Introduction
Colorectal cancer (CRC) is the third most common
type of cancer, and the leading cause of cancer-associated
mortality worldwide (1). Previous
studies suggest that ~20% of the patients diagnosed with CRC
exhibit metastatic disease, and an additional 30–35% develop
metastases later with tumor progression (2,3). Despite
significant therapeutic advances, ~60% of all patients receiving
curative resection will experience local recurrence or distant
metastases (4). Therefore, a
comprehensive understanding of the molecular mechanisms associated
with CRC metastasis is imperative to facilitate early diagnosis in
high-risk populations.
In previous years, rapid advances have occurred in
the use of microRNAs (miRNAs/miRs) as novel biomarkers in patients
with cancer. miRNAs are short non-coding RNA molecules, which have
been widely identified to be involved in malignant transformation
by inhibiting the expression of target genes through translational
inhibition or transcriptional silencing (5). In cancer, miRNAs may act as either
oncogenes or tumor suppressor genes that affect almost every basic
cellular process. However, the aberrant expression and potential
role of miRNAs in CRC remains largely unclear, particularly in the
metastatic process (6).
Of note, the dysregulation of the members of miR-34
family in various types of cancer has been associated with
tumorigenesis, growth and progression (7,8). For
instance, miR-34a as a tumor suppressor has been identified in
solid tumors including lung cancer (9), multiple myeloma (10), neuroblastoma (11,12),
glioblastoma (13,14), cervical carcinoma (15), breast cancer (16) and colon cancer (17). miR-34a promotes
epithelial-to-mesenchymal transition (EMT)-mediated invasion and
metastasis via feedback and feedforward loops with other miRNAs and
proteins (18–20). In addition, it has been suggested that
miR-34a mimics were a novel potential therapeutic agent targeting
multiple myeloma and pancreatic cancer (10,21). The
tumor suppressor miR-34a is a cell-fate determinant in early-stage
dividing colon cancer stem cells (22–24). It is
therefore critical to investigate the regulatory function of
miR-34a in cancer, including CRC.
In the present study, miR-34a expression was
determined in colon cancer cell lines and clinical CRC samples.
Functional studies on miR-34a demonstrated that it disrupted colon
cancer cell growth and invasion potentially via inhibition of
Notch1 and Jagged1 expression. Vimentin and fibronectin function as
the downstream molecules of these pathways, contributing to
progression of CRC. These studies provide novel insights into the
underlying molecular mechanisms of CRC cell metastasis,
highlighting the unique regulatory role of miRNAs.
Materials and methods
Cell lines and culture
Human colon cancer cell lines SW480, SW620, HT29,
and the human embryonic kidney HEK293 cell line were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and grown in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) (both from Hyclone, GE Healthcare Life Sciences, Logan,
UT, USA), 100 U/ml penicillin G and 100 mg/ml streptomycin (both
from Beyotime Institute of Biotechnology, Shanghai, China). The
human colonic epithelial cell line NCM460 was purchased from INCELL
Corporation LLC (San Antonio, TX, USA) and grown in M3:10 medium
(INCELL Corporation LLC). The cells were cultured at 37°C in a 5%
CO2 incubator.
Plasmids and reagents
Full-length Notch1 and Jagged1 cDNA lacking the
3′-untranslated region (UTR) were purchased from GeneCopoeia
(Rockville, MD, USA) and subcloned into the eukaryotic expression
vector pIRES (Clontech Laboratories, Inc., Mountain View, CA, USA).
The empty pIRES vector was used as a negative control. Rabbit
monoclonal anti-Notch1 (cat no. EP1238Y) and anti-Jagged1 (cat no.
EPR4290) were purchased from Epitomics (Abcam, Cambridge, UK),
mouse monoclonal anti-GAPDH (cat no. MAB374) was purchased from EMD
Millipore (Billerica, MA, USA), and mouse monoclonal anti-vimentin
(cat no. sc-6260), anti-fibronectin (cat no. C6F10) and horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat no. sc-2004)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Synthetic miR-34a mimic and negative control
(NC) were purchased from GenePharma Co., Ltd. (Shanghai, China).
The miR-34a LNA 5′-digoxigenin (DIG)-labeled (cat no. 612537-340)
and scrambled LNA 5′-DIG-labeled probes (cat no. 699004-340) were
purchased from Exiqon A/S (Vedbaek, Denmark). The probe sequences
are as follows: miR-34a, AGG GCA GTA TAC TTG CTG AT; and scramble,
GTG TAA CAC GTC TAT ACG CCC A. The probes were detected using an
enhanced sensitive ISH detection kit (cat no. MK1030; Wuhan Boster
Biological Technology, Ltd., Wuhan, China).
In situ hybridization (ISH)
A tissue microarray (TMA) containing 103 samples of
human CRC tissue specimens with their matched distant normal mucosa
tissues (>10 cm away from the primary tumor) was used in the
present study. The high-density CRC tissue microarray from Chinese
specimens collected in March 2006 from the Third Xiangya Hospital
of Central South University (Changsha, China) was constructed by
the study group. Written informed consent was obtained from
patients. The present study was approved by the Ethical Review
Committees of the Third Xiangya Hospital of Central South
University, Hunan Key Laboratory of Nonresolving Inflammation and
Cancer and the Central South University (Changsha, China).
Histology of all slides was reviewed by two expert pathologists.
The cancer stage was made according to the TNM classification
criteria (25). The TMAs (4 µm) were
dewaxed and treated with 3% H2O2 and
proteinase K to inactivate endogenous peroxidases. The slides were
treated with pepsin in 3% citric acid. The slides were hybridized
at 56°C overnight with 50 nM miR-34a LNA or scrambled LNA.
Following washing 3 times with 2X saline-sodium citrate buffer,
sections were blocked in blocking buffer (cat no. MK1030; Wuhan
Boster Biological Technology, Ltd.) at 37°C for 30 min and then
incubated with 1X anti-DIG Fab fragment antibody (cat no. MK1030;
Wuhan Boster Biological Technology, Ltd.) at room temperature for 2
h for the hybridization reaction. The immunoreaction was detected
using 3-diaminobenzidine tetrahydrochloride (cat no. 0031; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China). The sections were
counterstained with 5 g/l hematoxylin at room temperature for 3
min. Following dehydration and mounting, the sections were observed
under an inverted microscope (Nikon Corporation, Tokyo, Japan) with
magnifications of ×100 or ×200. The staining intensity of miR-34a
was scored as: 0–1, negative; 1–2, weak; 2–3, medium; and 3–4,
strong. The percentages of miR-34a cells in 3 representative
high-power fields of individual samples were determined. The
miR-34a expression was calculated in terms of the product of the
intensity scores and the percentage of positive cells. Individual
samples were evaluated by at least 2 blinded pathologists. Scores
≥2 were used to define specimens with a high expression, and a
score of <2 indicated low expression.
miRNA target prediction
Potential target genes of miR-34a were predicted and
analyzed using 4 web-based bioinformatics tools: TargetScan,
miRWalk, microRNA.org and RNA22. The number of
false positive predictions was markedly decreased by selecting the
putative target genes that were predicted by ≥2 programs.
Transient transfection of miRNA
precursors
The SW480 cells were seeded into 6-well plates. The
cells were transfected by nucleofection with 100 nM miR-34a mimic
or mimic NC using HiPerFect Transfection reagent (Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer's protocol. At 48
h post-transfection, the reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) was performed to verify the
transfection efficiency.
RT-qPCR
Total RNA from cells and tissues was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The Hairpin-it™ MicroRNA Quantitation PCR kit
was purchased from GenePharma Co., Ltd. The RT-qPCR was performed
according to the manufacturer's protocol. At least three biological
replicates were performed for each case. The qPCR analysis was
performed using the iQ5 Real-Time PCR system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The miRNA PCR quantification used the
2−ΔΔCq method (26)
against U6 for normalization.
The expression of mRNA was evaluated using the SYBR
Premix Ex Taq II (Tli RNaseH Plus) Real-Time qPCR kit (Takara
Biotechnology Co., Ltd., Dalian, China). The mRNA PCR
quantification used the 2−ΔΔCq method (26) against GAPDH for normalization. The
RT-qPCR primers, including E-cadherin, vimentin, fibronectin, zinc
finger protein SNAI2 (Slug), zinc finger protein SNAI1 (Snail),
GAPDH and zinc finger E-box-binding homeobox 1 (ZEB1), are
summarized in Table I.
 | Table I.Human primers for quantitative
polymerase chain reaction. |
Table I.
Human primers for quantitative
polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
|
Pre-microRNA-34a |
UGGCAGUGUCUUAGCUGGUUGU |
AACCAGCUAAGACACUGCCAUU |
| Notch1 |
AGCCTCAACATCCCCTACAA |
CCACGAAGAACAGAAGCACA |
| Jagged1 |
ACGGGAAGTGCAAGAGTCAG |
GTTTCACAGTAGGCCCCCTC |
| Epithelial
cadherin |
TTCTGGAAGGAATGGAGGAGTC |
ACCTGGAATTGGGCAAATGTG |
| Vimentin |
AGATGGCCCTTGACATTGAG |
TGGAAGAGGCAGAGAAATCC |
| Fibronectin |
GGTGACACTTATGAGCGTCCTAAA |
AACATGTAACCACCAGTCTCATGTG |
| ZEB1 |
GCACAACCAAGTGCAGAAGA |
GCCTGGTTCAGGAGAAGATG |
| Snail |
GCTGCAGGACTCTAATCCAGAGTT |
GACAGAGTCCCAGATGAGCATTG |
| Slug |
AGATGCATATTCGGACCCAC |
CCTCATGTTTGTGCAGGAGA |
| GAPDH |
AACGGATTTGGTCGTATTGG |
TTGATTTTGGAGGGATCTCG |
Luciferase reporter assay
Human Notch1 (accession number NM_017617) and
Jagged1 (NM_000214) 3′-untranslated regions (UTRs) are predicted to
contain two putative binding sites of miR-34a. Notch1 and Jagged1
wild-type (WT) 3′-UTRs and mutant 3′-UTRs with 6 base mutations at
putative seed regions were synthesized and cloned into the pIRES
vector with the restriction sites for HindIII and
SpeI at the two ends of the oligonucleotides. The WT and
mutant oligonucleotide sequences used are summarized in Table II. In total, 9 luciferase reporters
(LR) were constructed and referred to as LR-blank (no insertion),
LR-34a/Notch1w1 (where w1 indicates WT site 1), LR-34a/Notch1w2
(where w2 indicates WT site 2), LR-34a/Notch1m1 (where m1 indicates
site 1 mutant), LR-34a/Notch1 m2 (where m2 indicates site 2
mutant), LR-34a/Jagged1w1, LR-34a/Jagged1w2, LR-34a/Jagged1m1 and
LR-34a/Jagged1m2. HEK293 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS in 24-well plates at 37°C overnight, and
then co-transfected with 100 ng of one of the reporter plasmids and
10 pmol miR-34a mimic or mimic NC using MegaTran 1.0 Transfection
reagent (OriGene Technologies, Inc., Rockville, MD, USA). At 24 h
post-transfection, firefly and Renilla luciferase activities
were consecutively measured using the commercial dual-luciferase
reporter system (Promega Corporation, Madison, WI, USA). The
Renilla luciferase signal was normalized to the firefly
luciferase signal for each individual analysis.
 | Table II.Primers and oligonucleotides. |
Table II.
Primers and oligonucleotides.
|
| Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| 3′UTR of wild 1
Notch1 |
CGCGTTTTATTTATGTACTTTTATTT |
AGCTTTACATATAAATAAAAAGG |
|
|
TACACAGAAACACTGCCTTTTTATT |
CAGTGTTTCTGTGTAAAATAAA |
|
| TATATGTAA |
AGTACATAAATAAAA |
| 3′UTR of mutant1
Notch1 |
CGCGTTTTATTTATGTACTTTT |
AGCTTTACATATAAATAAAAAA |
|
|
ATTTTACACAGAAAAAAATTTTTTT |
AATTTTTTTCTGTGTAAAATAAAAG |
|
| TATTTATATGTAA | TACATAAATAAAA |
| 3′UTR of wild2
Notch1 |
CGCGTTACCCTTTTCTGGGGAA |
AGCTTGCTGGGGCCGCCACCG |
|
|
AGACACTGCCTGGGCTGACCCCGGT |
GGGTCAGCCCAGGCAGTGTCTTTC |
|
| GGCGGCCCCAGCA |
CCCAGAAAAGGGTAA |
| 3′UTR of mutant12
Notch1 |
CGCGTTACCCTTTTCTGGGGAAA |
AGCTTGCTGGGGCCGCCACCG |
|
|
GAAAAATTTTGGGCTGACCCCGGT |
GGGTCAGCCCAAAATTTTTCTTTCC |
|
| GGCGGCCCCAGCA | CCAGAAAAGGGTAA |
| 3′UTR of wild 1
Jagged1 |
CGCGTACCGCGGGCACTGCCGCCG |
AGCTTTTAAAGAACTACAAGC |
|
|
CTAGGTAGAGTCTGAGGGCTTGTAG |
CCTCAGACTCTACCTAGCG |
|
| TTCTTTAAA |
GCGGCAGTGCCCGCGGTA |
| 3′UTR of mutant1
Jagged1 |
CGCGTACCGCGGGAAAATTTGC |
AGCTTTTAAAGAACTACAAGC |
|
|
CGCTAGGTAGAGTCTGAGGGCTTGT |
CCTCAGACTCTACCTAGCGGC |
|
| AGTTCTTTAAA |
AAATTTTCCCGCGGTA |
| 3′UTR of wild2
Jagged1 |
CGCGTTTTAGATTTGCCATAGAGTA |
AGCTTCTTTGATTTCCTCACTTAA |
|
|
CACTGCCTGCCTTAAGTGAGGAA |
GGCAGGCAGTGTACTCTATG |
|
| ATCAAAGA | GCAAATCTAAAA |
| 3′UTR of mutant2
Jagged1 |
CGCGTTTTAGATTTGCCATAGAGTA |
AGCTTCTTTGATTTCCTCACTTAA |
|
|
AAAATTTTGCCTTAAGTGAGG |
GGCAAAATTTTTACTCTATG |
|
| AAATCAAAGA | GCAAATCTAAAA |
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer containing 1% protease inhibitor cocktail (Pierce; Thermo
Fisher Scientific, Inc.). The protein concentrations in the lysates
were measured using a bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Total protein (50 µg/lane) was
separated on a Bio-Rad Bis-Tris Gel system (Bio-Rad Laboratories,
Inc.) and electrotransferred onto polyvinylidene fluoride
membranes. Subsequent to blocking with 5% bovine serum albumin (cat
no. SW3015; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) at room temperature for 1 h, the membranes were
serially incubated with anti-Notch1 (1:500 dilution), anti-Jagged1,
anti-vimentin (1:200 dilution), anti-fibronectin (1:500 dilution)
and anti-GAPDH (1:1,000) primary antibodies at 4°C overnight,
followed by incubation with the HRP-conjugated goat anti-rabbit IgG
secondary antibody (1:1,000 dilution) at 37°C for 2 h.
Chemiluminescence using Luminata Forte Western horseradish
peroxidase substrate (Merck KGaA, Darmstadt, Germany) was
visualized using a ChemiDoc XRS+ system (Bio-Rad Laboratories,
Inc.). The densities of the bands were quantified using Image Lab
2.0 software (Bio-Rad Laboratories, Inc.).
Wound closure assay
SW480 cells (1×106 cells/well) were
cultured overnight at 37°C. SW480 cells were transfected with
miR-34a (or mimic NC) followed by Notch1 or Jagged1 transfection.
Following the cells in the 6-well dish attaining 90% confluence, a
wound was created using a 10 µl pipette tip. Following washing 3
times in PBS to remove cellular debris, cells were cultured in
RPMI-1640 medium with 2% serum. Cell migration at the wound sites
was documented using an inverted microscope (Nikon Corporation)
with a magnification of ×200 at the indicated time-points (0 and 48
h). Cells in 3 randomly selected areas were counted.
Invasion assay
Corning Costar Transwell 24-well plates (8-µm pores;
Corning Incorporated, Corning, NY, USA) coated with BD Matrigel
matrix (BD Biosciences, Franklin Lakes, NJ, USA) were maintained
for 1 h at 37°C, followed by the addition of 1×105
transfected cells suspended in 200 µl medium with 1% serum into the
top of each well insert. Normal growth medium was added to the
bottom wells. The cells were allowed to migrate for 24 h at 37°C.
The migrated cells were fixed with 10% methanol for 15 min. The
invading cells on the lower surface of the membrane were stained
with 0.5% crystal violet for 5 min at room temperature. The stained
cells were counted under a microscope (Nikon Corporation). To
minimize the bias, ≤5 randomly selected fields at ×100
magnification were counted, and the average number was
calculated.
Statistical analysis
Statistical analysis was performed using the SPSS
for Windows (version 17.0; SPSS, Inc., Chicago, IL, USA) and
GraphPad Prism (version 5; GraphPad Software, Inc., La Jolla, CA,
USA). χ2 tests were used for categorical variables,
including categorized miR-34a expression levels, clinical stage,
histological differentiation, sex, age and metastasis of the
colorectal tumors. Independent unpaired t-tests and one-way
analysis of variance with Bonferroni post hoc testing were
performed for the analysis of continuous variables in categories.
Survival analysis was determined using the Kaplan-Meier estimator
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Global repression of miR-34a
expression
RT-qPCR was used to detect the expression of miR-34a
in colon cancer cell lines SW480, SW620 and HT29, and normal
colonic epithelial cell line NCM460. Compared with the normal
colonic epithelial cells, miR-34a was identified to be
downregulated ~2.5-fold in SW480 cells, 3-fold in HT29 cells and
6-fold in SW620 cells (Fig. 1A). In
addition, compared with SW480, the relatively high metastatic SW620
and HT29 cells exhibited significantly decreased miR-34a
expression, particularly in SW620 cells (P=0.014).
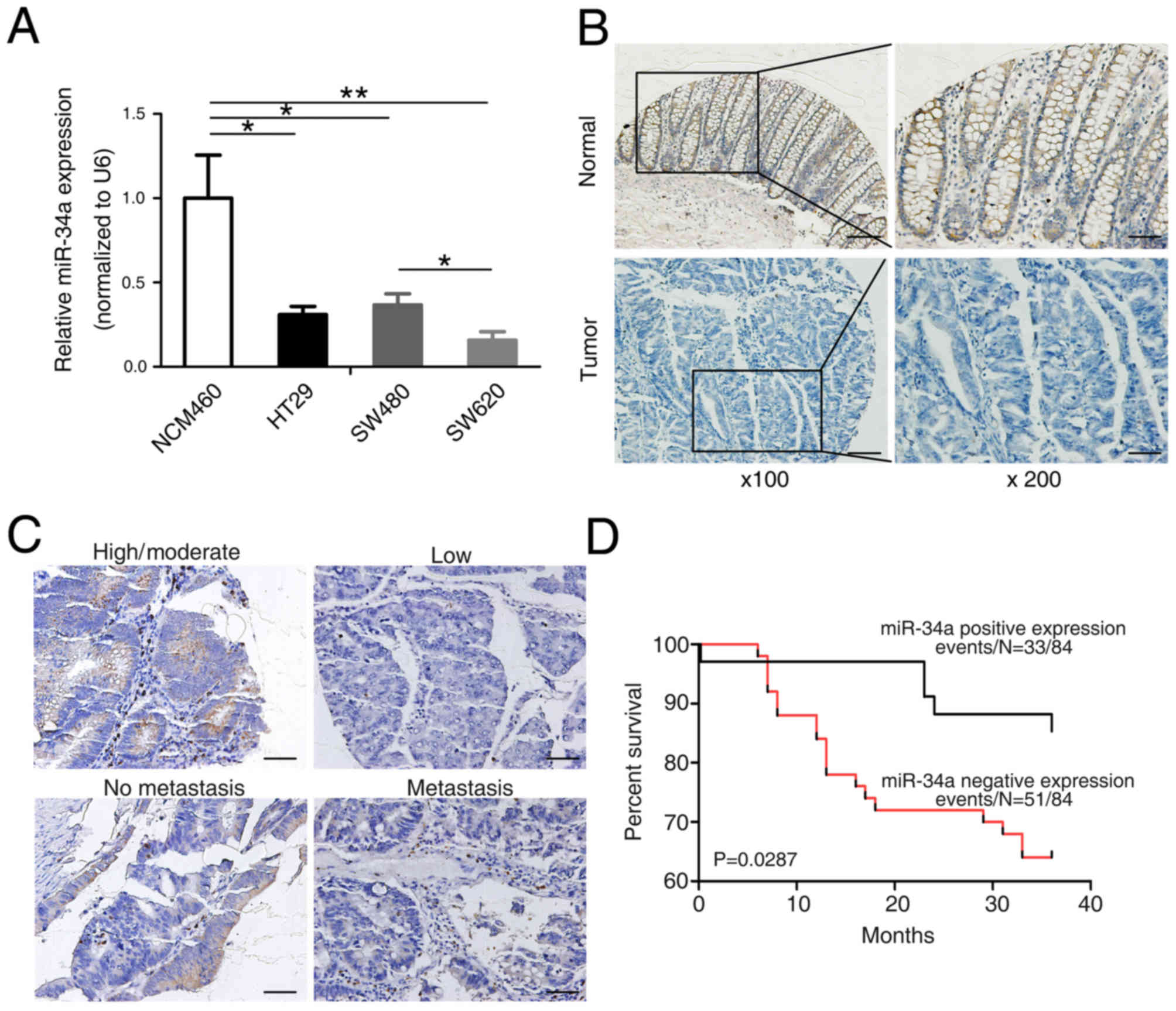 | Figure 1.Expression of mature miR-34a in
colorectal cancer cells and tissues. (A) Reverse
transcription-quantitative polymerase chain reaction analysis of
miR-34a expression in the potentially invasive colon cancer cell
lines HT29, SW480 and SW620, and the normal colonic epithelial cell
line NCM460. The data are expressed as the mean ± standard
deviation (n=3). *P<0.05 and **P<0.01 vs. NCM460. (B)
Colorectal cancer tissue microarray composed of normal colon tissue
and colon adenocarcinoma tissue from patients was analyzed using
in situ hybridization. Representative images of miR-34a
expression (left panel: Original magnification, ×100; scale bar,
100 µm; right panel: Original magnification, ×200; scale bar, 50
µm) (C) Representative images of miR-34a expression in
well-differentiated (high/moderate differentiation) colorectal
cancer, poorly differentiated (low differentiation) colorectal
cancer, non-metastatic tissues and metastasis tissues. Original
magnification, ×200; scale bar, 50 µm. (D) Kaplan-Meier estimator
survival curves for patients according to the expression levels of
miR-34a in tumors. miR, microRNA. |
To verify the altered expression of miR-34a in human
colorectal carcinogenesis, ISH was first conducted to evaluate
miR-34a expression levels in 103 pairs of CRC tissues and their
matched adjacent normal tissues using a TMA (representative images
are presented in Fig. 1B). It was
identified that miR-34a was apparently downregulated in the
colorectal tumors compared with the normal tissues (P=0.000;
Table III). Next, the potential
clinicopathological implications of altered miR-34a expression were
assessed. Clinical samples were divided into low- and
high-expression groups based on the miR-34a expression scores >2
or <2. Of the 103 normal colorectal samples, 88 (85%) exhibited
increased expression of miR-34a (Table
III). Thus, miR-34a is underexpressed in CRC tissue compared
with the normal colorectal mucosa (representative images are
presented in Fig. 1C). In the 103
individuals with CRC, the miR-34a level was inversely associated
with distant metastasis, and positively associated with
differentiation and age (P=0.045, 0.010 and 0.020, respectively;
Table III). However, no association
between miR-34a expression and sex and TNM stage was observed in
patients with CRC. To additionally assess the significance of
miR-34a in terms of clinical prognosis, a Kaplan-Meier estimator
survival analysis was conducted using overall survival (OS). The
results demonstrated that patients with a diminished miR-34a
expression exhibited shorter OS times compared with patients
expressing high miR-34a levels (P=0.029; Fig. 1D). These data demonstrated overt
downregulation of miR-34a in CRC, suggesting that the expression
levels of miR-34a was associated with the metastatic potential of
CRC.
 | Table III.Association of clinical and
pathological features with miR-34a expression. |
Table III.
Association of clinical and
pathological features with miR-34a expression.
|
|
| Expression of
microRNA-34a |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases (n) | Low, n (%) | High, n (%) | P-value |
|---|
| Histological
type |
|
|
| 0.000 |
| Normal
tissues | 103 | 15 (14.6) | 88 (85.4) |
|
|
Colorectal cancer tissues | 103 | 54 (52.4) | 49 (47.6) |
|
| Age, years |
|
|
| 0.020 |
|
≤56 | 52 | 39 (75.0) | 13 (25.0) |
|
|
>56 | 51 | 27 (52.9) | 24 (47.1) |
|
| Sex |
|
|
| 0.182 |
|
Male | 59 | 34 (57.6) | 25 (42.4) |
|
|
Female | 44 | 31 (70.5) | 13 (29.5) |
|
| Distant
metastasis |
|
|
| 0.045 |
| No | 78 | 32 (41.0) | 46 (59.0) |
|
|
Yes | 25 | 16 (64.0) | 9 (36.0) |
|
| Distant
differentiation |
|
|
| 0.010 |
|
High/moderate | 88 | 41 (46.6) | 47 (53.4) |
|
|
Low | 15 | 13 (86.7) | 2 (13.3) |
|
| TNM stage |
|
|
| 0.469 |
|
I–II | 48 | 29 (60.4) | 19 (39.6) |
|
|
III–VI | 55 | 37 (67.3) | 18 (32.7) |
|
miR-34a directly targets and inhibits
Notch1 and Jagged1
The biological function of miR-34a was explored
using several bioinformatics algorithms in order to identify
potential miR-34a target genes. Among the candidate target genes,
Notch1 and its ligand Jagged1 were identified. As presented in
Fig. 2A, 2 miR-34a-binding sites were
identified in the 3′-UTRs of Notch1 and Jagged1 mRNAs, with a
perfect base pairing between the seed sequence of mature miR-34a
and the miRNA-recognition element in the 3′-UTRs. A number of
cellular functions and biochemical processes associated with
tumorigenesis are modulated by Notch signaling, including EMT,
proliferation, apoptosis, adhesion and angiogenesis (27). A previous study has identified Notch1
overexpression in various solid tumors, including CRC (28). Previous studies have also suggested
that miR-34a suppressed tumor invasion via the downregulation of
Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells
(15). Therefore, an initial screen
of the effects of miR-34a on Notch1 and Jagged1 expression was
performed. Initially, miR-34a inhibition of the expression of
Notch1 and Jagged1 was determined by transfecting miR-34a mimic
into the SW480 cells. It was identified that the miR-34a mimic
significantly decreased the mRNA and protein levels of Notch1 and
Jagged1 compared with the mimic NC (Fig.
2B).
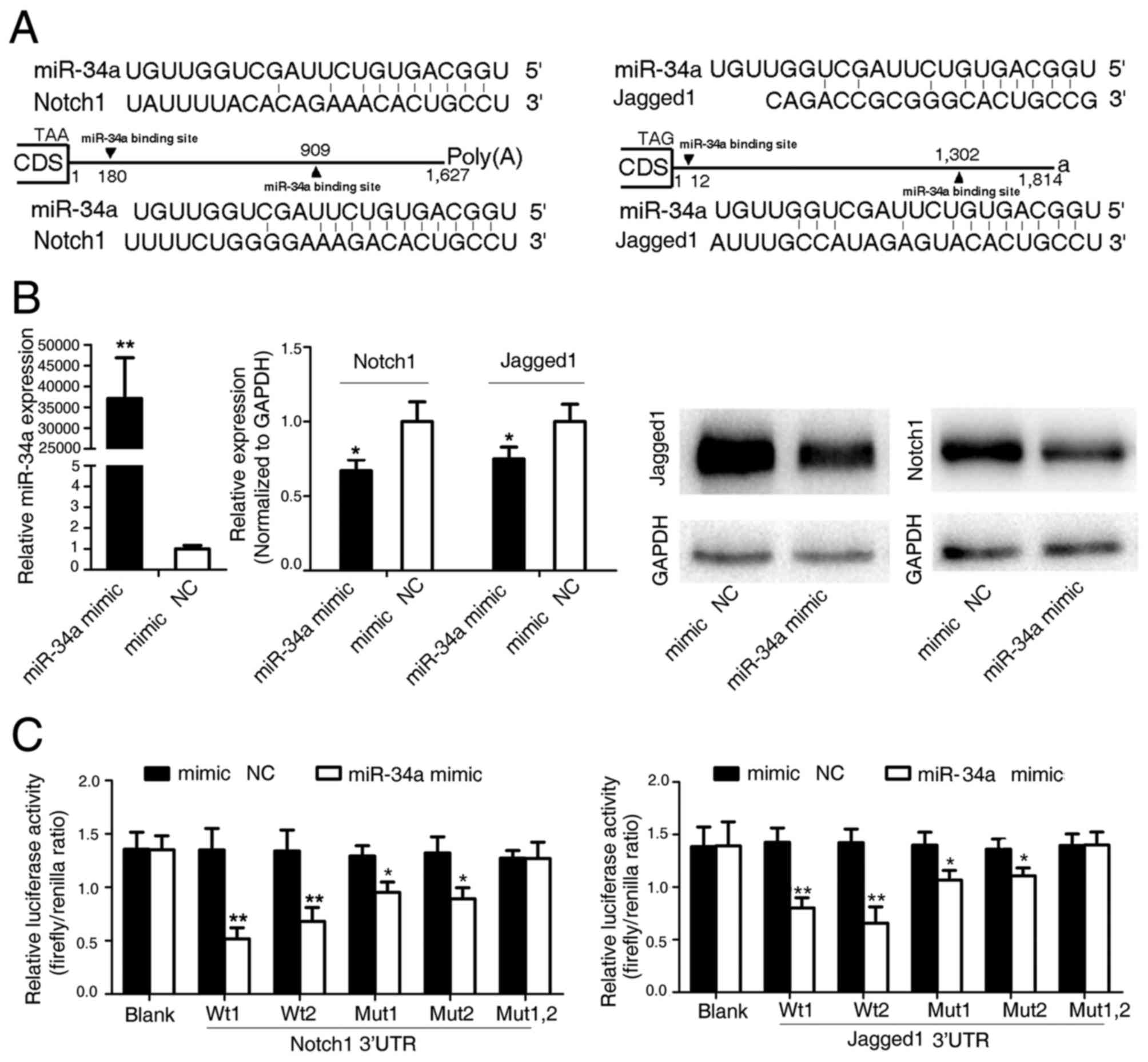 | Figure 2.Notch1 and Jagged1 are direct target
genes of human miR-34a. (A) Schematic representation of the
predicted binding sites of miR-34a in the Notch1 and Jagged1 3′
untranslated regions, and the sequence of the intact (wild-type)
miR-34a and their mutant binding sites within the luciferase
reporter vector. (B) miR-34a suppresses the expression of Notch1
and Jagged. Reverse transcription-quantitative polymerase chain
reaction (middle panel) and Western blot analyses (right panel) of
Notch1 and Jagged1 were performed following transfection of cells
with an miR-34a mimic or mimic negative control (NC) for 48 h (left
panel). The data are expressed as the mean ± SD (n=3). *P<0.05
and **P<0.01 vs. mimic NC. (C) A total of 9 luciferase reporter
constructs were created for the luciferase activity assay, namely
LR-blank (no insertion), LR-34a/Noth1w1, LR-34a/Noth1w2,
LR-34a/Jagged1w1, LR-34a/Jagged1w2, LR-34a/Noth1m1, LR-34a/Noth1m2,
LR-34a/Jagged1m2 and LR-34a/Jagged1m2. Each of the constructs was
co-transfected with an miR-34a mimic (or mimic NC) into HEK293
cells. Luciferase activities were measured 36 h post-transfection.
The data are expressed as the mean ± SD (n=3). *P<0.05 and
**P<0.01 vs. mimic NC. miR, microRNA; CDS, coding sequence; SD,
standard deviation; NC, negative control; w1/2, wild-type 1/2;
m1/m2, mutant 1/2. |
Whether Notch1 and Jagged1 were direct targets of
miR-34a was investigated using a luciferase reporter assay to test
the binding of miR-34a to the 3′-UTR of Notch1 and Jagged1. The
Notch1 and Jagged1 3′-UTRs containing the binding sites for miR-34a
were subcloned into a luciferase reporter vector. The addition of
an miR-34a mimic significantly suppressed the luciferase activity
of the Notch1 and Jagged1 3′-UTRs following co-transfection of the
luciferase vector (WT, mutant or blank control) with the miR-34a
mimic into SW480 cells (Fig. 2C).
Thus, these results confirm that miR-34a directly recognizes the
3′-UTRs of the Notch1 and Jagged1 mRNAs, leading to mRNA
degradation and translational inhibition.
miR-34a suppresses CRC metastasis by
targeting Notch1 and Jagged1
Considering the aforementioned data, the role of
miR-34a in the human colon cancer SW480 cell line was next
investigated. The effects of miR-34a were assessed using
wound-healing assays and Matrigel invasion assays following
transfection of the cells with miR-34a mimic or mimic NC. It was
identified that overexpressed miR-34a in SW480 cells significantly
attenuated cell migration and invasion. The re-expression of Notch1
or Jagged1 (lacking an endogenous 3′-UTR) prevented this
inhibition, suggesting that miR-34a specifically targets Notch1 or
Jagged1 to suppress CRC metastasis (Fig.
3A and B). Previous evidence suggests that the activation of
Notch1 and Jagged1 induces EMT and promoted the invasion and
dissemination of cancer cells (29–31).
Therefore, whether miR-34a affected EMT was investigated. A panel
of EMT markers, including epithelial cadherin, vimentin,
fibronectin, Slug, Snail and ZEB1, were detected in SW480 cells
following transfection with miR-34a mimic or mimic NC (Fig. 3C). Significant attenuation of the mRNA
expression levels of two key mesenchymal markers, vimentin and
fibronectin, was observed (Fig. 3C and
D). In addition, the re-expression of Notch1 or Jagged1
recovered the protein expression levels of vimentin and fibronectin
in miR-34a-overexpressing SW480 cells, suggesting that miR-34a
suppresses CRC metastasis by targeting Notch1/Jagged1, and the
downstream molecules vimentin and fibronectin.
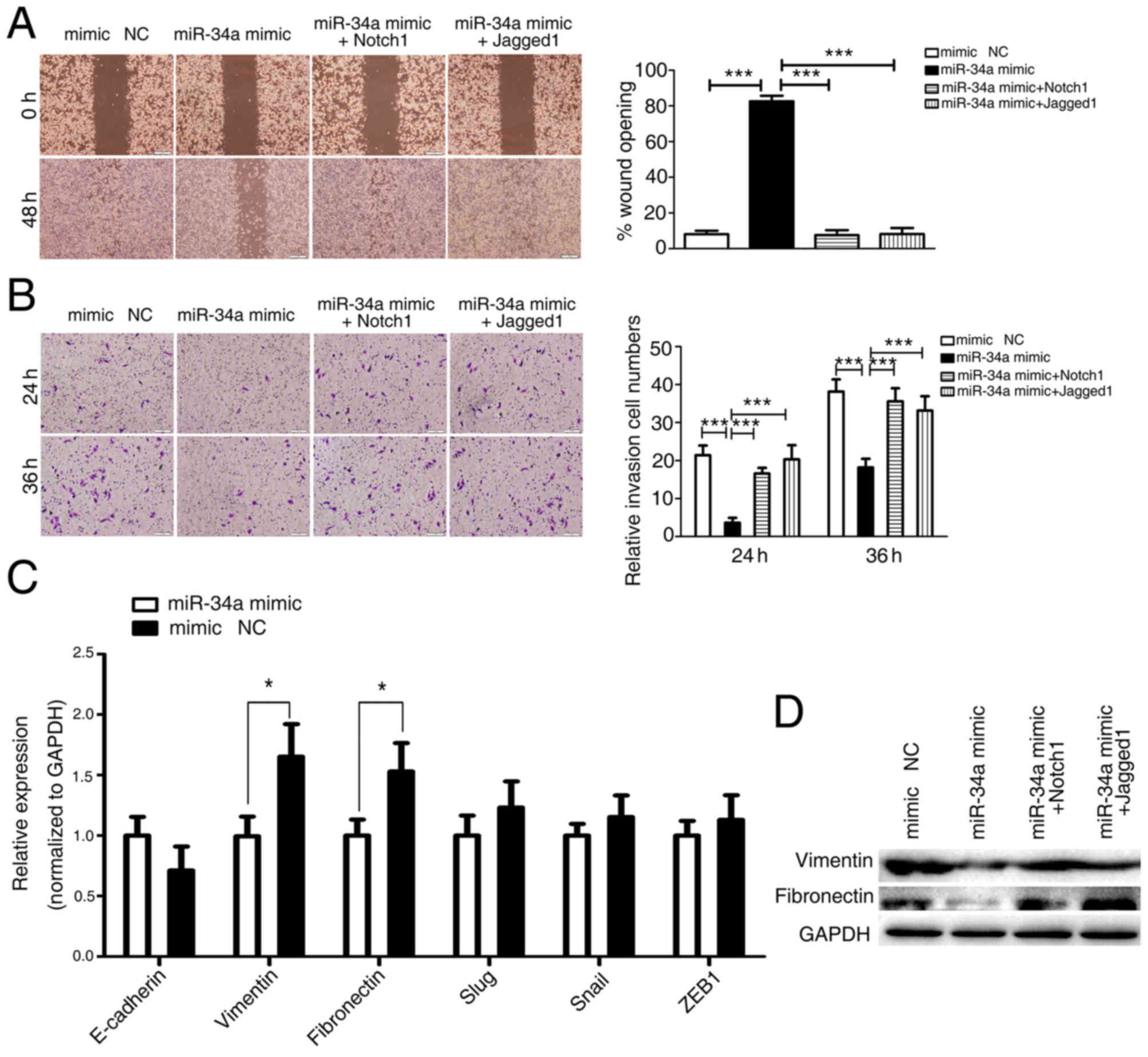 | Figure 3.miR-34a inhibits tumor cell migration
and invasion by targeting Notch1 and Jagged1. (A) SW480 cells were
transfected with miR-34a (or mimic NC) followed by Notch1 or
Jagged1 transfection. Cells were subjected to the wound closure
assay in a time-dependent manner. Images captured at 0 and 48 h are
provided. Representative images of the migrated stained cells are
also provided (left). Cells in 3 randomly selected areas were
counted and statistical analyses were performed. The data are
expressed as the mean ± SD (n=3) (right). *P<0.05, **P<0.01
and ***P<0.001 vs. miR-34a mimic. (B) Transwell migration assays
examining the effects of miR-34a on cell invasion ability. SW480
cells were transfected with miR-34a (or mimic NC) followed by
Notch1 or Jagged1 transfection. The Transwell migration assay was
performed 24 and 36 h later. Representative images of the migrated
stained cells are provided (left). SW480 cells in 5 randomly
selected areas were counted and statistical analyses were performed
(right). The data are expressed as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001 vs. miR-34a mimic. (C) Regulation of
the expression of E-cadherin, vimentin, fibronectin, Slug, Snail
and ZEB1 by miR-34a. SW480 cells were transfected with an miR-34a
mimic or mimic NC for 48 h. The expression of E-cadherin, vimentin,
fibronectin, Slug, Snail and ZEB1 was analyzed using the reverse
transcription-quantitative polymerase chain reaction. Values were
normalized to GAPDH as an internal control. The data are expressed
as the mean ± SD (n=3). *P<0.05 vs. mimic NC. (D) SW480 cells
were transfected with miR-34a (or mimic NC) followed by Notch1 or
Jagged1 transfection. The protein expression of vimentin and
fibronectin was assayed by western blot analysis. miR, microRNA;
SD, standard deviation; E-cadherin, epithelial cadherin; Slug, zinc
finger protein SNAI2; Snai1, zinc finger protein SNAI1; ZEB1, zinc
finger E-box-binding homeobox 1; NC, negative control. |
Discussion
Tumor progression in CRC is a complex process.
Metastasis is the primary characteristic of malignant tumors. It is
the primary cause of mortality in the majority of patients with
cancer (32). miRNAs have been
described as a novel class of molecular regulators of tumorigenesis
(33). Previous studies have
attempted to delineate their role in the regulation of metastasis
and disease progression (34–36).
MiR-34a is a member of a family of evolutionarily
conserved miRNAs that are regulated by the tumor suppressor tumor
protein 53 (37–39). Tazawa et al (17) have suggested that miR-34a expression
levels were downregulated by 36% in CRC relative to their levels in
normal tissues, although the underlying molecular mechanism for
tumorigenesis remains unclear. Previously, Liu et al
(40) identified that miR-34a
inhibited prostate cancer stem cells and metastasis by directly
repressing cluster of differentiation 44 expression. It was also
demonstrated that miR-34a inhibits colon cancer cell migration and
invasion by targeting Fra-1 (41). In
addition, it was suggested that miR-34a serves a key role in tumor
cell responses to chemotherapeutic agents, and may serve as a
target for antitumor therapy (10,42). In
the present study, by assaying the expression of miR-34a in CRC
cell lines and clinical specimens, it was confirmed that miR-34a
was downregulated in CRC samples compared with normal tissues, and
the levels of miR-34a expression were identified to be inversely
associated with tumor metastasis. Additionally, it was demonstrated
that increased survival was associated with high expression levels
of miR-34a.
A previous study confirmed that miR-34a targeted
multiple key pathways including the hepatocyte growth factor/c-Met
pathway, E2F pathway and cell cycle regulator cyclin-dependent
kinase 6 (17). However, the precise
underlying molecular mechanism for the role of miR-34a in
colorectal tumorigenesis remains unclear. Among the regulatory
mechanisms targeted by miR-34a, the Notch pathway serves a
prominent role in cell fate determination during cancer
proliferation, apoptosis, invasion and metastasis (43). There are 4 Notch receptors (Notch1-4)
and 5 Notch ligands (δ-like-1, −3 and −4, and Jagged1 and 2) in
mammals. Following specific ligand binding, the intracellular part
of the Notch receptor is cleaved and translocated to the nucleus,
where it binds to the transcription factor recombining binding
protein for immunoglobulin κ J region to activate Notch target
genes (44,45). Notch1 is one of the Notch receptors
involved in Notch signaling, which serves a key role in the
regulation of a number of fundamental cellular processes, including
proliferation, stem cell maintenance, differentiation and EMT
(27,46). Notch1 and Jagged1 are key factors in
the regulation of survival and invasion in a wide variety of
cancers including gastric, salivary, pancreatic and neuroendocrine
tumors, and adenoid cystic carcinoma and CRC (47–50). The
luciferase activity assays of the present study demonstrated that
miR-34a binds to the 3′-UTRs of Notch1 and Jagged1. Additionally,
when miR-34a oligonucleotides were transfected into CRC cells, an
inverse expression pattern was observed between miR-34a and Notch1
and Jagged1 at the gene and protein levels, implying that miR-34a
targets Notch1 and Jagged1 through translational arrest and mRNA
degradation. Thus, it was concluded that miR-34a directly targets
Notch1 and Jagged1 in CRC cells.
Considering that miR-34a and Notch1/Jagged1 are
closely associated with tumor invasion and metastasis, the effects
of miR-34a on these phenotypes in CRC cells were also investigated.
miR-34a overexpression in SW480 cells markedly attenuated the cell
migratory and invasive abilities, whereas the overexpression of
Notch1 or Jagged1 rescued the miR-34a inhibition of cell migration
and invasion caused by miR-34a, suggesting that miR-34a regulation
of CRC invasion and metastasis is a consequence of targeting
upstream Notch signaling. In addition, an overexpression of miR-34a
decreased the expression of mesenchymal markers, vimentin and
fibronectin in colon cancer cells. The expression of Notch1 or
Jagged1 in SW480 cells recovered the protein expression of vimentin
and fibronectin by miR-34a. It is possible that miR-34a suppresses
CRC metastasis by targeting Notch1/Jagged1 and the vimentin and
fibronectin downstream.
In conclusion, the results of present study support
the miR-34a-mediated suppression of metastasis in CRC by targeting
Notch1/Jagged1 and their downstream molecules vimentin and
fibronectin. However, additional studies including in vivo
assays are needed to confirm the understanding of miR-34a
regulation in CRC tumorigenesis and metastasis. In addition, due to
the multiple gene targets involved, miR-34a regulation of Notch
signaling and the associated pathways require comprehensive
investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472286).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Nordlinger B, Adam R, Köhne
CH, Pozzo C, Poston G, Ychou M and Rougier P; European Colorectal
Metastases Treatment Group, : Towards a pan-European consensus on
the treatment of patients with colorectal liver metastases. Eur J
Cancer. 42:2212–2221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies RJ, Miller R and Coleman N:
Colorectal cancer screening: Prospects for molecular stool
analysis. Nat Rev Cancer. 5:199–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schee K, Boye K, Abrahamsen TW, Fodstad Ø
and Flatmark K: Clinical relevance of microRNA miR-21, miR-31,
miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC
Cancer. 12:5052012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R, Ma J, Wu Q, Xia J, Miele L, Sarkar
FH and Wang Z: Functional role of miR-34 family in human cancer.
Curr Drug Targets. 14:1185–1191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agostini M and Knight RA: miR-34: From
bench to bedside. Oncotarget. 5:872–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Martino MT, Leone E, Amodio N, Foresta
U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli
E, et al: Synthetic miR-34a mimics as a novel therapeutic agent for
multiple myeloma: In vitro and in vivo evidence. Clin Cancer Res.
18:6260–6270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cole KA, Attiyeh EF, Mosse YP, Laquaglia
MJ, Diskin SJ, Brodeur GM and Maris JM: A functional screen
identifies miR-34a as a candidate neuroblastoma tumor suppressor
gene. Mol Cancer Res. 6:735–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Guessous F, Zhang Y, Dipierro C,
Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen
TD, et al: MicroRNA-34a inhibits glioblastoma growth by targeting
multiple oncogenes. Cancer Res. 69:7569–7576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WB, Ma MW, Dong LJ, Wang F, Chen LX and
Li XR: MicroRNA-34a targets notch1 and inhibits cell proliferation
in glioblastoma multiforme. Cancer Biol Ther. 12:477–483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang RT, Leung CO, Ye TM, Liu W, Chiu PC,
Lam KK, Lee KF and Yeung WS: MicroRNA-34a suppresses invasion
through downregulation of Notch1 and Jagged1 in cervical carcinoma
and choriocarcinoma cells. Carcinogenesis. 31:1037–1044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang S, Li Y, Gao J, Zhang T, Li S, Luo A,
Chen H, Ding F, Wang X and Liu Z: MicroRNA-34 suppresses breast
cancer invasion and metastasis by directly targeting Fra-1.
Oncogene. 32:4294–4303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:pp. 15472–15477. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lefort K, Brooks Y, Ostano P, Cario-André
M, Calpini V, Guinea-Viniegra J, Albinger-Hegyi A, Hoetzenecker W,
Kolfschoten I, Wagner EF, et al: A miR-34a-SIRT6 axis in the
squamous cell differentiation network. EMBO J. 32:2248–2263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hahn S, Jackstadt R, Siemens H, Hünten S
and Hermeking H: SNAIL and miR-34a feed-forward regulation of
ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J.
32:3079–3095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nalls D, Tang SN, Rodova M, Srivastava RK
and Shankar S: Targeting epigenetic regulation of miR-34a for
treatment of pancreatic cancer by inhibition of pancreatic cancer
stem cells. PLoS One. 6:e240992011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bu P, Chen KY, Chen JH, Wang L, Walters J,
Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al: A
microRNA miR-34a-regulated bimodal switch targets Notch in colon
cancer stem cells. Cell Stem Cell. 12:602–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng CY, Hwang CI, Corney DC,
Flesken-Nikitin A, Jiang L, Oner GM, Munroe RJ, Schimenti JC,
Hermeking H and Nikitin AY: miR-34 cooperates with p53 in
suppression of prostate cancer by joint regulation of stem cell
compartment. Cell Rep. 6:1000–1007. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C and Tang DG: MicroRNA regulation of
cancer stem cells. Cancer Res. 71:5950–5954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wittekind C: 2010 TNM system: On the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zagouras P, Stifani S, Blaumueller CM,
Carcangiu ML and Artavanis-Tsakonas S: Alterations in Notch
signaling in neoplastic lesions of the human cervix. Proc Natl Acad
Sci USA. 92:pp. 6414–6418. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L,
Wang H, Huang C and Sun S: Hypoxia-induced down-regulation of
microRNA-34a promotes EMT by targeting the Notch signaling pathway
in tubular epithelial cells. PLoS One. 7:e307712012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Wu B, Chamberlain AA, Lui W,
Koirala P, Susztak K, Klein D, Taylor V and Zhou B: Endocardial to
myocardial notch-wnt-bmp axis regulates early heart valve
development. PLoS One. 8:e602442013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: A pleiotropically acting microRNA, miR-31, inhibits
breast cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang TC, Wentzel EA, Kent OA,
Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M,
Ferlito M, Lowenstein CJ, et al: Transactivation of miR-34a by p53
broadly influences gene expression and promotes apoptosis. Mol
Cell. 26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 2:211–215. 2011.
View Article : Google Scholar
|
|
41
|
Wu J, Wu G, Lv L, Ren YF, Zhang XJ, Xue
YF, Li G, Lu X, Sun Z and Tang KF: MicroRNA-34a inhibits migration
and invasion of colon cancer cells via targeting to Fra-1.
Carcinogenesis. 33:519–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dotto GP: Notch tumor suppressor function.
Oncogene. 27:5115–5123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su BH, Qu J, Song M, Huang XY, Hu XM, Xie
J, Zhao Y, Ding LC, She L, Chen J, et al: NOTCH1 signaling
contributes to cell growth, anti-apoptosis and metastasis in
salivary adenoid cystic carcinoma. Oncotarget. 5:6885–6895. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu
CW, Wang K, Zheng S, Ng SS, Chan FK, et al: microRNA-139-5p exerts
tumor suppressor function by targeting NOTCH1 in colorectal cancer.
Mol Cancer. 13:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Wang X, Xu J and Sun Y: Notch1
activation is a poor prognostic factor in patients with gastric
cancer. Br J Cancer. 110:2283–2290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang J, Francois R, Iyer R, Seshadri M,
Zajac-Kaye M and Hochwald SN: Current understanding of the
molecular biology of pancreatic neuroendocrine tumors. J Natl
Cancer Inst. 105:1005–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|

















