Introduction
Thyroid cancer accounts for 30% of head and neck
cancers and its morbidity and mortality rates rank first among the
endocrine malignancies. In recent years, there has been a
significant upward trend in the incidence of thyroid cancer
(1). It has been reported that
thyroid cancer patients in the United States in 2010 included
33,930 females and 10,740 males, of which 1,690 cases succumbed to
the disease (2). Well-differentiated
papillary thyroid carcinoma accounted for 80% of the cases, and the
10-year survival percentage for those patients was 90%, although
>10% of patients had recurrences or metastases (3). Once thyroid cancer reaches a late or
recurrent level, there is often a poor prognosis, and surgical
treatment is not indicated in many of these patients. To make
matters worse, the available radiotherapy/chemotherapy has not only
poor efficacy, but is also highly toxic and produces side effects.
Therefore, it is necessary to find highly efficient and low
toxicity drugs to treat thyroid cancers. In recent years, a growing
number of studies have turned to natural medicines characterized by
their low toxicity, high security and abundant sources. Finding
high efficacy, low toxicity antitumor active ingredients in natural
medicines has become a target for the drug research and development
industries.
Hyperoside, an important active ingredient of
Hypericum perforatum L, is a flavonoid glycoside compound
(4). Studies have been carried out on
the toxicology and pharmacology of hyperoside and related compounds
showing low toxicity and few side effects, when used as an
antioxidant, analgesic, anti-inflammatory, or to protect the
cardiovascular system (5), but there
is currently little research on the antitumor activities of these
compounds.
The elucidation of the detailed mechanisms of
players during apoptosis may have far-reaching effects on the
general mechanism of apoptosis and greatly aid in the search of
targets for drug design. Fas and its ligand FasL are well-known
apoptosis-related cell membrane surface molecules. Survivin is a
new member of the family of inhibitors of apoptosis (IAPs) and the
most potent inhibitor of apoptosis found so far. Survivin has
complex functions including inhibiting apoptosis, promoting cell
transformation, participating in cell mitosis, angiogenesis, and
drug resistance in tumor cells (6).
In this study, a thyroid squamous cell carcinoma cell line was
treated with hyperoside, the resulting apoptosis in the cells was
quantified and the expression of Fas, FasL and survivin was
measured to elucidate possible mechanisms and provide a theoretical
basis for the clinical use of hyperoside against thyroid
cancer.
Materials and methods
Cell culture
Human thyroid squamous cell carcinoma SW579 cells
(Cell Bank of the Chinese Academy of Sciences, Shanghai, China)
were cultured in DMEM (Gibco Life Technologies, Carlsbad, CA, USA)
in an incubator at 37°C with 5% CO2. Once the cells
reached 80% confluence, the cells were removed with trypsin and
dissolved in DMEM (containing 10% calf serum) to a final
concentration of 2×108/l. The cells were counted and
seeded in corresponding culture plates for subsequent
experiments.
MTT detection of cell proliferation
inhibition rate
Cells were treated with hyperoside (Sigma, St.
Louis, MO, USA) and the cell viability was detected using the MTT
colorimetric assay (Sigma), and the cells were seeded in 96-well
plates at a concentration of 1×105/ml with 100 µl
DMEM/well. After 24 h, hyperoside was added (to final
concentrations of 0, 2.5, 5, 10 and 20 µg/ml, respectively). Five
wells were replica-plated for each concentration and the experiment
was repeated six times independently. The control group cells were
not treated with hyperoside. After incubating the cells at 37°C
with 5% CO2 for 24, 48 and 72 h, respectively, 10 µl MTT
was added into each well to a final concentration of 5 mg/ml, after
4 h, the optical density (OD) at 570 nm was measured using a
microplate reader. The cell inhibition rate was calculated
according to the formula: inhibition rate (%) = (OD value of the
control group - OD value of the experimental group/OD value of the
control group) × 100%.
Morphological observation
After hyperoside treatment for 24 h (with 0, 5, 10
and 20 mg/ml hyperoside), any morphological changes were observed
and images were captured with an inverted microscope (Nikon, Tokyo,
Japan).
Cell apoptosis analysis
SW579 cells in the logarithmic growth phase were
seeded in 6-well plates. After 12 h, the cells were treated with 0,
5, 10 and 20 µg/ml hyperoside, respectively. After 24 h, the cells
were washed three times with PBS, digested with trypsin and then
centrifuged. The specific protocol for the Annexin V-FITC apoptosis
detection kit (Biyuntian Biotechnology Research Institute, Jiangsu,
China) was carried out as per the manufacturers instructions.
Briefly, after centrifugation the cells were resuspended in 0.3 ml
of binding buffer. Then, 5 µl of Annexin V and 5 µl of PI were
added, and the cells were incubated at room temperature in the dark
for 15 min. Binding buffer (0.2 ml) was added to each sample and
cell apoptosis was detected by flow cytometry (Becton, Dickinson
and Company, Franklin Lakes, NJ, USA).
Expression of Fas and FasL mRNAs
detected by RT-qPCR
Cells were seeded in 6-well plates with
1×104 cells/well. After 24 h, the supernatant was
aspirated and cells were treated with 0, 5, 10 and 20 µg/ml
hyperoside, respectively. After 24 h, the cells were collected and
total RNA was extracted according to the protocol in the RNA
extraction kit (Invitrogen Life Technologies, Carlsbad, CA, USA).
The concentration and purity of total RNA were detected by UV-Vis
spectrophoto-meter (Hitachi, Tokyo, Japan) (A260/A280 >1.8
indicating pure RNA). cDNA was obtained via reverse transcription
from RNA and the expression of Fas and FasL mRNAs was detected by
RT-PCR based on the instruction of the RT-PCR kit (Invitrogen Life
Technologies). Primers were designed by Takara Bio (Dalian, China)
(Table I). Reaction conditions were
as follows: 94°C for 5 min; then 94°C for 30 sec, 57°C for 30 sec,
72°C for 30 sec, a total of 35 cycles of amplification; and a final
elongation at 72°C for 5 min. The Ct value was automatically
calculated by software, the Ct values were all normalized against
the abundance of the GAPDH control RNA, and the relative
quantification of gene expression was calculated by the
2−ΔCt method according to the formula: ΔCt (target gene)
= Ct (target gene) - Ct (control gene).
 | Table I.Primer sequences of Fas and FasL. |
Table I.
Primer sequences of Fas and FasL.
| Gene | Primer sequences |
|---|
| Fas | F:
5-GGCATCTGGACCCTCCTACCTCTG-3′ |
|
| R:
5-CCTTGGAGTTGATGTCAGTCACTTGG-3′ |
| FasL | F:
5-GGCCTGTGTCTCCTTGTGAT-3′ |
|
| R:
5-TGCCAGCTCCTTCTGAAGTA-3′ |
| GAPDH | F:
5-ATGGCACCGTCAAGGCTGAG-3′ |
|
| R:
5-GCAGTGATGGCATGGACTGT-3′ |
Expression of the survivin protein by
western blotting
Cells were seeded in 6-well plates with
104 cells/well. After 24 h, the supernatants were
aspirated and the cells were treated with 0, 5, 10 and 20 µg/ml
hyperoside, respectively. After 24 h, the cells were collected and
lysed with cell lysis buffer (Biyuntian Biotechnology Research
Institute), and then centrifuged for 15 min at high speed and low
temperature. After centrifugation at 10,050 × g for 15 min the
supernatant containing the soluble fraction was collected. The
extracted protein concentrations were determined by BCA kit
(Biyuntian Biotechnology Research Institute), 50 µg of protein was
separated on SDS-PAGE, and the separated protein was transferred to
a PVDF membrane. The membrane was incubated in blocking buffer for
1 h at room temperature and then primary rabbit monoclonal survivin
antibody (dilution, 1:500; cat. no. ab76424; Abcam, Cambridge, MA,
USA) was added and the membrane was incubated over-night at 4°C.
After washing the membrane with TTBS, the secondary goat
anti-rabbit (HRP) IgG antibody (dilution, 1:2,000; cat. no. ab6721;
Abcam) was added; and the membrane was incubated at room
temperature for 1 h. ECL was then added on the membrane and blots
were developed in the dark. Images were recorded with a gel imaging
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), GAPDH was
used as the internal reference and grayscale values were analyzed
and compared. All the antibodies were purchased from Wuhan Sanying
Biotechnology (Wuhan, China).
Statistical analysis
SPSS 17.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analyses. Data are presented as means ±
standard deviation, and analyzed using one-way ANOVA. Differences
with a P<0.05 were considered statistically significant.
Results
Effect of hyperoside on proliferation
inhibition of SW579 cells
After cells were treated with 0, 2.5, 5, 10 and 20
µg/ml hyperoside, the proliferation of SW579 cells was
significantly inhibited in all groups. The proliferation inhibition
rates were significantly increased with the increase in
concentration and time (P<0.05) showing an obvious
dose-dependent mechanism (Table II).
However, this study aimed to examine the mechanism of apoptosis,
and since 2.5 µg/ml hyperoside did not elicit a clear inhibitory
effect on cell proliferation, subsequent experiments were carried
out with 5 µg/ml hyperoside as the smallest concentration used. The
duration of treatment was 24 h.
 | Table II.Inhibitory effects of different
concentrations of hyperoside on proliferation of SW579 cells (means
± standard deviation, n=30). |
Table II.
Inhibitory effects of different
concentrations of hyperoside on proliferation of SW579 cells (means
± standard deviation, n=30).
|
| Cell proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Concentration
(µg/ml) | 24 h | 48 h | 72 h |
|---|
| Control group
(0) | 0 | 0 | 0 |
| 2.5 |
20.12±2.36a |
33.65±4.23a |
47.92±5.03a |
| 5 |
29.65±2.86a |
49.53±7.01a |
66.25±7.16a |
| 10 |
35.37±3.02a |
63.72±6.78a |
80.43±8.41a |
| 20 |
46.94±3.12a |
73.77±6.25a |
82.83±9.61a |
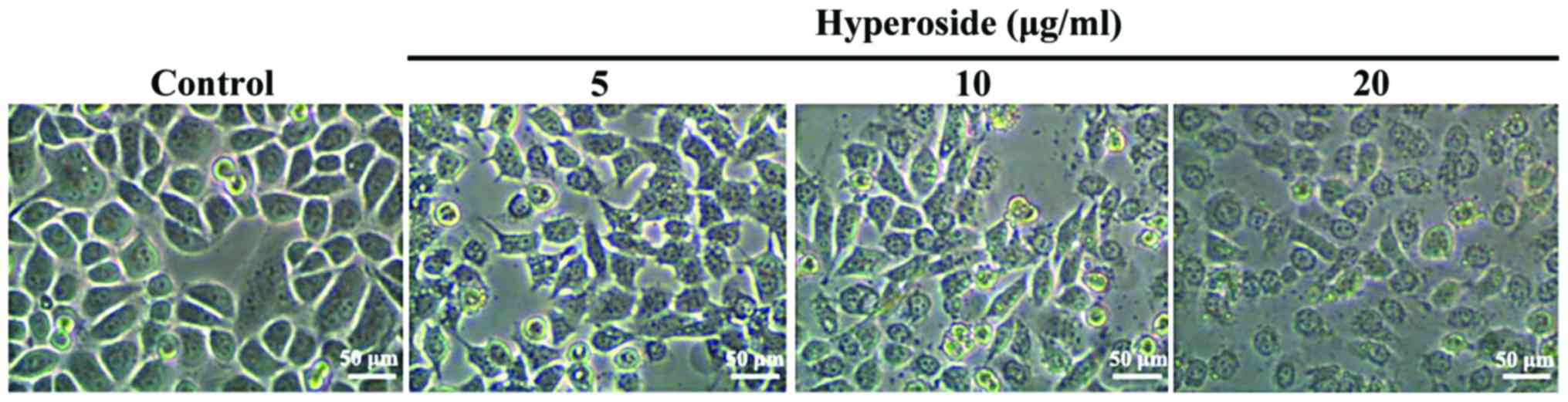
Effect of hyperoside on SW579 cell
morphology
As shown in Fig. 1,
after 24 h in the presence of 5, 10 and 20 µM hyperoside,
respectively, the cells underwent significant morphological changes
in a dose-dependent manner involving cell shrinkage, loose
adherence, a decrease in cell number and an increase in the number
of cell death.
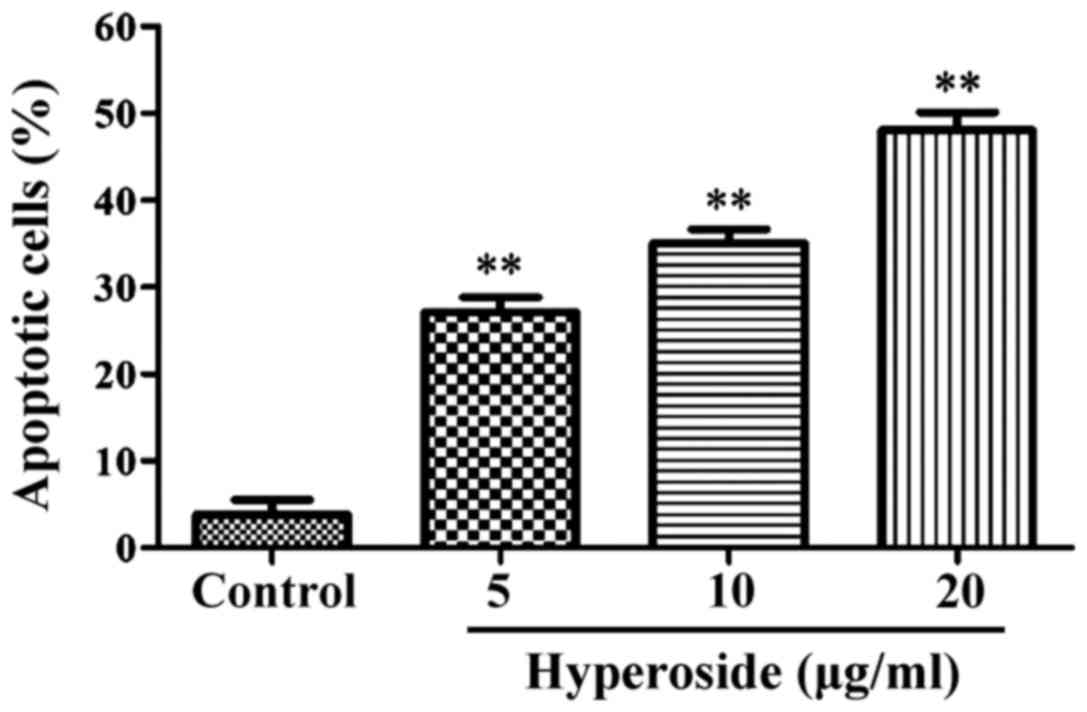
Effect of hyperoside on SW579 cell
apoptosis
As shown in Fig. 2,
flow cytometry results showed the apoptotic rates as 3.23±0.52,
27.34±3.18, 33.93±3.78 and 46.63±4.75% in the control 5, 10 and 20
µg/ml hyperoside groups, respectively. Compared with the control
group, the apoptotic rates of all the other groups were
significantly increased (P<0.01).
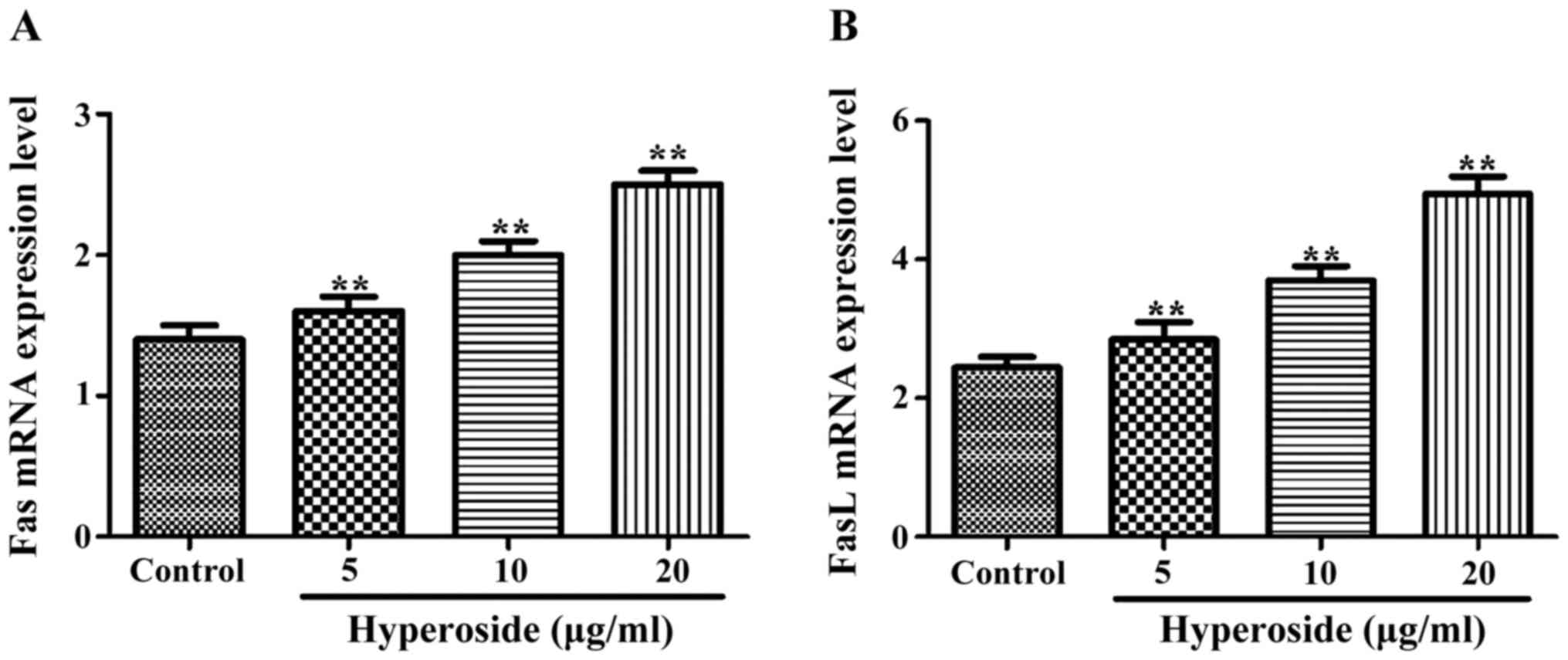
Effect of hyperoside on Fas and FasL
mRNA expression
As shown in Fig. 3,
the normalized expression of Fas and FasL mRNAs in each hyperoside
group was significantly higher than that in the control group, and
increased along with the increasing concentrations of hyperoside
treatment.
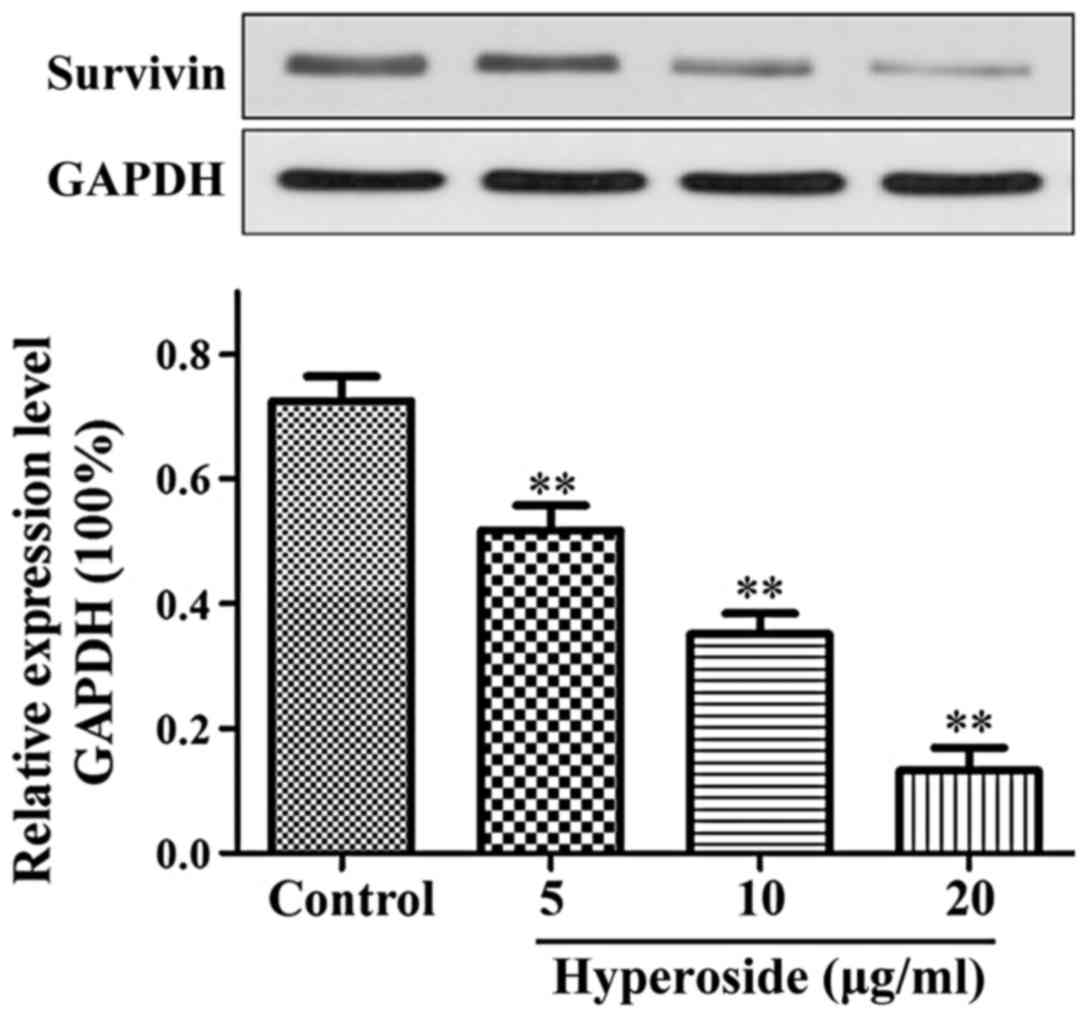
Effect of hyperoside on the expression
of survivin protein
As shown in Fig. 4,
the expression of survivin protein in each hyperoside group was
significantly lower than that in the control group and decreased in
a step-wise manner with each increase in the hyperoside
concentration applied (P<0.01).
Discussion
At present, the high incidence of thyroid cancer in
Chinese women has made it one of the most common malignancies
(1,2).
Furthermore, the incidence and mortality rates of thyroid cancer
are experiencing upward trends in China (3). The treatment based on chemical drugs is
expensive and not very effective and carries with it significant
side effects. Therefore, it is necessary to find a highly
efficient, low toxicity and affordable treatment. In this study, we
explored the effect of hyperoside on the apoptosis of the human
thyroid squamous cell carcinoma SW579 cell line and the possible
mechanisms leading to it.
Fas is a cell membrane receptor capable of
activating intracellular related apoptosis signaling pathways to
induce apoptosis when bound to its ligand FasL (7,8). The
normal Fas system plays an important role in the homeostasis of
organisms, maintaining a dynamic balance between apoptosis and
proliferation rates, and its abnormal expression can cause immune
system diseases and tumors and affects transplantation immunity
(9). The Fas/FasL signaling pathway
plays an important role in the process of tumor inhibition and its
expression has been found to be downregulated or lost in malignant
tumor cells. Furthermore, malignant tumor cells have been shown to
downregulate the expression of Fas, while they upregulate the
expression of FasL, which would lead to apoptosis induction in
tumor-fighting activated T cells, ultimately favouring the
proliferation of tumor cells (10,11). The
expression of Fas protein in gastric cancer has been associated
with the differentiation status of the cells, whereby poorly
differentiated gastric cancer cells express lower quantities of Fas
than well-differentiated cancer cells. Furthermore, studies have
confirmed that deletion of the Fas gene reduces apoptosis rates and
accelerates the proliferation of tumor cells (12). The survivin protein is an important
member of the family of apoptosis inhibitors and has the strongest
inhibitory effect on apoptosis currently identified (13). Survivin can inhibit apoptosis by
interfering with the function of cysteine proteolytic enzymes, and
its overexpression is closely related to the poor prognosis of
tumors and resistance to chemotherapeutic drugs (14).
In this study, the thyroid squamous cell carcinoma
SW579 cells were treated with different concentrations of
hyperoside for 24, 48 and 72 h, respectively. Results of MTT assays
showed that hyperoside significantly inhibited the activity of the
cells treated with hyperoside compared with the activity of cells
in the control group. With increasing concentrations and treatment
time, the proliferation inhibition rates were significantly
increased in a dose-dependent manner. The morphological changes
experienced by apoptotic SW579 cells included cell shrinkage, loose
adherence, a decrease in cell number and an increase in the number
of cell deaths. Additionally, the results of RT-PCR showed the
expression levels of Fas and FasL mRNAs increased with increasing
hyperoside concentrations suggesting that hyperoside can upregulate
the expressions of Fas and FasL and induce apoptosis. Shimada et
al (15) found a similar scenario
in non-small cell lung cancer cells where the expression of Fas in
tumor cells was decreased possibly leading to unlimited cell
proliferation (15). Their results
support our hypothesis that hyperoside can induce apoptosis of
SW579 cells by upregulating the expressions of Fas and FasL mRNAs.
Finally, results of western blotting in our study showed that the
expression of survivin was inhibited to a higher degree with
increasing hyperoside concentrations. Of note, no survivin gene
expression has been found in normal thyroid tissues, however,
survivin expression is active in thyroid cancer cells, and its
expression and the degree of malignancy are positively correlated
(16,17). Taken together, our findings suggest
that hyperoside-induced apoptosis is probably carried out via
inhibition of the expression of survivin protein and upregulation
of the Fas/FasL system. Our results provide a theoretical basis for
the therapeutic effect of hyperoside on thyroid squamous cell
carcinoma.
References
|
1
|
Pacini F: How far should we go in the
search and treatment of recurrent or persistent lymph node
metastases during follow-up of thyroid cancer patients? Eur Thyroid
J. 2:145–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brehar AC, Brehar FM, Bulgar AC and
Dumitrache C: Genetic and epigenetic alterations in differentiated
thyroid carcinoma. J Med Life. 6:403–408. 2013.PubMed/NCBI
|
|
4
|
Zou Y, Lu Y and Wei D: Antioxidant
activity of a flavonoid-rich extract of Hypericum perforatum L. in
vitro. J Agric Food Chem. 52:5032–5039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song BW, Ma CG and Xu SY: Hyperin: A new
peripheral analgesic agent. Asia Pac J Pharm. 3:1–5. 1998.
|
|
6
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin - prognostic tumor biomarker in human
neoplasms - review. Ginekol Pol. 83:537–540. 2012.PubMed/NCBI
|
|
7
|
Myong NH: Tissue microarray analysis of
Fas and FasL expressions in human non-small cell lung carcinomas;
with reference to the p53 and bcl-2 overexpressions. J Korean Med
Sci. 20:770–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang JS, Hsu YL, Kuo PL, Chiang LC and
Lin CC: Upregulation of Fas/Fas ligand-mediated apoptosis by
gossypol in an immortalized human alveolar lung cancer cell line.
Clin Exp Pharmacol Physiol. 31:716–722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryan AE, Shanahan F, Oconnell J and
Houston AM: Fas ligand promotes tumor immune evasion of colon
cancer in vivo. Cell Cycle. 5:246–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korkolopoulou P, Saetta AA, Levidou G,
Gigelou F, Lazaris A, Thymara I, Scliri M, Bousboukea K,
Michalopoulos NV, Apostolikas N, et al: c-FLIP expression in
colorectal carcinomas: Association with Fas/FasL expression and
prognostic implications. Histopathology. 51:150–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel RM, Chan FK, Chun HJ and Lenardo
MJ: The multifaceted role of Fas signaling in immune cell
homeostasis and autoimmunity. Nat Immunol. 1:469–474. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morimoto Y, Hizuta A, Ding EX, Ishii T,
Hongo T, Fujiwara T, Iwagaki H and Tanaka N: Functional expression
of Fas and Fas ligand on human intestinal intraepithelial
lymphocytes. Clin Exp Immunol. 116:84–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shariat SF, Ashfaq R, Karakiewicz PI,
Saeedi O, Sagalowsky AI and Lotan Y: Survivin expression is
associated with bladder cancer presence, stage, progression, and
mortality. Cancer. 109:1106–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beardmore VA, Ahonen LJ, Gorbsky GJ and
Kallio MJ: Survivin dynamics increases at centromeres during G2/M
phase transition and is regulated by microtubule-attachment and
Aurora B kinase activity. J Cell Sci. 117:4033–4042. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada T, Nishimura Y, Nishiuma T,
Rikitake Y, Hirase T and Yokoyama M: Adenoviral transfer of rho
family proteins to lung cancer cells ameliorates cell proliferation
and motility and increases apoptotic change. Kobe J Med Sci.
53:125–134. 2007.PubMed/NCBI
|
|
16
|
Olie RA, Simões-Wüst AP, Baumann B, Leech
SH, Fabbro D, Stahel RA and Zangemeister-Wittke U: A novel
antisense oligonucleotide targeting survivin expression induces
apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer
Res. 60:2805–2809. 2000.PubMed/NCBI
|
|
17
|
Wu YF, Cao MF, Gao YP, Chen F, Wang T,
Zumbika EP and Qian KX: Down-modulation of heat shock protein 70
and up-modulation of Caspase-3 during schisandrin B-induced
apoptosis in human hepatoma SMMC-7721 cells. World J Gastroenterol.
10:2944–2948. 2004. View Article : Google Scholar : PubMed/NCBI
|