using ImageJ
software (version 1.6.0_20; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Experiments were performed independently ≥3 times
and values were expressed as the mean ± standard deviation.
Multiple comparison tests were performed for different dose groups.
The Levene's test was used for examination of variance homogeneity.
If the Levene's test indicated no significant deviations from
variance homogeneity, the obtained data were analyzed using an
independent t-test and a one way analysis of variance test followed
by the least-significant differences multi-comparison test to
determine which pairs of the group comparison were significantly
different. Statistical analysis was performed using SPSS software
(version 14.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
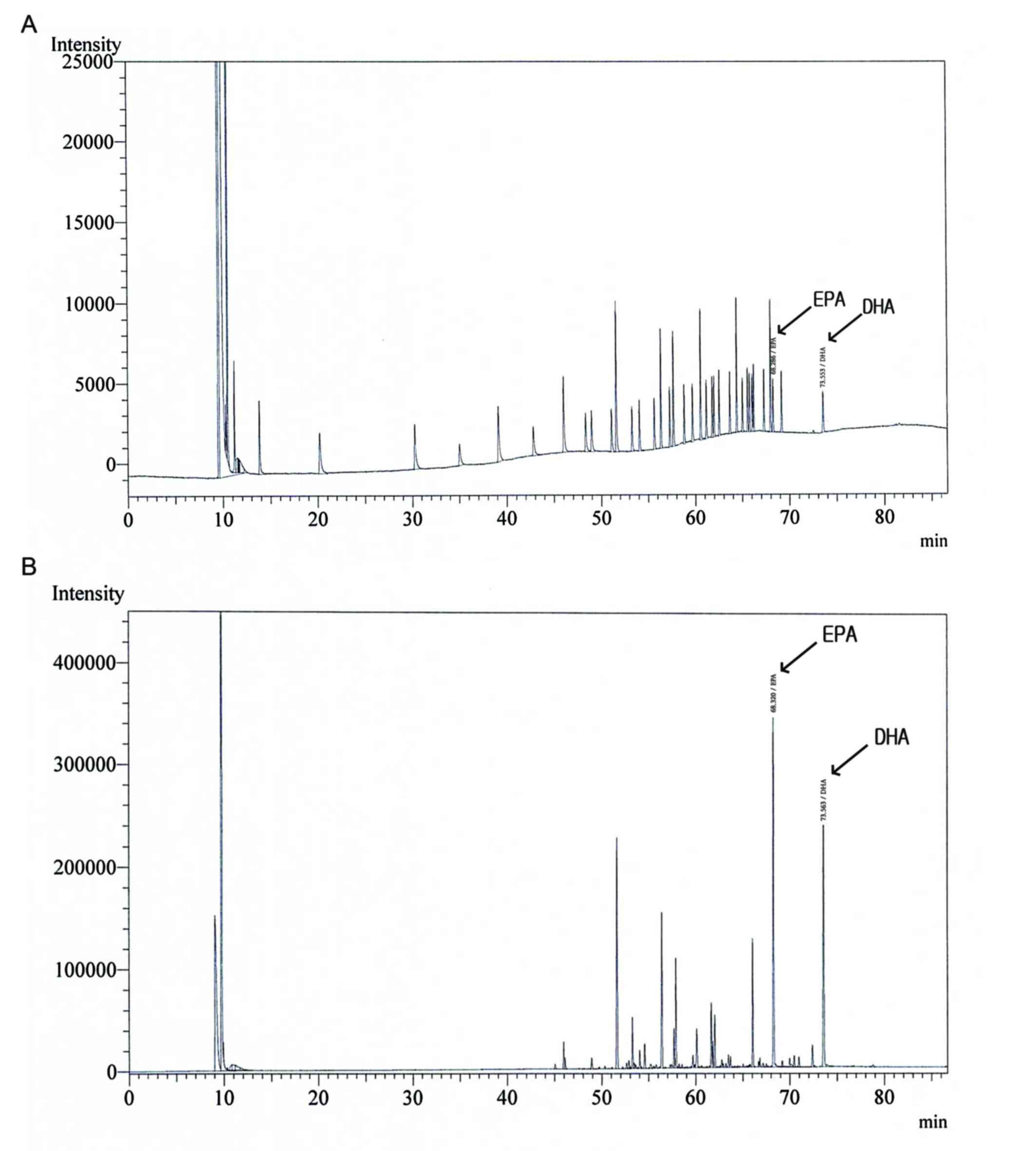
Gas chromatography analysis of SE
The profiles of DHA and EPA in SE were determined
using gas chromatography (Fig. 1 and
Table I). Validation with a set of 37
standards verified the reliability and stability of the method, and
use of the method resulted in successive separation of DHA and EPA
in SE samples.
 | Table I.Content of DHA and EPA in SE samples
analyzed by gas chromatography (n=3). |
Table I.
Content of DHA and EPA in SE samples
analyzed by gas chromatography (n=3).
| ω-3 polyunsaturated
fatty acid | Content (mg/100
g) |
|---|
| DHA |
665.2±0.01 |
| EPA | 1,177.1±0.06 |
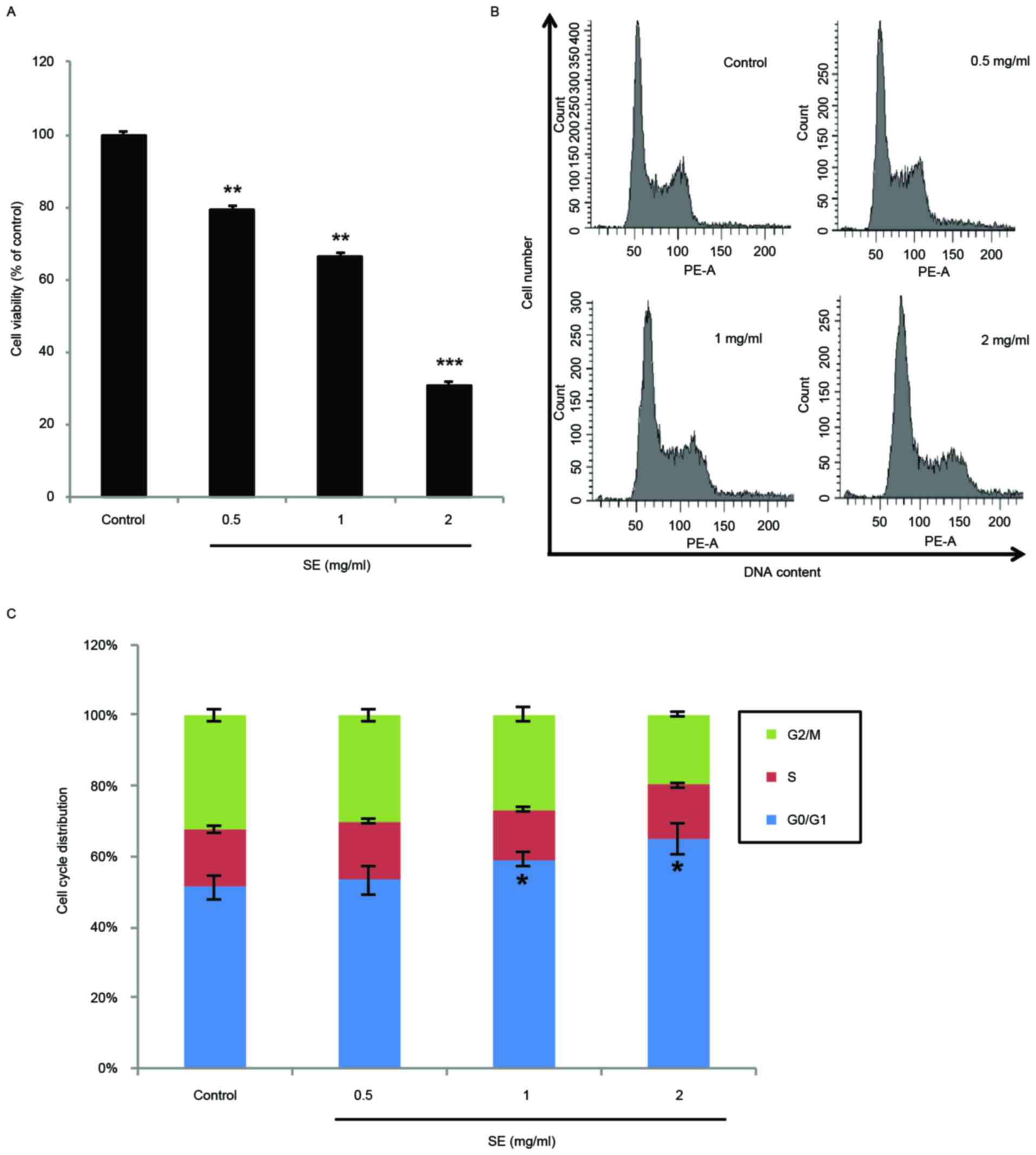
Effects of SE on MCF-7 cell
proliferation and cell cycle arrest
The antiproliferative effects of SE on human breast
cancer were investigated in the MCF-7 breast cancer cell line. As
demonstrated by the data in Fig. 2A,
SE significantly inhibited the proliferation of MCF-7 cells in a
dose-dependent manner (0.5 and 1 mg/ml SE, P<0.01; 2 mg/ml SE,
P<0.001). In addition, the DNA contents of MCF-7 cells treated
with SE were examined using flow cytometry cell cycle distribution
analysis. Treatment with SE induced a dose-dependent accumulation
of MCF-7 cells in the G0/G1 phase. In particular, the accumulation
of cells in the G0/G1 phase was significantly increased at the
concentrations of 1 and 2 mg/ml, compared with the control group (1
and 2 mg/ml SE, P<0.05; Fig. 2B and
C).
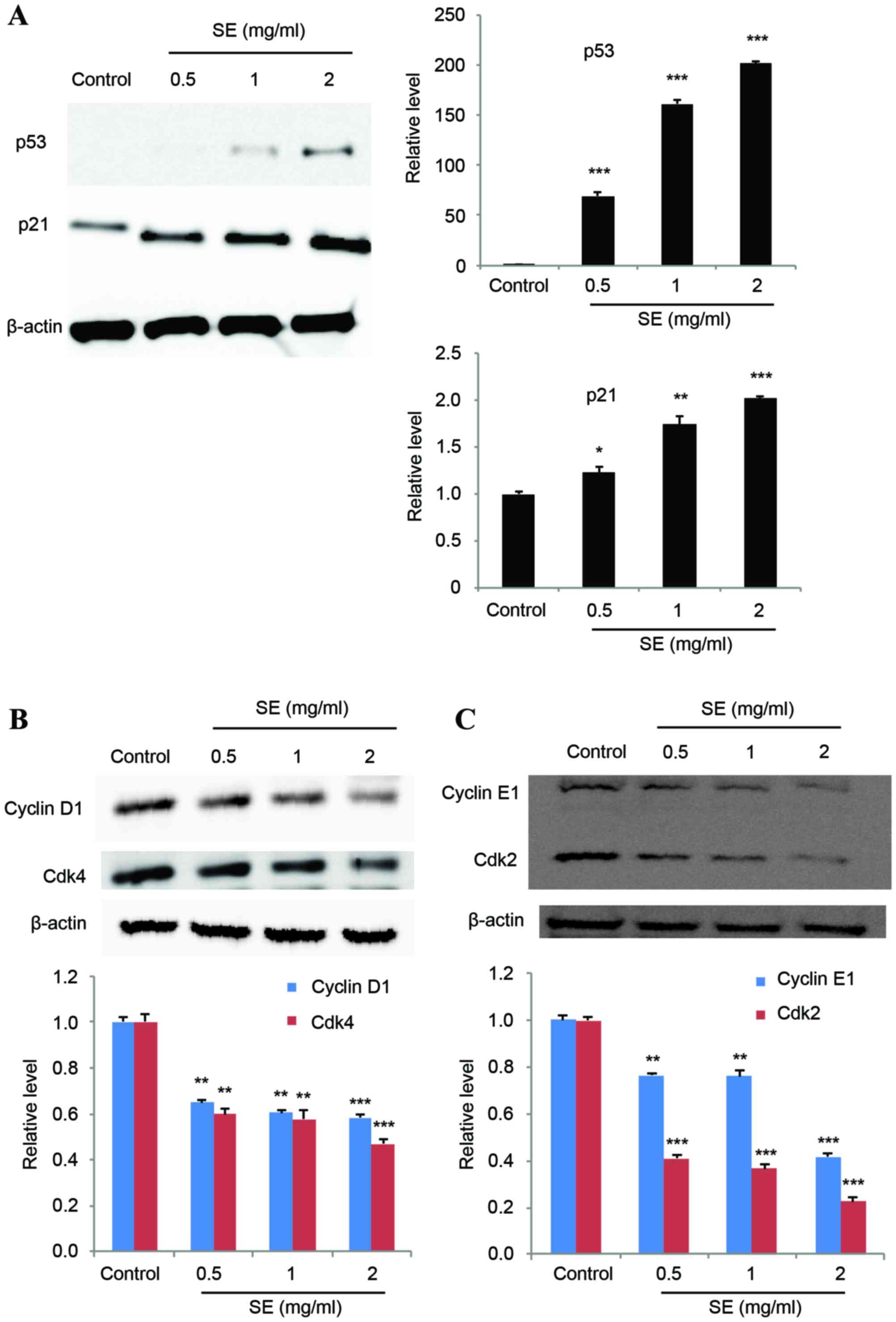
Effect of SE on proteins involved in
the cell cycle
The expression levels of p53 and p21 were
investigated to determine their potential association with SE
treatment. The results of a western blot assay showed that p53
expression was increasingly induced with increasing concentrations
of SE, and p21 expression was correspondingly increased (p53: 0.5,
1 and 2 mg/ml SE, P<0.001; p21: 0.5 mg/ml SE, P<0.05; 1 mg/ml
SE, P<0.01; 2 mg/ml SE, P<0.001; Fig. 3A). In addition, treatment with SE
resulted in a significantly decreased expression of cyclins D1 and
E1, and Cdks 2 and 4, in a dose-dependent manner, compared with the
control group (cyclins D1: 0.5 and 1 mg/ml SE, P<0.01; 2 mg/ml
SE, P<0.001; cyclins E1: 0.5 and 1 mg/ml SE, P<0.01; 2 mg/ml
SE, P<0.001; Cdk 4: 0.5 and 1 mg/ml SE, P<0.01; 2 mg/ml SE,
P<0.001; Cdk 2: 0.5, 1 and 2 mg/ml SE, P<0.001; Fig. 3B and C).
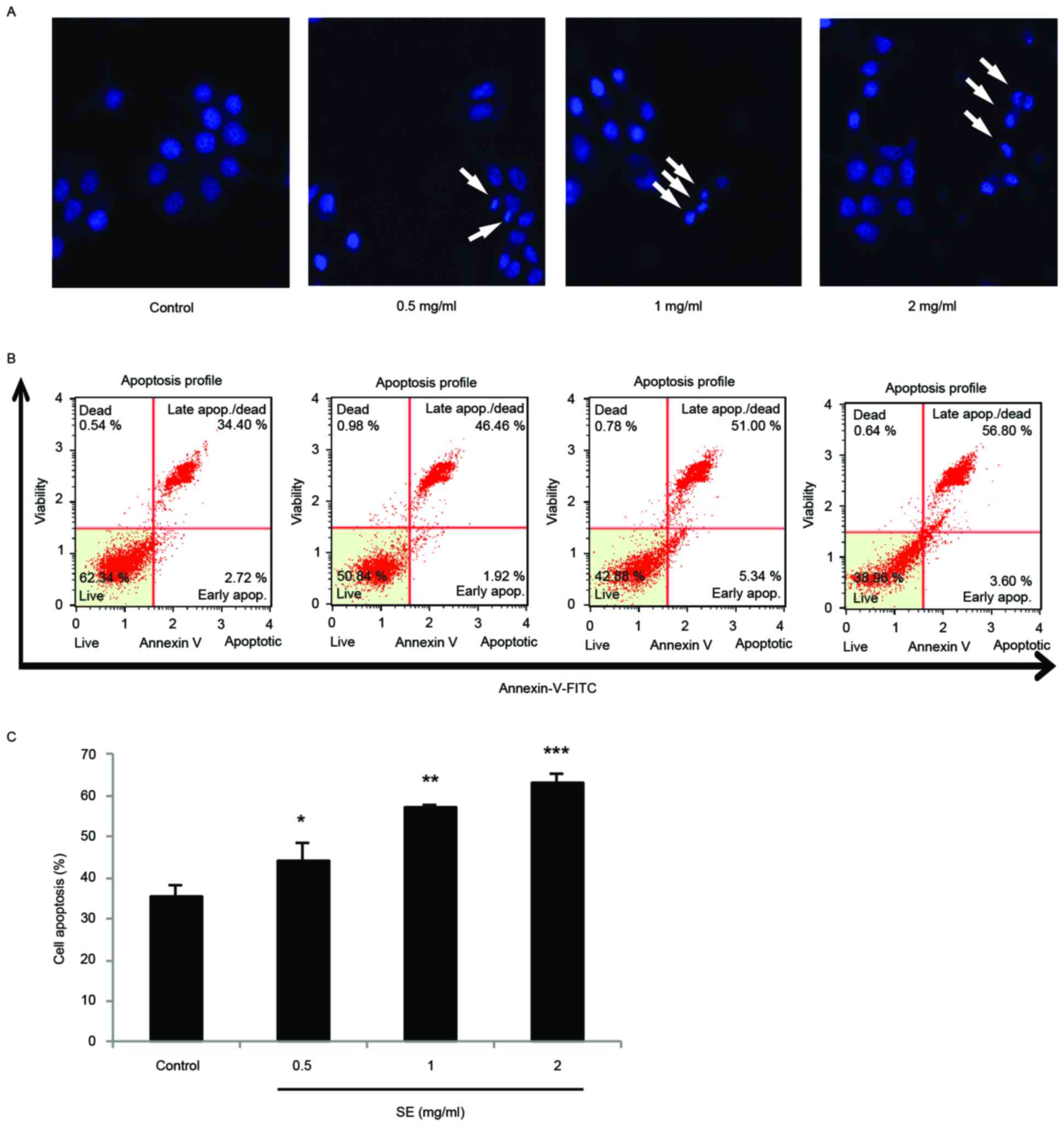
Apoptosis is induced by SE in MCF-7
cells
DAPI staining and a confocal microscope were used to
examine the morphological changes of MCF-7 cells treated with SE.
The staining showed fragmented and condensed chromatin,
characteristic of apoptotic cell death (Fig. 4A). In addition, in a flow cytometry
experiment with Annexin V/PI staining, there were significant
increases to the proportion of apoptotic cells at increasing
concentrations of SE treatment (0.5 mg/ml SE, P<0.05; 1 mg/ml
SE, P<0.01; 2 mg/ml SE, P<0.001; Fig. 4B and C).
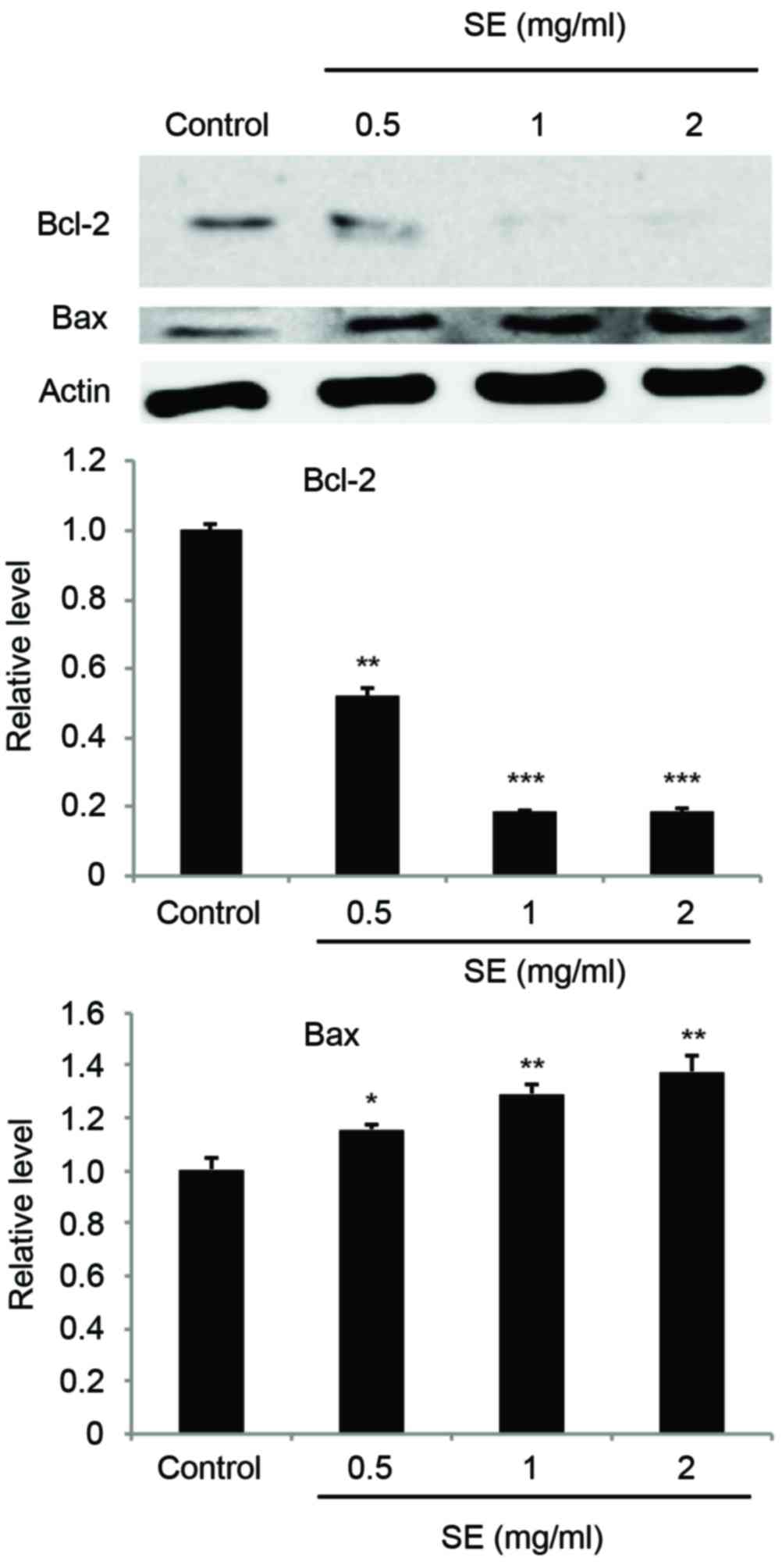
Regulation of Bcl-2 and Bax expression
caused by SE in MCF-7 cells
Expression of Bcl-2 and Bax was examined using a
western blot assay. The results indicated that treatment with SE
suppressed the expression of Bcl-2, an anti-apoptotic protein, and
increased the expression level of Bax, a pro-apoptotic protein, in
a dose-dependent manner (Bcl-2: 0.5 mg/ml SE, P<0.01; 1 and 2
mg/ml SE, P<0.001; Bax: 0.5 mg/ml SE, P<0.05; 1 and 2 mg/ml
SE; P<0.01; Fig. 5).
Effects of SE on the expression levels
of FADD, caspases, PARP, and cytochrome c in MCF-7 cells
The expression of FADD was increased in a
dose-dependent manner as compared with the control group (0.5 mg/ml
SE, P<0.05; 1 and 2 mg/ml SE, P<0.01; Fig. 6A). In addition, involvement of
caspases in apoptosis induction of SE was evaluated. The expression
of procaspase-3, cleaved caspase-8, and cleaved caspase-9 was also
examined by a western blot assay (Fig. 6A
and B). Our results showed that treatment with SE resulted in a
significantly decreased expression of procaspase-3 (0.5 and 1 mg/ml
SE, P<0.01; 2 mg/ml SE, P<0.001; Fig. 6A) and an increased expression of
cleaved caspase-8, cleaved caspase-9, and cleaved PARP in a
dose-dependent manner as compared with the control group (cleaved
caspase-8: 0.5 and 1 mg/ml SE, P<0.01; 2 mg/ml SE, P<0.001;
cleaved caspase-9: 0.5 mg/ml SE, P<0.05; 1 mg/ml SE, P<0.01;
2 mg/ml SE, P<0.001; cleaved PARP: 0.5 and 1 mg/ml SE,
P<0.05; 2 mg/ml SE, P<0.001; Fig.
6A and B). Treatment with SE resulted in the activation of
caspase-3, −8, and −9, as demonstrated by the increase in cleaved
caspase-3, −8, and −9 levels, leading to a significant increase in
the levels of cleaved PARP in MCF-7 cells. Furthermore, the
expression of cytochrome c was analyzed to examine whether
cytochrome c had been released from mitochondrial membrane.
The results indicated that the amount of cytochrome c was
significantly increased by SE, in a dose-dependent manner, compared
with the control group (cytochrome c: 0.5 and 1 mg/ml SE,
P<0.01; 2 mg/ml SE, P<0.001; Fig.
6B).
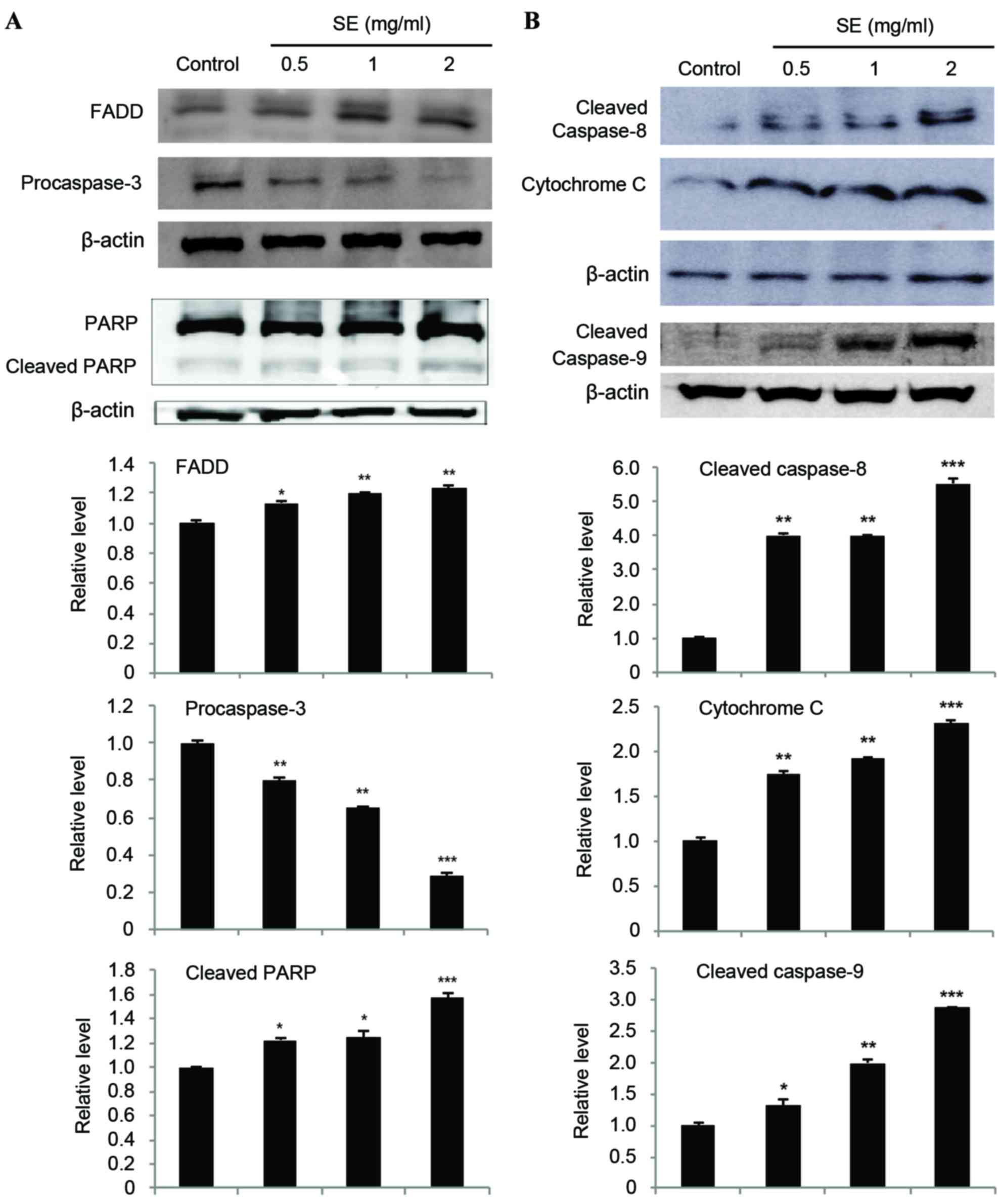 | Figure 6.(A and B) Effects of SE on the
expression of FADD, caspases, PARP, and cytochrome c in
MCF-7 cells. Cells were treated with the indicated concentrations
of SE for 24 h. Western blot analysis was performed for
determination of protein levels of FADD, caspases, PARP, and
cytochrome c. β-actin was used as a loading control. The
blots included in the fig. are representative of three blots
yielding similar results. The relative levels (each protein vs.
β-actin) were measured via densitometry. Data present the mean ±
standard deviation from three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. control group. SE, scallop flesh
extract; FADD, Fas-associated via death domain; PARP, poly
(ADP-ribose) polymerase. |
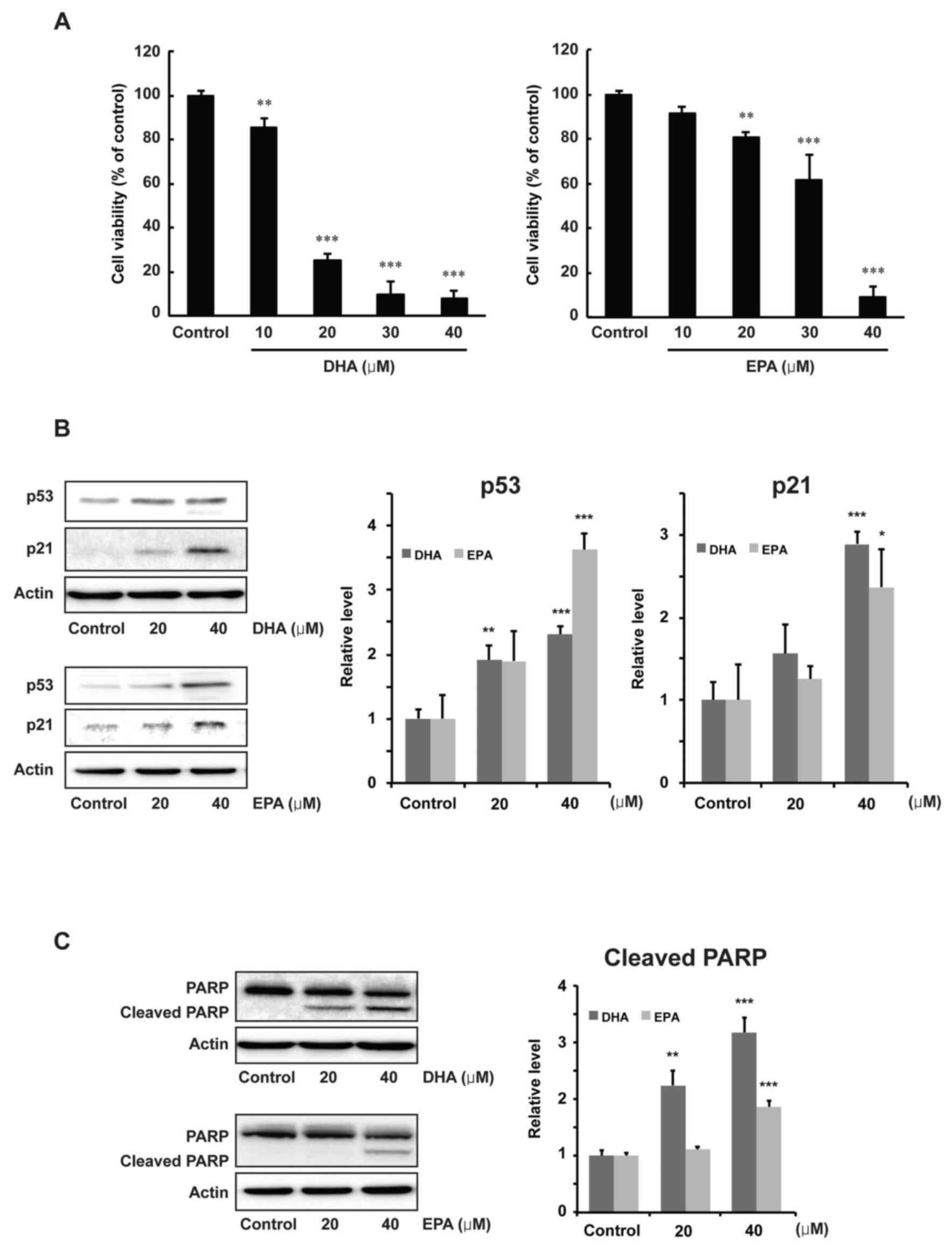
Effects of DHA and EPA on
proliferation and protein expression levels of p53, p21 and PARP in
MCF-7 cells
The antiproliferative effects of DHA and EPA on
human breast cancer were examined by using MCF-7 cells. As shown in
Fig. 7A, DHA or EPA significantly
inhibited the proliferation of MCF-7 cells, in a dose-dependent
manner, at the concentrations of 20, 30 and 40 µM compared with the
control group (20, 30 and 40 µM DHA, P<0.001; 20 µM EPA,
P<0.01; 30 and 40 µM EPA, P<0.001). Alterations to the
expression of p53, p21 and PARP were then examined to determine the
regulatory effects of DHA or EPA. The results of a western blot
analysis indicated that p53 was significantly induced by a 40-µM
concentration of DHA or EPA (P<0.001). p21 was also
significantly increased at a 40-µM concentration of DHA or EPA
compared with the control group (P<0.001 for DHA; P<0.05 for
EPA, Fig. 7B). Moreover, the level of
cleaved PARP was also significantly increased at 40 µM of DHA or
EPA compared with the control group (P<0.001, Fig. 7C).
Discussion
In traditional East Asian medicine, the scallop
flesh was commonly used as a drug for the treatment of diabetes,
pollakisuria, and indigestion (1).
Shellfish proteins are also considered a potential resource for
antitumor drug development (17).
However, the molecular action and mechanism of inhibitory effects
of SE on MCF-7 human breast cancer cells have not been elucidated.
Therefore, the present study investigated the antiproliferative
effects of SE on MCF-7 cells.
Cancerous cells commonly exhibit a high rate of
growth, due to deregulation of the apoptosis and cell cycle
pathways (8). Accordingly, the
induction of cell cycle arrest is considered to be a therapeutic
target in cancer (20–22). The present study has demonstrated that
SE significantly inhibited the proliferation of MCF-7 cells in a
dose- dependent manner. In addition, treatment with SE induced a
dose-dependent accumulation of MCF-7 cells in the G0/G1 phase of
the cell cycle. In particular, the accumulation of cells was
significantly increased compared with the control group at the
concentrations of 1 and 2 mg/ml. These results suggest that the
inhibitory effect of SE on MCF-7 breast cancer cell proliferation
may be associated with the induction of cell cycle arrest in the
G0/G1 phase.
The p53 tumor suppressor gene serves a major role in
mediating the response of cells to diverse stressors by repressing
or inducing various genes involved in apoptosis, cell cycle arrest,
and DNA repair (10,23–26). In
addition, p53 induces the transcription of a variety of genes,
including p21, an important CKI and regulator of the cell cycle.
The induction of p21 results in the inhibition of cyclin-Cdk
complexes and cell cycle arrest (7,8,20,27,28). The
cyclin-Cdks complexes control cell cycle progression; their
inactivation causes cell cycle arrest (20). The results from the present study
revealed that p53 was induced, and p21 was correspondingly
increased, following treatment with SE in a dose-dependent manner.
Furthermore, treatment with SE resulted in a significant,
dose-dependent decrease in the expression of cyclins D1 and E1, and
Cdks 2 and 4, compared with the control group. These data indicate
that treatment with SE affected G0/G1 cell cycle checkpoints via
these proteins to cause a block to cell cycle progression.
Caspases, cysteine-class aspartyl-specific
proteases, are indicated as inactive precursors (procaspases) that
require proteolytic cleavage for activation (29–33). In
apoptosis, this process proceeds via activation of the intrinsic
mitochondrial pathway or the extrinsic death receptor pathway
(34–36). The intrinsic pathway involves the
regulation of Bcl-2 family members (including Bcl-2 and Bax), the
release of cytochrome c and the subsequent activation of
caspases (including caspase-3, −7 and −9) (37–40). The
extrinsic pathway involves transmembrane death receptors that bind
to pro-apoptotic ligands and cause the subsequent activation of
caspases (including caspase-3, −7 and −8) (40,41). The
results of the present study demonstrated that treatment with SE
suppressed the expression of Bcl-2 (an anti-apoptotic protein) and
increased the expression levels of Bax (a pro-apoptotic protein) in
a dose-dependent manner. Furthermore, the amount of cytochrome
c was significantly increased by SE in a dose-dependent
manner. The decrease in Bcl-2, the increase in Bax and the release
of cytochrome c from the mitochondria into the cytosol may
be caused by SE-induced intrinsic apoptosis. Further results from
the present study indicated that treatment with SE significantly
decreased the expression of procaspase-3, and increased the
expression of cleaved caspase-8 and −9, FADD, and cleaved PARP in a
dose-dependent manner. These results indicate that treatment with
SE results in activation of caspase-3, −8, and −9, leading to a
significant increase in the level of cleaved PARP in MCF-7 cells.
Taken together, the results of the present study suggest that
intrinsic and extrinsic pathways of apoptosis may be associated
with the antiproliferative effects of SE on MCF-7 cells.
A number of studies have also reported on anticancer
agents from the flesh of marine organisms (42–44).
Previous studies have particularly focused on the antitumor effects
of DHA and EPA (45–49). Dietary ω-3 fatty acids have exhibited
significant tumor-suppressing effects; DHA has been identified as
the primary tumor-suppressing fatty acid (50). ω-3 polyunsaturated fatty acids (DHA
and EPA) may inhibit breast cancer growth via the activation of a
neutral sphingomyelinase-mediated pathway and DHA has been
demonstrated to synergistically enhance the cytotoxic activities of
docetaxel in cancer cells via an increase in apoptosis (51,52). EPA
was also previously demonstrated to inhibit the liver metastasis of
mouse MC-26 colorectal cancer cells injected into a mouse model
through the inhibition of PGE2-dependent cell motility
(53). The data of the present study
demonstrated that DHA or EPA significantly inhibited the
proliferation of MCF-7 cells in a dose-dependent manner at the
concentrations 20, 30 and 40 µM, compared with the control group. A
western blot analysis revealed that p53 and p21 were significantly
induced at a 40-µM concentration of DHA or EPA compared with the
control group. In addition, the expression of cleaved PARP was also
significantly increased at a 40-µM concentration of DHA or EPA
compared with the control group. By utilizing gas chromatographic
analysis, the present study has demonstrated that DHA and EPA are
the most prominent markers of SE. Taking all of the data together,
we hypothesize that the antiproliferative effect of SE on MCF-7
human breast cancer cells is caused by DHA and EPA.
In conclusion, the results demonstrate that SE
induced a growth inhibition of MCF-7 human breast cancer cells, in
a dose-dependent manner, by inducing G0/G1 phase arrest. In
addition, the cell cycle arrest was associated with the
upregulation of p53 and p21, and the downregulation of G1
phase-associated cyclin D1-Cdk 4 and cyclin E1-Cdk2 complexes.
SE-mediated cell cycle arrest was also linked with promotion of
apoptosis, as demonstrated by the expression of apoptosis-related
proteins and changes to nuclear morphology. SE apparently induced
the mitochondrial apoptotic cascade, as indicated by the decreased
expression of Bcl-2, activation of Bax, release of cytochrome
c, decrease in procaspase-3, and subsequent increase in
cleaved-PARP. The expression levels of FADD and cleaved caspase-8,
proteins associated with the extrinsic pathway of apoptosis, were
also increased in a SE dose-dependent manner. Taken together, the
data suggest that the intrinsic and extrinsic pathways are
associated with the antiproliferative effects of SE on MCF-7 cells.
Thus, SE may be a beneficial candidate for use in the treatment and
prevention of human breast cancer. Further studies are to be
conducted to confirm the anticancer effects of SE in
vivo.
Acknowledgements
This study was supported by the Research Program
grant funded by the National Fisheries Research and Development
Institute of Korea (grant no. R2017002) and by the National
Research Foundation of Korea grant from the Korean government
(grant no. 2012 R1A5A2A42671316).
References
|
1
|
Xiao PG: Atlas of Chinese Herbs. The
Commercial Press Ltd.; Hong Kong: 7. pp. 2021990
|
|
2
|
Nam M, Lee C, Moon TS and Huh MK: Genetic
diversity and population structure of the scallop patinopecten
yessoensis in Korea, China and Japan by random amplified
polymorphic DNA markers. J Life Sci. 22:466–471. 2012. View Article : Google Scholar
|
|
3
|
Saad ED: Endpoints in advanced breast
cancer: Methodological aspects & clinical implications. Indian
J Med Res. 134:413–418. 2011.PubMed/NCBI
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsao AS, Kim ES and Hong WK:
Chemoprevention of cancer. CA Cancer J Clin. 54:150–180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunter T and Pines J: Cyclins and cancer
II Cyclin D and CDK inhibitors come of age. Cell. 79:573–582. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harper JW and Elledge SJ: CDK inhibitors
in development and cancer. Curr Opin Genet Dev. 6:56–64. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
10
|
Meek DW: The p53 response to DNA damage.
DNA Repair (Amst). 3:1049–1056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weng MS, Ho YS and Lin JK: Chrysin induces
G1 phase cell cycle arrest in C6 glioma cells through inducing
p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated
protein kinase. Biochem Pharmacol. 69:1815–1827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muppidi J, Porter M and Siegel RM:
Measurement of apoptosis and other forms of cell death. Curr Protoc
Immunol. 3:3–17. 2004.
|
|
13
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MacKenzie SH and Clark AC: Targeting cell
death in tumors by activating caspases. Curr Cancer Drug Targets.
8:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Repicky A, Jantova S and Milata V: Signal
pathways of cell proliferation and death as targets of potential
chemotherapeutics. Ceska Slov Farm. 57:4–10. 2008.(In Slovak).
PubMed/NCBI
|
|
16
|
Ireland CM, Copp BR, Foster MP, et al:
Biomedical potential of marine natural productsPharmaceutical and
bioactive natural products. Attaway DH and Zaborsky OR: Springer
US; New York, USA: 1. pp. 1–43. 1993, View Article : Google Scholar
|
|
17
|
Lv S, Gao J, Liu T, Zhu J, Xu J, Song L,
Liang J and Yu R: Purification and partial characterization of a
new antitumor protein from Tegillarca granosa. Mar Drugs.
13:1466–1480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasaki T, Uchida H, Uchida NA, et al:
Antitumor activity and immunomodulatory effect of glycoprotein
fraction from scallop Patinopecten yessoensis. Nippon Suisan
Gakkaishi. 53:267–272. 1987. View Article : Google Scholar
|
|
19
|
Zhang H, Wang K, Lin G and Zhao Z:
Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer
therapy. BMC Complement Altern Med. 14:2702014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagle AA, Gan FF, Jones G, So CL, Wells G
and Chew EH: Induction of tumor cell death through targeting
tubulin and evoking dysregulation of cell cycle regulatory proteins
by multifunctional cinnamaldehydes. PLoS One. 7:e501252012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bellamy CO: p53 and apoptosis. Br Med
Bull. 53:522–538. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Didenko VV, Wang X, Yang L and Hornsby PJ:
Expression of p21(WAF1/CIP1/SDI1) and p53 in apoptotic cells in the
adrenal cortex and induction by ischemia/reperfusion injury. J Clin
Invest. 97:1723–1731. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Zhang HW, Hu R, Yang Y, Qi Q, Lu
N, Liu W, Chu YY, You QD and Guo QL: Wogonin induces G1 phase
arrest through inhibiting CDK4 and cyclin D1 concomitant with an
elevation in p21Cip1 in human cervical carcinoma HeLa cells.
Biochem Cell Biol. 87:933–942. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zorn JA, Wolan DW, Agard NJ and Wells JA:
Fibrils colocalize caspase-3 with procaspase-3 to foster
maturation. J Biol Chem. 287:33781–33795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Donepudi M and Grütter MG: Structure and
zymogen activation of caspases. Biophys Chem 101–102. 145–153.
2002. View Article : Google Scholar
|
|
31
|
Stennicke HR and Salvesen GS: Caspases.
Controlling intracellular signals by protease zymogen activation.
Biochim Biophys Acta. 1477:299–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gray DC, Mahrus S and Wells JA: Activation
of specific apoptotic caspases with an engineered small
molecule-activated protease. Cell. 142:637–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Putt KS, Chen GW, Pearson JM, Sandhorst
JS, Hoagland MS, Kwon JT, Hwang SK, Jin H, Churchwell MI, Cho MH,
et al: Small-molecule activation of procaspase-3 to caspase-3 as a
personalized anticancer strategy. Nat Chem Biol. 2:543–550. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salvesen GS and Riedl SJ: Caspase
mechanisms. Adv Exp Med Biol. 615:13–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Wang LJ, Qiu GF, Yu JQ, Liang SC and
Hu XM: Apoptosis of Hela cells induced by extract from
Cremanthodium humile. Food Chem Toxicol. 45:2040–2046. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schwartsmann G, da Rocha A Brondani,
Berlinck RG and Jimeno J: Marine organisms as a source of new
anticancer agents. Lancet Oncol. 2:221–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mitsiades CS, Ocio EM, Pandiella A, Maiso
P, Gajate C, Garayoa M, Vilanova D, Montero JC, Mitsiades N,
McMullan CJ, et al: Aplidin, a marine organism-derived compound
with potent antimyeloma activity in vitro and in vivo. Cancer Res.
68:5216–5225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Suarez-Jimenez GM, Burgos-Hernandez A and
Ezquerra-Brauer JM: Bioactive peptides and depsipeptides with
anticancer potential: Sources from marine animals. Mar Drugs.
10:963–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chapkin RS, Seo J, McMurray DN and Lupton
JR: Mechanisms by which docosahexaenoic acid and related fatty
acids reduce colon cancer risk and inflammatory disorders of the
intestine. Chem Phys Lipids. 153:14–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fukui M, Kang KS, Okada K and Zhu BT: EPA,
an omega-3 fatty acid, induces apoptosis in human pancreatic cancer
cells: Role of ROS accumulation, caspase-8 activation, and
autophagy induction. J Cell Biochem. 114:192–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maleek MI: Omega-3 fatty acids decrease
the proliferation of Rhabdomyosarcoma (RD) and Vero cell lines. J
Cancer Sci Ther. 5:85–88. 2013. View Article : Google Scholar
|
|
48
|
Park JM, Kwon SH, Han YM, Hahm KB and Kim
EH: Omega-3 polyunsaturated fatty acids as potential
chemopreventive agent for gastrointestinal cancer. J Cancer Prev.
18:201–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang P, Cartwright C, Chan D, Ding J,
Felix E, Pan Y, Pang J, Rhea P, Block K, Fischer SM, et al:
Anticancer activity of fish oils against human lung cancer is
associated with changes in formation of PGE2 and PGE3 and
alteration of Akt phosphorylation. Mol Carcinog. 53:566–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kato T, Hancock RL, Mohammadpour H,
McGregor B, Manalo P, Khaiboullina S, Hall MR, Pardini L and
Pardini RS: Influence of omega-3 fatty acids on the growth of human
colon carcinoma in nude mice. Cancer Lett. 187:169–177. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu M, Harvey KA, Ruzmetov N, Welch ZR,
Sech L, Jackson K, Stillwell W, Zaloga GP and Siddiqui RA: Omega-3
polyunsaturated fatty acids attenuate breast cancer growth through
activation of a neutral sphingomyelinase-mediated pathway. Int J
Cancer. 117:340–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shaikh IA, Brown I, Schofield AC, Wahle KW
and Heys SD: Docosahexaenoic acid enhances the efficacy of
docetaxel in prostate cancer cells by modulation of apoptosis: The
role of genes associated with the NF-kappaB pathway. Prostate.
68:1635–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hawcroft G, Volpato M, Marston G, Ingram
N, Perry SL, Cockbain AJ, Race AD, Munarini A, Belluzzi A, Loadman
PM, et al: The omega-3 polyunsaturated fatty acid eicosapentaenoic
acid inhibits mouse MC-26 colorectal cancer cell liver metastasis
via inhibition of PGE2-dependent cell motility. Br J Pharmacol.
166:1724–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|