Introduction
Glycosylation, one of the post-translational
modifications, commonly regulates protein stability, folding and
secretion (1–3). Certain types of glycosylation exist,
including N-linked and O-linked glycosylations,
C-mannosylation, and the formation of
glycosylphosphatidylinositol anchors. C-mannosylation is a
unique type of glycosylation in which α-D-mannose is attached
directly to the indole C2 carbon atom of a tryptophan
residue via a C-C linkage (4,5).
C-mannosylation occurs at the first tryptophan residue in
the consensus sequence W-X-X-W/C (X represents any amino acid)
(6). Certain C-mannosylated
proteins, including ribonuclease 2 (7), interleukin-12 (8), properdin (9) and mindin (10), have been identified; however, the
biological roles of C-mannosylation remain unclear. A
previous study reported that Caenorhabditis elegans (C.
elegans) dpy-19 is the C-mannosyltransferase for the
thrombospondin type-1 repeat (TSR-1)-derived peptide (11) and that human dpy-19 like 3 (DPY19L3),
one of the homologs of C. elegans dpy-19, catalyzes
C-mannosylation of R-spondin (Rspo) 1 at the W156
residue (12).
RPE-spondin (RPESP) is a protein that has unknown
functions and exists in the aorta extracellular matrix (13,14). RPESP
has certain domains: An N-terminal signal peptide toward the
secretory pathway, followed by a somatomedin B (SMB) domain and
TSR-1. Specific proteins containing the SMB domain have been
reported to bind plasminogen activator inhibitor-1 and
ectonucleotide pyrophosphatase/phosphodiesterase 1 (15–18);
however, the physiological functions of its association remain
unclear. In the TSR-1 domain, RPESP has two putative
C-mannosylation sites, the W80 and W83
residues. Previously, it was demonstrated that
C-mannosylation of TSR-1 in Rspo1 and Rspo3 regulates these
functions (12,19). The present study focused on RPESP
protein and examined the existence of C-mannosylation in
RPESP. The results suggested that RPESP is C-mannosylated at
both prediction sites and that DPY19L3 catalyzes
C-mannosylation of RPESP at W83 specifically.
Therefore, it was indicated that DPY19L3 has substrate specificity
and that C-mannosylation at W80 is catalyzed by
an unidentified C-mannosyltransferase. Furthermore, the
present study demonstrated that the expression of RPESP was
observed in certain human tumor cell lines, suggesting the
malignant roles of RPESP in tumorigenesis.
Materials and methods
Cell culture
Human HT1080 (JCRB Cell Bank, Osaka, Japan)
fibrosarcoma, A549 (RIKEN BioResource Center, Tsukuba, Japan)
non-small-cell lung cancer, HepG2 (RIKEN BioResource Center)
hepatocellular cancer, HT29 [American Type Culture Collection
(ATCC), Manassas, VA, USA] colon cancer and WM266-4 (ATCC) melanoma
cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM; Nissui, Tokyo, Japan) supplemented with 10% (v/v) fetal
bovine serum (FBS; BioWest S.A.S, Nuaillé, France), 100 U/ml
penicillin G, 100 mg/l kanamycin, 600 mg/l L-glutamine, and 2.25
g/l NaHCO3 at 37°C in a humidified incubator with 5%
CO2. Human ES2 (which was kindly donated by the
Department of Obstetrics and Gynecology, Keio University School of
Medicine, Tokyo, Japan) ovarian cancer and HCT116 (RIKEN
BioResource Center) colon cancer cell lines were cultured in DMEM
supplemented with 10% (v/v) heat-inactivated FBS, 100 U/ml
penicillin G, 100 mg/l kanamycin, 600 mg/l L-glutamine, and 2.25
g/l NaHCO3 at 37°C in a humidified incubator with 5%
CO2. Human COLO 205, CW-2 and LoVo colon cancer cell
lines (RIKEN BioResource Center) were cultured in RPMI-1640
(Nissui) supplemented with 10% (v/v) FBS, 100 U/ml penicillin G,
100 mg/l kanamycin, 300 mg/l L-glutamine, and 2.25 g/l
NaHCO3 at 37°C in a humidified incubator with 5%
CO2. The human Jurkat (ATCC) acute leukemia cell line
was cultured in RPMI-1640 supplemented with 10% (v/v)
heat-inactivated FBS, 100 U/ml penicillin G, 100 mg/l kanamycin,
300 mg/l L-glutamine, and 2.25 g/l NaHCO3 at 37°C in a
humidified incubator with 5% CO2. The S2 Drosophila
melanogaster embryonic cell line (RIKEN BioResource Center) was
cultured in Schneider's Drosophila medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% (v/v)
heat-inactivated FBS, 100 U/ml penicillin G and 100 mg/l kanamycin
at 25°C.
Plasmid construction
The synthetic DNA encoding C-terminally
Myc-His6-tagged human RPESP, optimized to the human
codon and introduced with N-terminal XhoI and C-terminal
NotI restriction enzyme sites, respectively, was purchased
from Thermo Fisher Scientific, Inc. The synthetic DNA was cloned
into the XhoI/NotI restriction sites of pCI-neo
vector (Promega Corporation, Madison, WI, USA). For expression in
S2 cells, C-terminal Myc-His6-tagged RPESP cDNA was
amplified by polymerase chain reaction (PCR) from
pCI-neo-RPESP-Myc-His6 using PrimeSTAR® Max DNA
Polymerase (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. The sequences of the primers used were as
follows: Forward, 5′-TTTTAGATCTGGATGTGCCGAAGCCGGCAGAT-3′ (Eurofins
Genomics, Ebersberg, Germany) and reverse,
5′-TTTTACGCGTCTAATGGTGATGGTGATGAT-3′ (Thermo Fisher Scientific,
Inc.). The reaction was conducted in a C1000™ thermal cycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the cycling
conditions were as follows: 30 cycles at 98°C for 10 sec, 55°C for
5 sec and 72°C for 5 sec. Subsequently, the
RPESP-Myc-His6 cDNA was subcloned into the
BglII/MluI restriction sites of pMT-PURO (RIKEN
BioResource Center) (20), resulting
in the expression of RPESP-Myc-His6 protein. Human
DPY19L1, DPY19L2, DPY19L3 and DPY19L4 cDNAs, which were subcloned
into pIZ/V5-his vectors (Thermo Fisher Scientific, Inc.), were
constructed previously (12).
Establishment of an
RPESP-overexpressing cell line
The permanent cell line expressing wild-type
RPESP-Myc-His6 was established by transfecting the
vectors using Lipofectamine® LTX (Thermo Fisher Scientific, Inc.)
into HT1080 cells for 24 h at 37°C, followed by 400 µg/ml G418
(Wako Pure Chemical Pure Industries, Ltd., Osaka, Japan) selection.
The clone that expressed high levels of Myc-His6-tagged
wild-type RPESP was designated HT1080-RPESP-MH. The cells that were
transfected with pCI-neo were designated HT1080-neo as control
(21).
Western blotting
Western blot analysis was performed according to a
previously described method with slight modification (22–26).
HT1080-neo (control) and HT1080-RPESP-MH cells were cultured for 24
h at 37°C and lysed in a lysis buffer [50 mM Tris-HCl (pH 7.5), 150
mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Triton X-100, 1% (w/v) sodium
deoxycholate and 1 mM phenylmethylsulfonyl fluoride] with
sonication (20 kHz, 50 W, 10 sec, twice) in an ultrasonic
homogenizer (UH-50; SMT Co., Ltd., Tokyo, Japan) at 4°C. The
lysates were centrifuged at 16,100 × g for 10 min at 4°C and the
amount of protein in each lysate was evaluated by staining with
Coomassie Brilliant Blue (CBB) G-250 (Bio-Rad Laboratories, Inc.).
A loading buffer [350 mM Tris-HCl (pH 6.8), 30% (w/v) glycerol,
0.012% (w/v) bromophenol blue, 6% (w/v) SDS and 30% (v/v)
2-mercaptoethanol] was added to each lysate, which was subsequently
boiled for 3 min. Proteins (15 µg per lane) were loaded and
electrophoresed on 12.5% SDS-polyacrylamide gels. The proteins were
transferred to polyvinylidene difluoride membranes. Membranes were
blocked with TBS-Tween-20 [TBST; 20 mM Tris-HCl (pH 7.6), 137 mM
NaCl and 0.1% (v/v) Tween-20] containing 5% Difco™ skim milk (cat.
no. 232100; BD Biosciences, Franklin Lakes, NJ, USA) for 30 min at
room temperature, and immunoblotted with anti-c-myc [dilution, 1:50
(9E10 hybridoma cultured supernatant in TBST containing 5% Difco™
skim milk; cat. no. 9E10 hybridoma; Developmental Studies Hybridoma
Bank, Iowa City, IA, USA) antibody for 1 h at room temperature.
Subsequently, membranes were incubated with TBST containing 5%
Difco™ skim milk secondary horseradish peroxidase (HRP)-conjugated
sheep polyclonal anti-mouse IgG (dilution, 1:3,000; cat. no.
NA931V; GE Healthcare Life Sciences, Little Chalfont, UK) for 1 h
at room temperature. Detection was performed using Immobilon
Western Chemiluminescent HRP substrates (EMD Millipore, Billerica,
MA, USA) on an ImageQuant LAS4000mini (GE Healthcare Life
Sciences). To detect all loading control proteins, the membrane was
stained by Coomassie brilliant blue solution at room temperature
for 5 min (27). Western blotting was
conducted in three independent experiments.
Purification of recombinant wild-type
RPESP from whole-cell lysate
HT1080-RPESP-MH cells were lysed using the
aforementioned lysis buffer, and the cell lysate was used for
purification. Initially, solid ammonium sulfate was slowly added
with agitation of the lysate preparations (125 ml) until 30%
saturation was achieved. The pH of the lysate was maintained at pH
8.0 by dropwise addition of 5 N NaOH and the mixture was incubated
on ice for 30 min. Subsequently, the precipitates were separated by
centrifugation at 16,100 × g for 30 min at 4°C and the supernatant
was collected. Solid ammonium sulfate was slowly added to the
supernatant until 60% saturation was achieved and the solution was
preserved on ice for another 30 min prior to centrifugation at
16,100 × g for 30 min at 4°C. The precipitates were collected and
dissolved in a small volume (~2 ml) of PBS, and added to 1% (w/v)
SDS. The mixture was sonicated (20 kHz, 50 W, 15 sec, 15 times) at
4°C and centrifuged at 16,100 × g for 15 min at 4°C. The
supernatant was subjected to buffer exchange with PBS using PD-10
Desalting Columns (GE Healthcare Life Sciences). Following the
addition of 8 M urea, the mixture was incubated with Ni-NTA agarose
(Qiagen GmbH, Hilden, Germany) for 2 h at 4°C. The Ni-NTA agarose
was washed three times with buffer A (900 mM NaCl, 2.7 mM KCl, 10
mM Na2HPO4, 1.8 mM
KH2PO4 and 20 mM imidazole) and Ni-NTA
agarose-bound RPESP was eluted with 100 µl of 500 mM imidazole.
Eluates (20 µl per lane) were loaded and electrophoresed on a 12.5%
SDS-PAGE and the protein bands were visualized using CBB staining
for 1 h at room temperature. The stained gel was scanned with
CanoScan LiDE 200 (Canon, Tokyo, Japan) using MP Navigator EX
software (Ver. 2.0.7; Canon).
Liquid chromatography-mass
spectrometry (LC-MS)
In order to determine the C-mannosylation
sites, the present study used the ultra-sensitive ‘Q-Exactive’ nano
LC-MS/MS system (28). Purified RPESP
samples were subjected to 12.5% SDS-PAGE. Following CBB staining
for 1 h at room temperature, the visible band was excised and
de-stained. The band was reduced using 50 mM dithiothreitol (Wako
Pure Chemical Industries, Ltd.) at 37°C for 2 h, followed by
carboxymethylated with 100 mM iodoacetate (Sigma-Aldrich; Merck
KGaA) at 25°C for 30 min. In-gel digestion was performed using
trypsin (TPCK-treated; Worthington Biochemical Corporation,
Lakewood, NJ, USA) at 37°C for 12 h. The digestion mixture was
separated on a nanoflow LC (Easy nLC; Thermo Fisher Scientific,
Inc.) using a nano-electrospray ionization spray column (NTCC
analytical C18 column, ϕ75 µm ×100 mm, 3 µm; Nikkyo Technos Co.,
Ltd., Tokyo, Japan) with a linear gradient of 5–60% buffer B (100%
acetonitrile and 0.1% formic acid) at a flow rate of 300 nl/min
over 10 min and subjected on-line to a Q-Exactive mass spectrometer
(Thermo Fisher Scientific, Inc.), which was equipped with a
nanospray ion source. MS and MS/MS data were acquired using the
data-dependent TOP10 method (28).
Obtained MS/MS data were searched against an in-house database,
including the RPESP sequence, using the MASCOT program (Matrix
Science, Inc., Boston, MA) with variable modifications:
Gln→pyro-Glu (N-term Q), oxidation (M), propionamide (C) and Hex
(W). MS/MS chromatograms and spectra were acquired using the
targeted MS/MS method (28).
Purification of RPESP from S2
cells
S2 cells were plated on 100 mm dishes and
transfected with pMT-RPESP-MH and pIZ-DPY19L1, pIZ-DPY19L2,
pIZ-DPY19L3, pIZ-DPY19L4 or pIZ as a control using transIT-Insect
Transfection reagent (Mirus Bio, LLC, Madison, WI, USA) at 25°C.
Following 24 h, the cells were washed and cultured in Schneider's
Drosophila medium at 25°C with 700 µM CuSO4 to induce
RPESP expression. Following 48 h of induction, the cells were lysed
in 1 ml of the aforementioned lysis buffer with sonication (20 kHz,
50 W, 15 sec, four times) at 4°C and 50 µl of Ni-NTA agarose was
added to the lysates. After 2 h, Ni-NTA agarose-bound RPESP was
eluted with 100 µl of 500 mM imidazole. Eluates (20 µl per lane)
were loaded and electrophoresed on 12.5% SDS-polyacrylamide gels.
The protein bands were visualized using CBB for 1 h at room
temperature and the visible band was excised and de-stained. The
samples were treated with N-(iodoethyl)-trifluoroacetamide for
cysteine-aminoethylation, followed by LC-MS analysis, as
aforementioned.
Reverse
transcription-semi-quantitative PCR (RT-sqPCR)
Reverse transcribed cDNAs of A549, ES2, HepG2,
HT1080, HT29, Jurkat, LNCaP, WM266-4, COLO 205, CW-2, HCT116 and
LoVo human cancer cell lines were used for PCR amplification using
Quick Taq HS Dye mix (Toyobo, Co., Ltd., Osaka, Japan), according
to the manufacturer's protocol. The sequences of the primers used
(synthesized by Thermo Fisher Scientific, Inc.) for RT-sqPCR were
as follows: RPESP forward, 5′-GACAGGGTCTACGGGACGTGTTTC-3′ and
reverse, 5′-TGCAGAGGTAGTTATAAAGGCAG-3′; β-actin forward,
5′-CTTCGAGCACGAGATGGCCA-3′ and reverse, 5′-CCAGACAGCACTGTGTTGGC-3′.
The cycling conditions were as follows: RPESP, 94°C for 2 min,
followed by 25 cycles at 94°C for 30 sec, 60°C for 30 sec and 68°C
for 30 sec; β-actin, 94°C for 2 min, followed by 20 cycles at 94°C
for 30 sec, 58°C for 30 sec and 68°C for 30 sec. β-actin was used
for the loading control. PCR products were electrophoresed on 2%
agarose gels, stained with ethidium bromide for 10 min at room
temperature and visualized using an ultraviolet illuminator. The
expression levels of transfected DPY19L1-L4 genes in S2 cells were
normalized to GAPDH and confirmed according to a previously
described method (12). RT-sqPCR was
conducted in three independent experiments.
Results
RPESP is C-mannosylated in cells
The amino acid sequence of human RPESP contains two
possible C-mannosylation sites at the W80 and
W83 residues in the TSR-1 domain (Fig. 1A). Although the physiological
functions of RPESP remain unclear, the Rspo family and certain
proteins have been reported to be C-mannosylated in the
TSR-1, and C-mannosylation of these proteins regulates their
functions, including secretion and Wnt/β-catenin signal-enhancing
activity (12,19). Therefore, C-mannosylation of
RPESP may regulate its functions. To determine whether RPESP is
C-mannosylated, the present study established an
RPESP-overexpressing HT1080 cell line, HT1080-RPESP-MH cells
(Fig. 1B). Although RPESP has a
predicted N-terminal signal peptide, the present study did not
detect RPESP secretion in the conditioned medium in the
HT1080-RPESP-MH cells (data not shown). Therefore, the present
study purified recombinant RPESP protein from whole-cell lysates of
HT1080-RPESP-MH cells for LC-MS analysis (Fig. 2A). The obtained recombinant RPESP was
treated with trypsin and the resulting mixture of peptides was
analyzed using the targeted MS/MS method. According to the
inclusion list of three quadruple-protonated parent ions of
un-mannosylated (m/z, 797.09), mono-mannosylated
(m/z, 837.60) and di-mannosylated (m/z, 878.11)
69ACPARPCFVGEWSPWSGCADQCKPTTR95 peptides,
MS/MS spectra were obtained. The selected ion chromatograms of
y5 ion of these parent ions were determined (Fig. 2B). Fig.
2B demonstrated that un- and mono-mannosylated peptides were
not detected, which suggests that all RPESP protein is
C-mannosylated at W80, and W83. The
MS/MS spectrum of the quadruple-charged di-mannosylated peptide ion
(m/z, 878.11) is presented in Fig.
2C. LC-MS/MS analysis of the peptide, modified by two mannose
residues, revealed that the differences in the theoretical
m/z values for non-mannosylated tryptophan between
b5-b11 and y3-y12 ions
were not observed. This result indicates that modification occurs
at the peptide 80WSPW83. Glycosylation at a
proline residue has not been reported. Furthermore, addition of
hexose at a serine residue by O-glycosylation has previously
been reported (29), including
O-glucosylation of epidermal growth factor-like repeats and
O-mannosylation of α-dystroglycan; however, these
modifications were site-specific, and RPESP did not satisfy the
requirements (29). Additionally, the
W80 and W83 residues met the requirements of
the C-mannosylation consensus sequence W-X-X-W/C, which
suggests that RPESP may be C-mannosylated at W80
and W83.
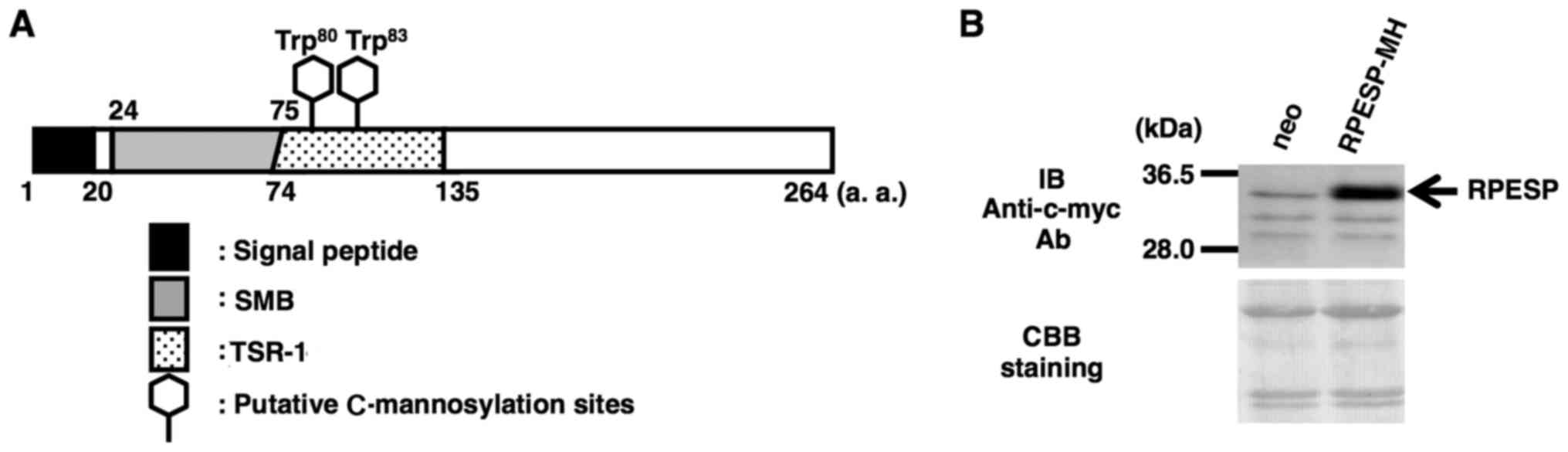 | Figure 1.Establishment of an
RPESP-overexpressing cell line. (A) Schematic diagram of human
RPESP. RPESP has two possible C-mannosylation sites within
TSR-1. A black box, gray box, dotted box and two hexagonal shapes
denote the signal peptide, SMB, TSR-1 and the putative
C-mannosylation sites, respectively. (B) Establishment of an
RPESP-overexpressing cell line, HT1080-RPESP-MH. HT1080-neo and
HT1080-RPESP-MH cells were lysed and each cell lysate was
electrophoresed, and immunoblotted with anti-c-myc antibody. The
membrane was stained by CBB solution following immunoblotting.
RPESP, RPE-spondin; TSR-1, thrombospondin type-1 repeat; SMB,
somatomedin B; CBB, Coomassie Brilliant Blue; a.a, amino acid; neo,
HT1080 cells expressing the pCI-neo vector. |
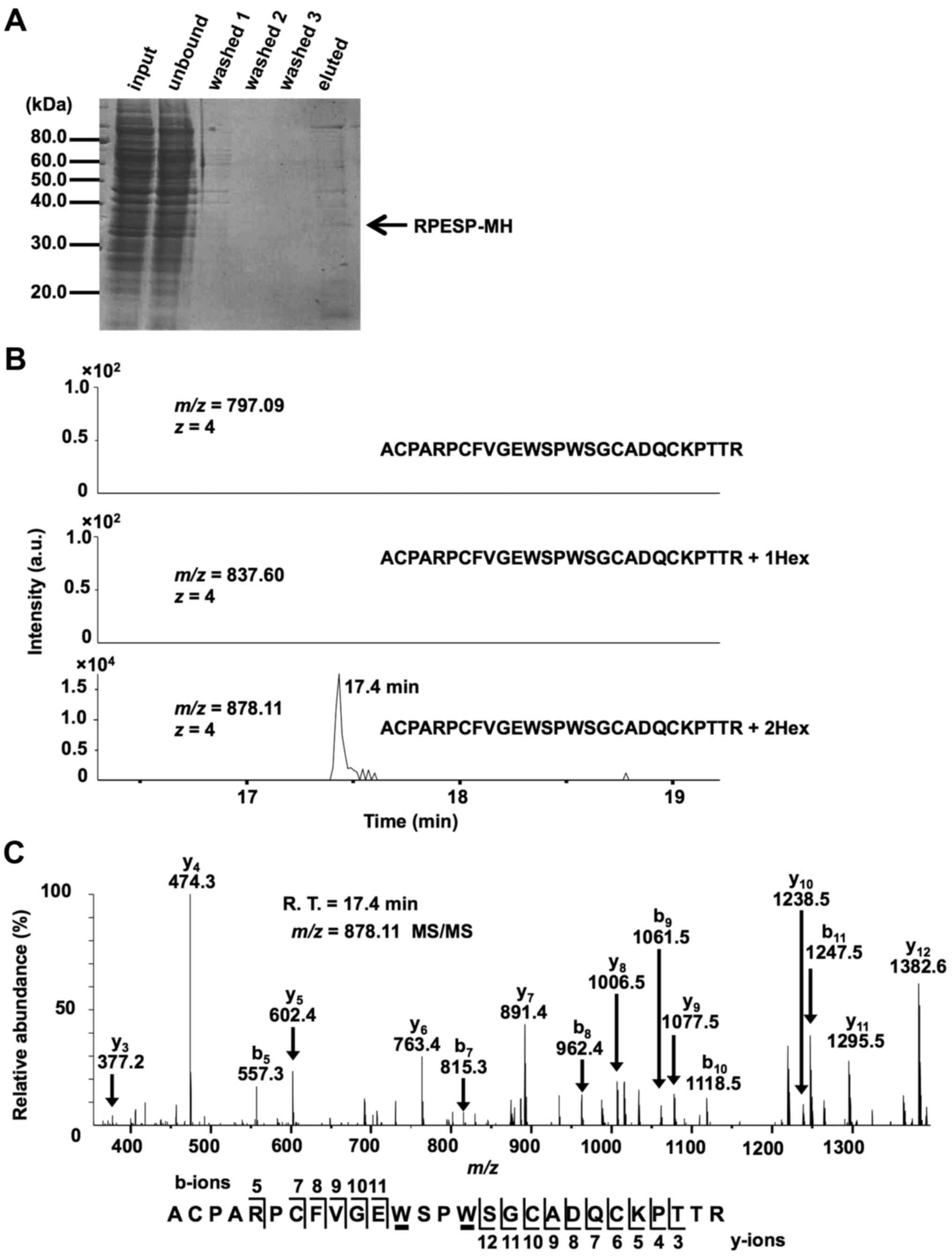 | Figure 2.RPESP is C-mannosylated in
cells. (A) Purification of recombinant RPESP-MH from whole-cell
lysate of HT1080-RPESP-MH. HT1080-RPESP-MH cells were lysed and
cell lysate was treated with ammonium sulfate. The precipitate
between 30–60% saturation of ammonium sulfate was dissolved in PBS
(input) and recombinant RPESP-MH was purified using Ni-NTA agarose.
These samples were electrophoresed and detected by CBB staining. (B
and C) Determination of C-mannosylation sites within RPESP.
The samples were digested with trypsin and the resulting peptides
were analyzed using the targeted MS/MS method. According to the
inclusion list of three quadruple-protonated parent ions of
un-mannosylated (m/z, 797.09), mono-mannosylated
(m/z, 837.60) and di-mannosylated
69ACPARPCFVGEWSPWSGCADQCKPTTR95 peptides
(m/z, 878.11), MS/MS spectra were obtained. Selected ion
chromatograms of y5 ion (602.366±20 ppm) of these parent
ions were determined (B). The MS/MS spectrum of the
quadruple-charged di-mannosylated peptide ion (m/z, 878.11)
is presented (C). The indicated b- and y-series ions were detected
as singly charged ions and C-mannosylation at the
W80 and W83 residues of RPESP were suggested.
C-mannosyltryptophans are underlined. RPESP, RPE-spondin;
CBB, Coomassie Brilliant Blue; MS, mass spectrometry; Hex, hexose;
R.T., retention time. |
Identification of DPY19L3 as the
C-mannosyltransferase of RPESP at W83
A previous report demonstrated that C.
elegans dpy-19 was identified as a C-mannosyltransferase
for TSR-1 (11), and that DPY19L3
catalyzes C-mannosylation of human Rspo1 at the
W156 residue in human cells (12). Since C-mannosylation of RPESP
occurred in TSR-1, the present study hypothesized that at least one
of the human homologs (DPY19L1-L4) of C. elegans dpy-19 may
be a C-mannosyltransferase of RPESP. In order to identify
the C-mannosyltransferase(s) of RPESP, the present study
performed gain-of-function experiments. Drosophila S2 cells
have no C-mannosyltransferase activity (11,30,31) but
harbor dolichol-phosphate-mannose, which is the donor substrate for
C-mannosylation. The present study transiently transfected
human RPESP cDNA with the probable C-mannosyltransferase
DPY19L1-L4 or empty vector (mock), respectively, into S2 cells. The
expression of transfected DPY19L1-L4 mRNAs in S2 cells was
confirmed by RT-sqPCR (Fig. 3A).
Recombinant RPESP proteins were purified from each S2 cell lysate
and analyzed using LC-MS. The obtained recombinant RPESP were
aminoethylated, digested with trypsin and the resulting peptides
were analyzed using the targeted MS/MS method. According to the
inclusion list of three doubly protonated parent ions of un-, mono-
and di-mannosylated 76FVGEWSPWSGC86 peptides,
MS/MS spectra were obtained. Selected ion chromatograms of
y5 ions of these parent ions were determined (Fig. 3A). Mono-mannosylated peptide was
observed only when RPESP was produced in DPY19L3-expressing S2
cells; conversely, di-mannosylated peptide was not observed in any
of the samples (Fig. 3A). These
results suggested that human DPY19L3 catalyzes
C-mannosylation of RPESP at a tryptophan residue. The MS/MS
spectra of the doubly charged un- and mono-mannosylated peptide ion
derived from DPY19L3-expressing S2 cells were presented (Fig. 3B). The y3 ion corresponded
well in un- and mono-mannosylated peptides; however,
y4-y6 ions were observed at an increased
position of m/z 162 in mono-mannosylated peptide compared
with the unmannosylated peptide (Fig.
3B). These results suggested that DPY19L3 may be the
C-mannosyltransferase of RPESP at only the W83
residue.
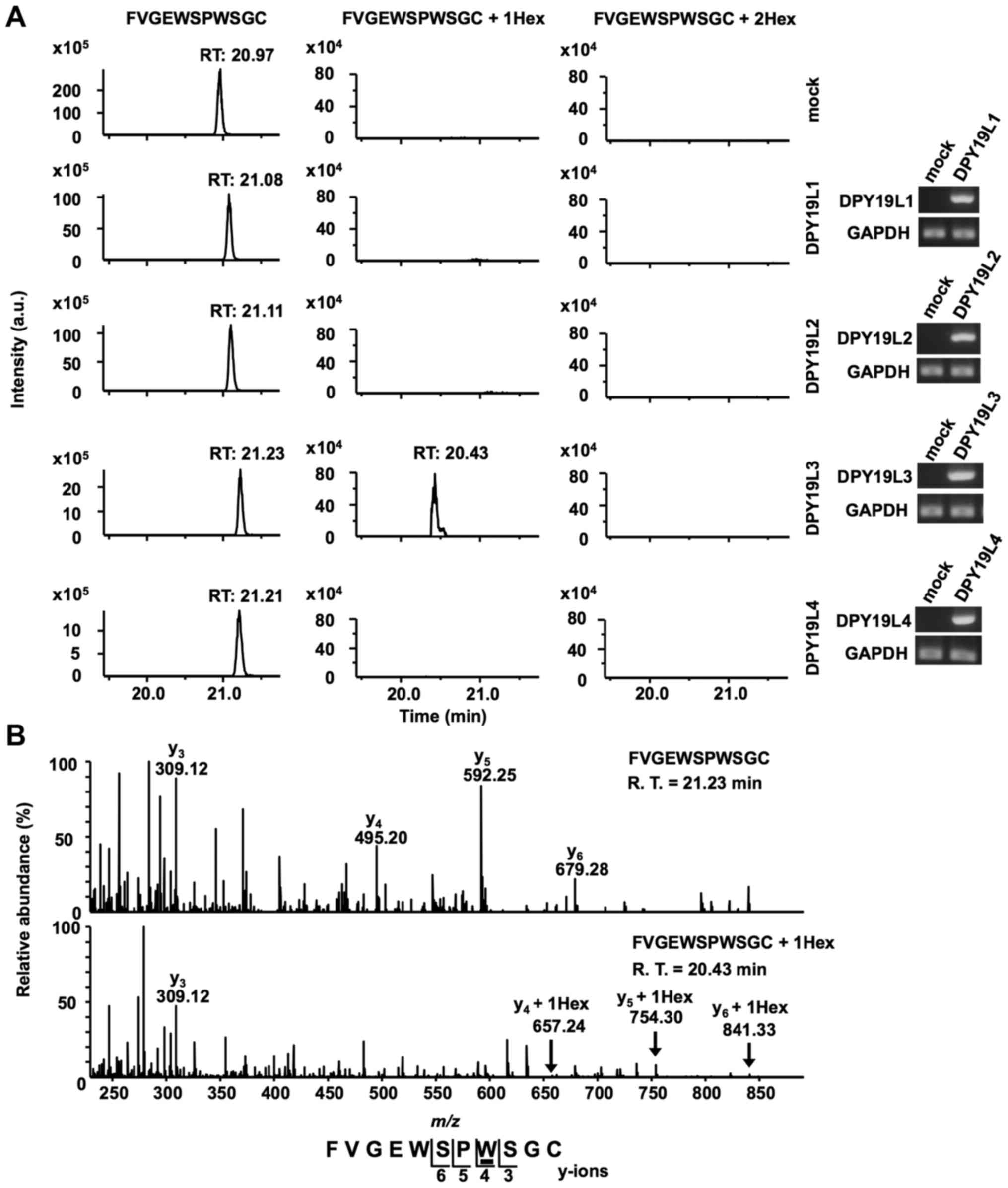 | Figure 3.DPY19L3 is the
C-mannosyltransferase of RPESP at only W83. (A
and B) Identification of C-mannosyltransferase of RPESP.
Human DPY19L1-L4 or empty vector (mock) and pMT-RPESP-MH were
transiently transfected into Drosophila S2 cells and protein
expression was induced using treatment with 700 µM CuSO4
for 48 h. RPESP-MH protein was purified with Ni-NTA agarose.
Following aminoethylation, the samples were digested with trypsin,
and the resulting peptides were analyzed by targeted MS/MS method.
According to the inclusion list of three doubly protonated parent
ions of un-mannosylated (m/z, 649.28), mono-mannosylated
(m/z, 730.31) and di-mannosylated
76FVGEWSPWSGC86 peptides (m/z,
811.34), MS/MS spectra were obtained. Selected ion chromatograms of
y5 ion of these parent ions were determined (A, left
panel). The expression of transfected DPY19L1-L4 mRNAs in S2 cells
was confirmed by reverse transcription-semi-quantitative polymerase
chain reaction (A, right panel). The MS/MS spectra of the doubly
charged un- (B, upper panel) and mono- (B, lower panel)
mannosylated peptide ion derived from DPY19L3-expressing S2 cells
are presented. The indicated y-series ions were detected as singly
charged ions and only the W83 residue of RPESP was
C-mannosylated. C-mannosyltryptophan is underlined.
DPY19L, dpy-19 like; MS, mass spectrometry; RPESP, RPE-spondin;
Hex, hexose; R.T., retention time. |
Expression level of RPESP in human
tumor cell lines
To examine the roles of RPESP in tumor malignancy,
the present study analyzed RPESP mRNA expression levels using
RT-sqPCR in a number of human tumor cell lines. Among 8 cell lines,
the expression level of RPESP in HT29 cells was high; however,
RPESP mRNA in other cell lines was undetectable under the assay
conditions maintained (Fig. 4A).
Since endogenous RPESP mRNA in HT29, a colon cancer cell line, was
detected, the present study evaluated the expression levels of
other human colon cancer cell lines. As presented in Fig. 4B, HT29, CW-2 and LoVo cells expressed
RPESP mRNA, although COLO 205 and HCT116 did not. Therefore, it was
suggested that RPESP may serve certain roles in tumorigenesis,
particularly in colon cancer.
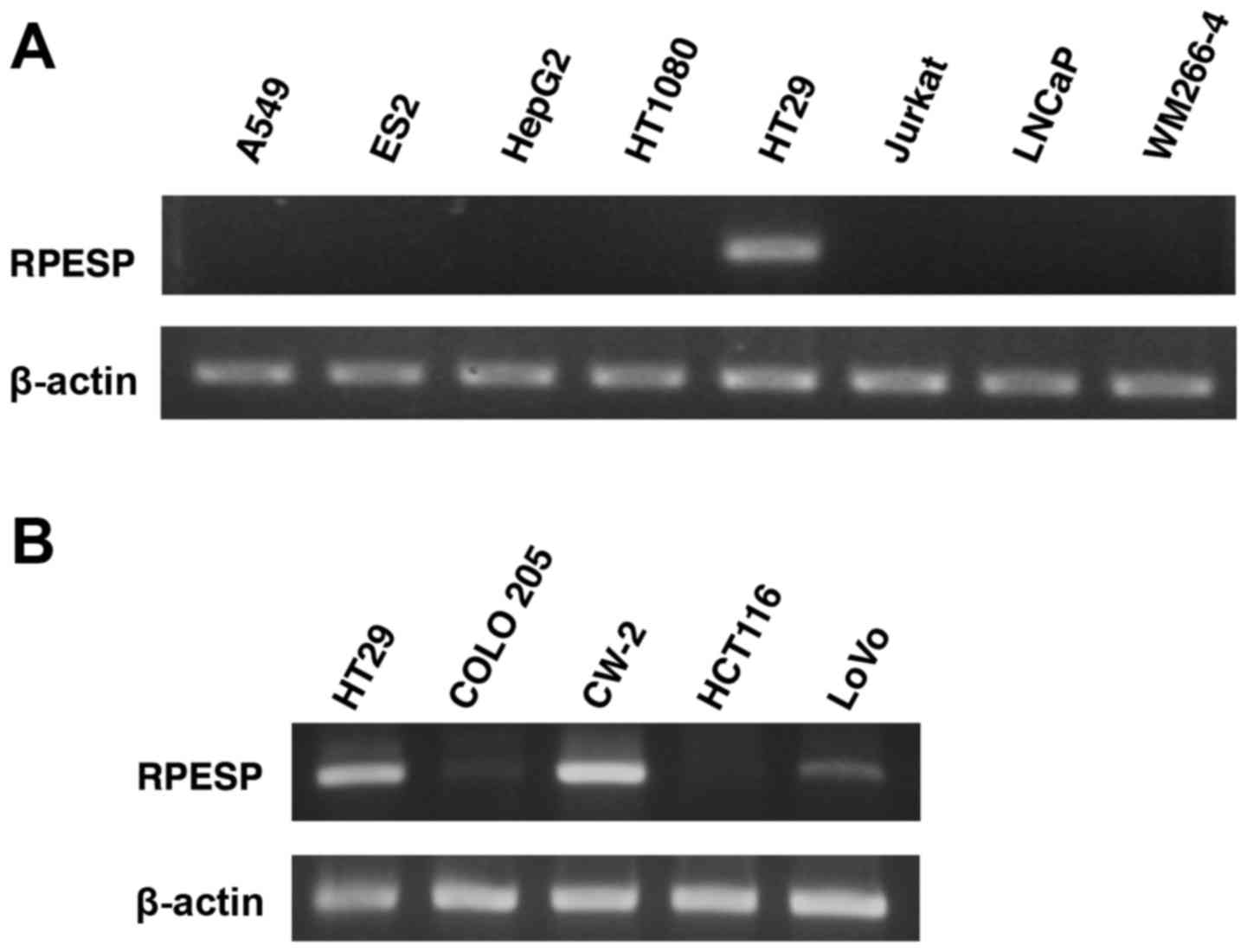 | Figure 4.RPESP is expressed in certain colon
cancer cell lines. (A) Expression of RPESP mRNA in human tumor cell
lines. Total RNAs were isolated from A549 lung adenocarcinoma, ES2
ovarian cancer, HepG2 hepatoma, HT1080 fibrosarcoma, HT29 colon
cancer, Jurkat leukemia, LNCaP prostate cancer and WM266-4 melanoma
cells and RT-sqPCR analysis was performed. (B) Expression of RPESP
mRNA in human colon cancer cell lines. Total RNAs were isolated
from human colon cancer cell lines, HT29, COLO 205, CW-2, HCT116
and LoVo, and RT-sqPCR analysis was performed. RT-sqPCR, reverse
transcription-semi-quantitative polymerase chain reaction; RPESP,
RPE-spondin. |
Discussion
The present study suggested that human RPESP may be
C-mannosylated at W80 and W83.
Previous studies have suggested that C-mannosylation has
potential functions, including protein folding, stability,
secretion and enzymatic activity (12,19,22,32,33).
Certain TSR-1 superfamily proteins are reported to be
C-mannosylated, particularly in Rspo1 and Rspo3; the
modifications regulate their secretion and the agonistic activity
of canonical Wnt signaling pathways (12,19);
however, the physiological functions of RPESP have not yet been
investigated. In our preliminary experiment, the present study
investigated the mRNA expression level of RPESP among various types
of cancer cell lines. RPESP was expressed in certain colon
adenocarcinoma cell lines (HT29, CW-2 and LoVo) but not in any
other cancer cell line investigated, which suggests an importance
of RPESP expression in colorectal cancer. Previously it was
reported that RPESP exists in the aorta extracellular matrix
(14); however, the physiological
functions remain unclear. Considering RPESP is expressed in colon
adenocarcinoma cell lines, RPESP may be involved in malignant
alteration of cancer. As the present study was not able to
elucidate the association between the physiological functions of
RPESP or the role of C-mannosylation on RPESP and cancer,
further studies are required.
A previous study demonstrated that C. elegans
dpy-19 was a C-mannosyltransferase of TSR-1 (11). Based on this study, the present study
investigated the human C-mannosyltransferase(s) of Rspo1,
which has two C-mannosylation sites at W153 and
W156, and identified human DPY19L3, one of the homologs
of C. elegans dpy-19, as a C-mannosyltransferase of
Rspo1 at W156 (12). The
present study also investigated the C-mannosyltransferase(s)
of RPESP, which has two C-mannosylation sites at
W80 and W83, and revealed that human RPESP
was C-mannosylated at W83 by DPY19L3. DPY19L3,
and not DPY19L1, DPY19L2 or DPY19L4, acted as a
C-mannosyltransferase of RPESP at the W83
residue, and C-mannosylation of RPESP at W80 was
not catalyzed by all DPY19 family proteins, suggesting the
existence of other C-mannosyltransferase member(s) in human
cells. Further studies are required in order to identify the novel
C-mannosyltransferase of RPESP at W80.
In conclusion, the present study demonstrated that
RPESP was C-mannosylated by DPY19L3 in human cells. Although
the physiological functions of RPESP and the significance of its
C-mannosylation remain unclear, our previous and present
studies suggest that human DPY19 family members have substrate
specificity, and that DPY19L3 may have a selective amino acid
sequence for C-mannosylation. These studies may aid in
furthering the understanding of DPY19L3-mediated
C-mannosylation and RPESP functions in cancer.
Acknowledgements
The present study was supported in part by
Grants-in-Aid for Scientific Research (B) (grant no. 24310167) and
Japan Society for the Promotion of Science Fellowship (grant no.
254256).
Glossary
Abbreviations
Abbreviations:
|
TSR-1
|
thrombospondin type-1 repeat
|
|
Rspo
|
R-spondin
|
|
RPESP
|
RPE-spondin
|
|
SMB
|
somatomedin B
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
CBB
|
Coomassie Brilliant Blue
|
|
LC-MS
|
liquid chromatography-mass
spectrometry
|
|
ER
|
endoplasmic reticulum
|
References
|
1
|
Dwek RA, Butters TD, Platt FM and Zitzmann
N: Targeting glycosylation as a therapeutic approach. Nat Rev Drug
Discov. 1:65–75. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simizu S, Ishida K, Wierzba MK and Osada
H: Secretion of heparanase protein is regulated by glycosylation in
human tumor cell lines. J Biol Chem. 279:2697–2703. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simizu S, Takagi S, Tamura Y and Osada H:
RECK-mediated suppression of tumor cell invasion is regulated by
glycosylation in human tumor cell lines. Cancer Res. 65:7455–7461.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furmanek A and Hofsteenge J: Protein
C-mannosylation: Facts and questions. Acta Biochim Pol. 47:781–789.
2000.PubMed/NCBI
|
|
5
|
Doucey MA, Hess D, Cacan R and Hofsteenge
J: Protein C-mannosylation is enzyme-catalysed and uses
dolichyl-phosphate-mannose as a precursor. Mol Biol Cell.
9:291–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krieg J, Hartmann S, Vicentini A, Gläsner
W, Hess D and Hofsteenge J: Recognition signal for C-mannosylation
of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol Biol
Cell. 9:301–309. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Beer T, Vliegenthart JF, Löffler A and
Hofsteenge J: The hexopyranosyl residue that is C-glycosidically
linked to the side chain of tryptophan-7 in human RNase Us is
alpha-mannopyranose. Biochemistry. 34:11785–11789. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doucey MA, Hess D, Blommers MJ and
Hofsteenge J: Recombinant human interleukin-12 is the second
example of a C-mannosylated protein. Glycobiology. 9:435–441. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartmann S and Hofsteenge J: Properdin,
the positive regulator of complement, is highly C-mannosylated. J
Biol Chem. 275:28569–28574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Cao C, Jia W, Yu L, Mo M, Wang Q,
Huang Y, Lim JM, Ishihara M, Wells L, et al: Structure of the
F-spondin domain of mindin, an integrin ligand and pattern
recognition molecule. EMBO J. 28:286–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buettner FF, Ashikov A, Tiemann B, Lehle L
and Bakker H: C. elegans DPY-19 is a C-mannosyltransferase
glycosylating thrombospondin repeats. Mol Cell. 50:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niwa Y, Suzuki T, Dohmae N and Simizu S:
Identification of DPY19L3 as the C-mannosyltransferase of
R-spondin1 in human cells. Mol Biol Cell. 27:744–756. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulz HL, Rahman FA, El Moula FM Fadl,
Stojic J, Gehrig A and Weber BH: Identifying differentially
expressed genes in the mammalian retina and the retinal pigment
epithelium by suppression subtractive hybridization. Cytogenet
Genome Res. 106:74–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Didangelos A, Yin X, Mandal K, Baumert M,
Jahangiri M and Mayr M: Proteomics characterization of
extracellular space components in the human aorta. Mol Cell
Proteomics. 9:2048–2062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou A, Huntington JA, Pannu NS, Carrell
RW and Read RJ: How vitronectin binds PAI-1 to modulate
fibrinolysis and cell migration. Nat Struct Biol. 10:541–544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamikubo Y, De Guzman R, Kroon G, Curriden
S, Neels JG, Churchill MJ, Dawson P, Ołdziej S, Jagielska A,
Scheraga HA, et al: Disulfide bonding arrangements in active forms
of the somatomedin B domain of human vitronectin. Biochemistry.
43:6519–6534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayasundari A, Whittemore NA, Serpersu EH
and Peterson CB: The solution structure of the N-terminal domain of
human vitronectin: Proximal sites that regulate fibrinolysis and
cell migration. J Biol Chem. 279:29359–29366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kato K, Nishimasu H, Okudaira S, Mihara E,
Ishitani R, Takagi J, Aoki J and Nureki O: Crystal structure of
Enpp1, an extracellular glycoprotein involved in bone
mineralization and insulin signaling. Proc Natl Acad Sci USA.
109:pp. 16876–16881. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujiwara M, Kato S, Niwa Y, Suzuki T,
Tsuchiya M, Sasazawa Y, Dohmae N and Simizu S: C-mannosylation of
R-spondin3 regulates its secretion and activity of Wnt/β-catenin
signaling in cells. FEBS Lett. 590:2639–2649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwaki T and Castellino FJ: A single
plasmid transfection that offers a significant advantage associated
with puromycin selection in Drosophila Schneider S2 cells
expressing heterologous proteins. Cytotechnology. 57:45–49. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niwa Y, Suzuki T, Dohmae N, Umezawa K and
Simizu S: Determination of cathepsin V activity and intracellular
trafficking by N-glycosylation. FEBS Lett. 586:3601–3607. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goto Y, Niwa Y, Suzuki T, Dohmae N,
Umezawa K and Simizu S: C-mannosylation of human hyaluronidase 1:
Possible roles for secretion and enzymatic activity. Int J Oncol.
45:344–350. 2014.PubMed/NCBI
|
|
23
|
Goto Y, Niwa Y, Suzuki T, Uematsu S,
Dohmae N and Simizu S: N-glycosylation is required for secretion
and enzymatic activity of human hyaluronidase1. FEBS Open Bio.
4:554–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simizu S, Suzuki T, Muroi M, Lai NS,
Takagi S, Dohmae N and Osada H: Involvement of disulfide bond
formation in the activation of heparanase. Cancer Res.
67:7841–7849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simizu S, Umezawa K, Takada M, Arber N and
Imoto M: Induction of hydrogen peroxide production and Bax
expression by caspase-3(−like) proteases in tyrosine kinase
inhibitor-induced apoptosis in human small cell lung carcinoma
cells. Exp Cell Res. 238:197–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyazaki S, Sasazawa Y, Mogi T, Suzuki T,
Yoshida K, Dohmae N, Takao K and Simizu S: Identification of
seco-clavilactone B as a novel small-molecule actin polymerization
inhibitor. FEBS Lett. 590:1163–1173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Welinder C and Ekblad L: Coomassie
staining as loading control in western blot analysis. J Proteome
Res. 10:1416–1419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Michalski A, Damoc E, Hauschild JP, Lange
O, Wieghaus A, Makarov A, Nagaraj N, Cox J, Mann M and Horning S:
Mass spectrometry-based proteomics using Q exactive, a
high-performance benchtop quadrupole Orbitrap mass spectrometer.
Mol Cell Proteomics. 10:M111.011015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moremen KW, Tiemeyer M and Nairn AV:
Vertebrate protein glycosylation: Diversity, synthesis and
function. Nat Rev Mol Cell Biol. 13:448–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krieg J, Gläsner W, Vicentini A, Doucey
MA, Löffler A, Hess D and Hofsteenge J: C-mannosylation of human
RNase 2 is an intracellular process performed by a variety of
cultured cells. J Biol Chem. 272:26687–26692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hofsteenge J, Huwiler KG, Macek B, Hess D,
Lawler J, Mosher DF and Peter-Katalinic J: C-mannosylation and
O-fucosylation of the thrombospondin type 1 module. J Biol Chem.
276:6485–6498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perez-Vilar J, Randell SH and Boucher RC:
C-mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology.
14:325–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasazawa Y, Sato N, Suzuki T, Dohmae N and
Simizu S: C-mannosylation of thrombopoietin receptor (c-Mpl)
regulates thrombopoietin-dependent JAK-STAT signaling. Biochem
Biophys Res Commun. 468:262–268. 2015. View Article : Google Scholar : PubMed/NCBI
|


















