Introduction
Inflammation is a hallmark of cancer, not only
triggering tumor development, but also promoting tumor progression,
therapy resistance and metastasis (1,2). Breast
cancer is a common type of malignancy, with >1.67 million new
cases and 522,000 mortalities reported in 2012 worldwide (3). Approximately 30% of females with breast
cancer experience recurrence within 5 years, with a 50% chance of
developing distant metastases (4,5). Cancer is
aptly described as a wound that never heals since solid tumors are
chronically inflamed and immune cells in concert with other stromal
components influence cancer cell behavior. Immune infiltrates
represent a significant component of the mass in solid tumors
(6) and aid in regulating cancer cell
growth, angiogenesis and invasion via the production of an array of
cytokines, reactive oxygen species, and proteases (7–10).
Tumor-associated macrophages (TAMs) are a prominent component,
serving a central role in promoting tumor growth and metastasis
(1). Accordingly, a greater abundance
of TAMs has been associated with metastasis and poor prognosis in
numerous types of solid tumors including breast (11,12), lung
(13,14), prostate (15), colorectal, and pancreatic cancer
(16–19). TAMs are recognized as potent producers
of growth factors (transforming growth factor-β, fibrobast growth
factors and epidermal growth factor), pro-angiogenic factors
[vascular endothelial growth factor, tumor necrosis factor-α
(TNF-α), interleukin (IL)-8, matrix metalloproteinase and platelet
derived growth factor], proteases (cathepsin and serine proteases)
and cytokines (IL-10), which profoundly affect epithelial cancer
cell growth, angiogenesis, local invasion, extracellualr matrix
degradation, epithelial-mesenchymal transition, metastasis, therapy
response, and immunosuppression (20–22).
Macrophages originate from peripheral blood
mononuclear cells derived from bone marrow and are recruited into
the tumor via colony stimulating factor 1 and C-X-C motif chemokine
ligand 12, released from cancer cells or the tumor microenvironment
(23). For successful tissue
migration, circulating immune cells undergo a sequential multistep
adhesion cascade initiated by adhesion to the vessel surface
(24–28). Vascular expression of selectin family
member proteins aids physical interaction with counter-receptor
ligands expressed on immune cells, including sialyl
Lewisx (sLex), sialyl LewisA
(sLeA), cluster of differentiation (CD)44, cutaneous
lymphocyte-associated antigen and P-selectin glycoprotein ligand-1
(29–31). E-selectin (also known as CD62E,
endothelial cell leukocyte adhesion-1 or Leukocyte-endothelial cell
adhesion molecule 2) is exclusively expressed on the luminal
surface of inflamed vessels and serves a role in the catch bond
that switches from rolling adhesion to integrin-mediated firm
adhesion (32). Thus, elevated
vascular E-selectin expression levels have been reported in a range
of solid tumors, including breast (11,12), lung
(13,14) and pancreatic (16,18)
cancer. E-selectin expression often synchronizes with an abundance
of sLex- or sLeA-positive immune infiltrates
in the tumor (33,34). The present study investigated the
abundance of tumor vascular E-selectin and macrophage marker CD68
expression levels in order to understand the association between
inflamed tumor vessels and TAM infiltration, as well as their role
in breast cancer prognosis.
Materials and methods
Tumors
Surgical whole mounts from a total of 100 human
breast carcinoma specimens from females diagnosed between January
1987 and December 1988 from the pathology archives at Thomas
Jefferson University (Philadelphia, USA) were used in the present
study. The average age of patients at the time of surgery was
61.8±16.4 years. Cases with tissue containing ductal carcinoma
in situ (DCIS) only, inflammatory breast cancer or other
concurrent malignancies were excluded from the present study. Cases
with no reactivity to vimentin staining were eliminated from the
study. Only cases with fully annotated information regarding
demographics, estrogen receptor (ER) expression, histology grade
and overall survival (OS) were used in final analyses.
Immunohistochemistry
For quality control, cases were first
immunohistochemically stained with anti-vimentin monoclonal
antibody (cat. no. 550513; BD Biosciences, San Jose, CA, USA) at a
1:250 dilution overnight at 4°C. Those without vimentin reactivity
were removed from the study. Double immunohistochemistry was
performed using formalin-fixed paraffin-embedded tumor sections (4
µm thickness). Briefly, following deparaffinization and
rehydration, antigen retrieval was performed using Envision Flex
Target Retrieval Solution (pH 6.1; Dako; Santa Clara, CA, USA) in a
pressure cooker for 20 min at 102°C. Endogenous peroxidase and
nonspecific epitopes were blocked with 0.3% hydrogen peroxide in
absolute methanol for 30 min at room temperature and 5% normal
horse serum and 1% normal goat serum (Sigma-Aldrich; St. Louis, MO,
USA) for 1 h at room temperature. Sections were incubated with
mouse anti-E-selectin monoclonal antibody at 1:100 (cat. no.
MO20039; Neuromics, Inc., Minneapolis, MN, USA) overnight at room
temperature. Following washing with PBS and subsequent blocking
with 5% normal horse serum and 1% normal goat serum for 5 min at
room temperature, the slides were incubated with pre-dilute
secondary horseradish peroxidase (HRP)-polymer conjugated
anti-mouse IgG (cat. no. K4001; Dako) for 30 min at room
temperature. HRP was detected using 3–3′-diaminobenzidine (DAB;
Biocare Medical LLC, Paheco, CA, USA) substrate for 10 min at room
temperature and enhanced using DAB Sparkle (Biocare Medical LLC)
for 1 min at room temperature. Residual antibodies were eluted
using Denaturing solution (Biocare Medical LLC) at a 1:3 dilution
for 3 min at room temperature to ensure no cross reaction between
the first and second staining. Slides were blocked with 5% normal
horse serum and 1% normal goat serum for 5 min at room temperature
and then incubated with mouse anti-CD68 monoclonal antibody at 1:25
(cat. no. M0876; Dako) overnight at 4°C. Following a brief wash
with PBS, the slides were incubated with pre-dilute secondary
alkaline phosphatase-polymer conjugated MACH2 anti-mouse IgG (cat.
no. MALP521; Biocare Medical LLC) for 1 h at room temperature and
then visualized using Fast-Red (Biocare Medical LLC) for 7 min,
followed by counterstain with Mayer Hematoxylin (Dako) for 4 min
both at room temperature. The slides were air-dried and mounted. As
a negative control, breast carcinoma tissues were immunostained
with the secondary IgG only.
Pathologic evaluation
All immunohistochemically stained slides were
evaluated by a board certified surgical pathologist (Department of
Pathology, School of Medicine, University of Oklahoma Health
Sciences Center, Oklahoma City, OK, USA) for breast pathology. The
tumors were classified and graded according to the protocol from of
the College of American Pathologists (Protocol for the Examination
of Specimens from Patients with Invasive Carcinoma of the Breast
according to InvasiveBreast 3.3.0.0.) (35). Tumor inflammation was defined as
positive or negative, as characterized by the presence of
lymphocyte clusters. Immunohistochemical reaction to E-selectin was
graded using intensity as a score of 0, 1+, 2+ or 3+ for no, weak,
moderate or strong reaction in endothelial cells within the tumor,
respectively. Immunohistochemical staining of CD68 was
quantitatively categorized as a score of 0, 1+, 2+ or 3+ for no
CD68+ cells, ≤10 CD68+ cells, 11–20
CD68+ cells or ≥21 CD68+ cells in the
observing field at ×200 magnification within the tumor,
respectively. All images were viewed under a light microscope
(DM2500; Leica, Buffalo Grove, IL, USA) and images were captured
using digital cooling color camera (DFC450; Leica).
Statistical analysis
The Fisher's exact test was used to analyze the
association between age and tumor pathological parameters using
CD68 and E-selectin expression levels. Spearman's rho was used to
determine the correlation between CD68+ TAMs, and
E-selectin expression level and tumor inflammation. The
Kaplan-Meier method was used to estimate OS as a function of time,
and differences were analyzed using the log-rank test. Cox
proportional hazards regression analysis was used for multivariable
analysis of prognostic factors in relation to OS. The statistical
software SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA) was
used to perform statistical analyses. GraphPad Prism version 6
(Graphpad Software, Inc., La Jolla, CA, USA) was used to generate
Kaplan-Meier curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
Tumor characteristics
Following exclusions, a total of 53 invasive breast
cancer cases that had been first-time diagnosed by surgical
resection in 1986–1988 at Thomas Jefferson University were used in
the present study. Tumor characteristics of these patients are
presented in Table I. Cases were
categorized as either high (score 3+) or low (score 0–2+) CD68
expression level according to the abundance of CD68+
TAMs in the tumor core and high (score 3+), or low (score 0–2+)
expression level of E-selectin on vessels in the tumor area.
CD68+ TAMs were significantly associated with tumor
inflammation (P=0.005) and ER status (P=0.037). E-selectin
expression level was associated with age (P=0.016) and was
significantly higher among females over the age of 60 years
(Table I).
 | Table I.Association between clinicopathologic
parameters, and CD68 and E-selectin expression in procedure-naïve
invasive breast carcinoma tissues. |
Table I.
Association between clinicopathologic
parameters, and CD68 and E-selectin expression in procedure-naïve
invasive breast carcinoma tissues.
|
|
| CD68
expression | E-selectin
expression |
|---|
|
|
|
|
|
|---|
| Clinicopathologic
parameters | Total no.
cases | Low | High | P-value | Low | High | P-value |
|---|
| All cases | 53 | 34 | 19 |
| 41 | 12 |
|
| Age |
|
|
| 0.349 |
|
| 0.016a |
|
≤60 | 20 | 14 | 6 |
| 19 | 1 |
|
|
>60 | 33 | 20 | 13 |
| 22 | 11 |
|
| Tumor
inflammation |
|
|
| 0.005a |
|
| 0.540 |
|
(−) | 25 | 21 | 4 |
| 19 | 6 |
|
|
(+) | 28 | 13 | 15 |
| 22 | 6 |
|
| Nottingham
histological grade |
|
|
| 0.561 |
|
| 0.455 |
| Grade
I | 3 | 8 | 4 |
| 3 | 0 |
|
| Grade
II + III | 50 | 26 | 15 |
| 38 | 12 |
|
| ER status |
|
|
| 0.037a |
|
| 0.521 |
|
Negative | 11 | 4 | 7 |
| 9 | 2 |
|
|
Positive | 42 | 30 | 12 |
| 32 | 10 |
|
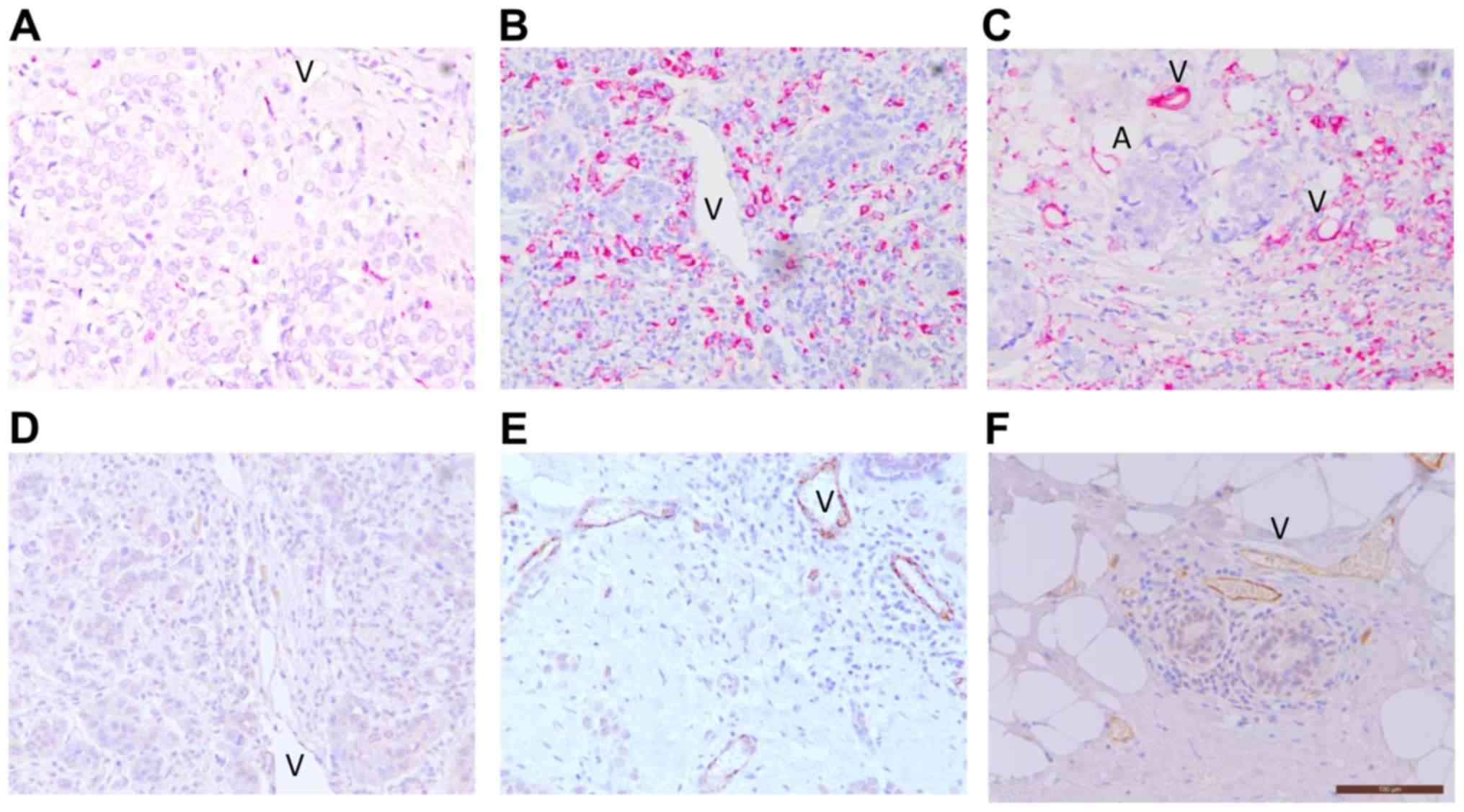
Pattern of CD68+ TAM infiltration and
E-selectin+ vessel inflammation in breast tumors
Representative images of high and low expression
levels of CD68+ TAMs, and E-selectin+
inflamed vessels are presented in Fig.
1. Various expression levels of CD68+ TAMs were
consistently present in the tumor stroma and core of all 53 cases
(Fig. 1A-C). Considerable,
multilayered CD68+ TAM deposition in the necrotic area
of the tumors was observed. CD68+ TAMs were also
abundant in the mammary fat adjacent to the tumor, as well as
around the luminal surface of vessels (Fig. 1C). E-selectin was predominantly
expressed on vessels within the tumor stroma (Fig. 1D and E). No positive signal for
E-selectin was detected in other tumor components, including cancer
cells, fibroblasts, immune infiltrates or the apical side of the
vessels. The size of E-selectin-expressing vessels varied from
small capillaries to large vessels in the stroma and fat adjacent
to the invasive front of tumors. Vessel structure was well retained
within the stroma but often compressed, crushed or even absent in
the tumor core. Overall, 88.7% of the breast carcinoma cases
exhibited E-selectin expression on their tumor vessels. Consistent
with inflammation of the adipose tissue adjacent to the tumor,
E-selectin expression level was also high in vessels of the
neighboring peripheral adipose tissue. Of note, vascular E-selectin
expression level was present in the stroma surrounding the mammary
duct in invasive carcinomas that retained a ductal structure
(Fig. 1F). A similar pattern of
E-selectin expressing vessels in the stroma around the mammary duct
in DCIS only cases was also revealed (data not shown), suggesting
the presence of peritumoral inflammation at the pre-invasive
stage.
Association between vessel
inflammation and TAM infiltration
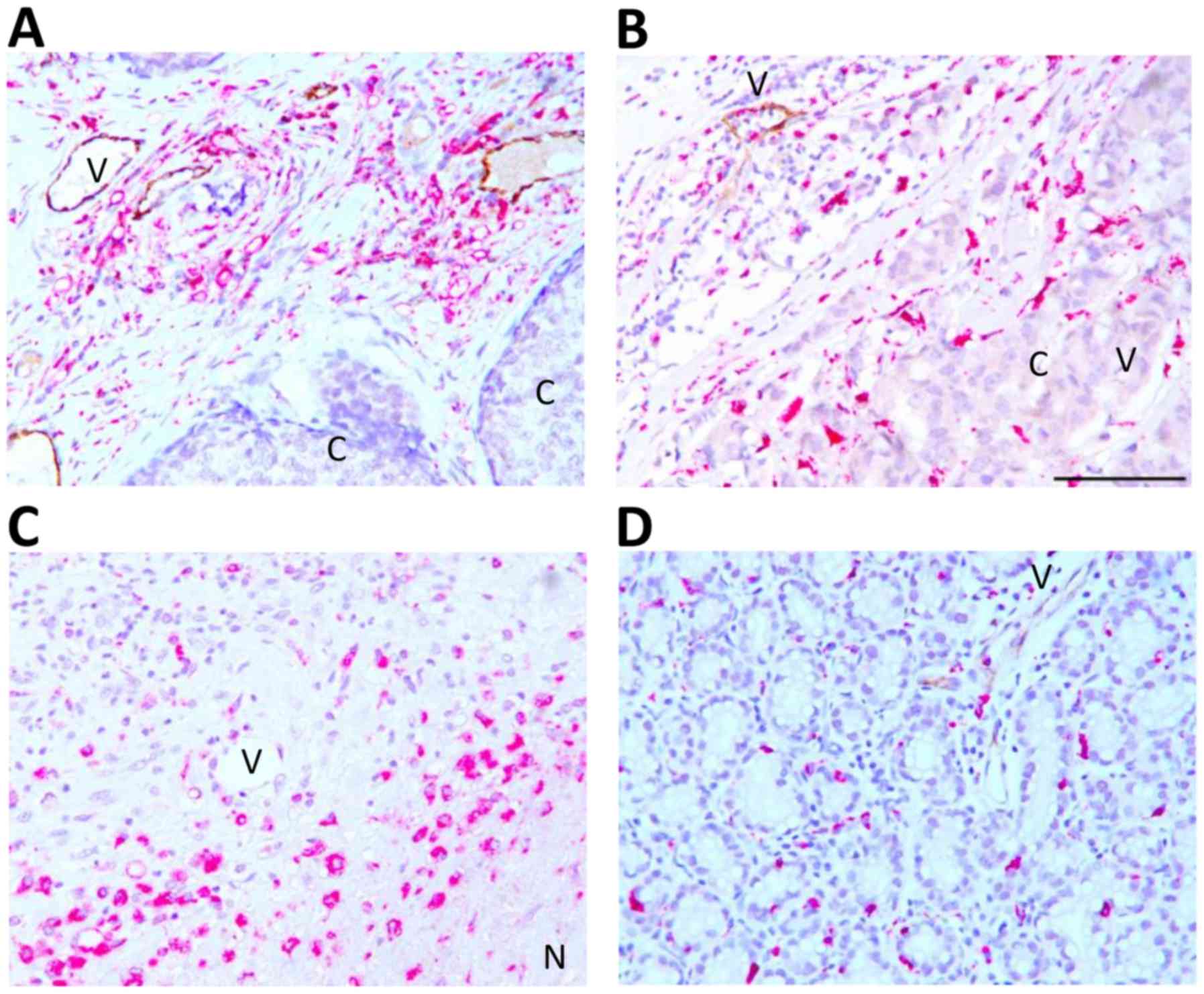
Double immunohistochemistry for CD68 (pink) and
E-selectin (brown) was performed to evaluate their association.
CD68+ TAMs were abundant in close proximity to
E-selectin-expressing vessels in the tumor stroma and peripheral
tissue adjacent to the tumor (Fig. 2A and
B). CD68+ TAMs were sparsely present in carcinoma
cell rich areas; however, E-selectin expression was limited to the
surrounding inflamed area and absent or weakly present in the tumor
core (Fig. 2B). CD68+ TAMs
were also highly abundant in necrotic areas, but E-selectin was
absent within and adjacent to the necrotic core (Fig. 2C). TAMs and E-selectin were present at
the location where the ductal structure was retained (Fig. 2D). Association between the abundance
of CD68+ TAMs and E-selectin+ vessels was
evaluated using a 4-level scoring scale (0, 1+, 2+, 3+) for the
expression level of each marker. High abundance of CD68+
TAMs and E-selectin+ vessels (3+/3+) was demonstrated in
7.5% of the overall analyzed samples. CD68+ TAMs and
E-selectin expression levels were positively correlated (r=0.30,
P=0.030; Table II). Additionally,
CD68+ TAMs were significantly correlated with tumor
inflammation (r=0.54, P=0.001; Table
II).
 | Table II.Association between CD68+
TAMs, and E-selectin expressing vessels and tumor inflammation in
procedure-naïve invasive breast carcinoma tissues. |
Table II.
Association between CD68+
TAMs, and E-selectin expressing vessels and tumor inflammation in
procedure-naïve invasive breast carcinoma tissues.
|
| E-selectin | Inflammation |
|---|
|
|
|
|
|---|
| CD68 | 0 | 1+ | 2+ | 3+ | − | + |
|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1+ | 3 | 5 | 4 | 2 | 13 | 1 |
| 2+ | 1 | 6 | 10 | 3 | 8 | 12 |
| 3+ | 2 | 2 | 8 | 7 | 4 | 15 |
|
|
| *P=0.030,
r=0.302 |
|
| *P<0.001,
r=0.541 |
|
Abundance of markers and clinical
outcome
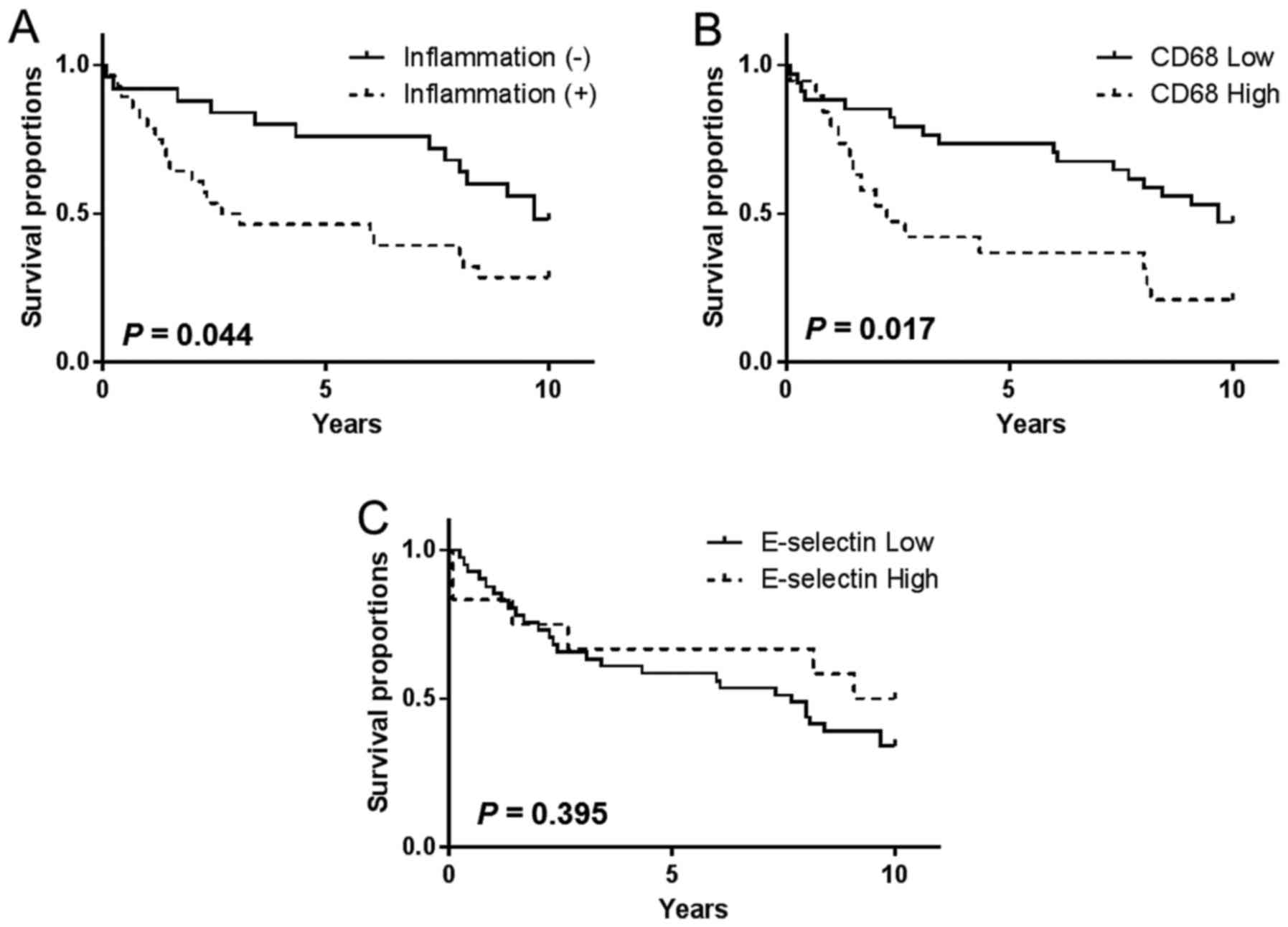
OS was determined and graphically presented using
the Kaplan-Meier method, and a Cox proportional hazards regression
model was used for multivariable analysis of the association
between clinicopathological parameters and marker expression level
with OS. Tumor inflammation was significantly associated with OS
among patients with breast cancer [hazard ratio (HR), 2.00;
confidence interval (CI) 95%, 1.03–4.06; P=0.044; Fig. 3A]. However, inflammation in the tumor
periphery lacked association with OS (HR, 1.20; CI 95%, 0.560–2.61;
P=0.629; data not shown). Compared with tissue samples with lower
expression levels of CD68+ TAMs, higher expression
levels of CD68+ TAMs in the tumor core were
significantly associated with shorter OS at the 10-year follow-up
(HR, 2.23; CI 95%, 1.12–5.54; P=0.017; Fig. 3B). Conversely, CD68+ TAMs
in the tumor periphery were not significantly associated with OS
(HR, 1.39; CI 95%, 0.59–3.50; P=0.420; data not shown). Although
the abundance of E-selectin+ vessels and
CD68+ TAMs were positively correlated, E-selectin
expression level alone did not impact OS at the tumor (HR, 0.71; CI
95%, 0.32–1.57; P=0.395; Fig. 3C) or
periphery. Kaplan-Meier analysis demonstrated that OS was
unaffected by age for CD68, E-selectin or tumor inflammation
statuses (data not shown). Finally, multivariable analysis of
procedure-naïve breast tumor tissues revealed that the presence of
abundant CD68+ TAMs was an independent predictor of OS
(HR, 2.37; 95% CI, 1.02–5.36; P=0.045) following adjustment for ER
status, tumor inflammation and E-selectin expression level
(Table III).
 | Table III.Multivariable Cox proportional hazard
regression analysis of overall survival. |
Table III.
Multivariable Cox proportional hazard
regression analysis of overall survival.
| Variables | P-value | Hazard ratio | 95% CI |
|---|
| Tumor inflammation
(ref. positive) | 0.326 | 1.48 | 0.68–3.26 |
| ER status (ref.
negative) | 0.341 | 1.50 | 0.65–3.42 |
| E-selectin
expression (ref. high) | 0.112 | 0.46 | 0.18–1.20 |
| CD68 expression
(ref. high) | 0.045a | 2.34 | 1.02–5.36 |
Discussion
Tissue infiltration by circulating leukocytes occurs
in response to tissue damage and injury. In the context of solid
tumors, cell death arises from intrinsic and extrinsic inducers,
initiating an inflammatory cascade in an attempt to scavenge
debris, and repair damaged tissue. Intrinsic cell death is
hypoxia-derived necrosis or DNA-damage-associated apoptosis,
whereas extrinsic cell death is associated with external stimuli,
including chemotherapy, biopsy, surgery or radiation therapy. The
standard of care for breast cancer has altered significantly over
the past 3 decades (35). Diagnostic
needle biopsy became popular in the 1990s (36) and neoadjuvant chemotherapy for
relatively large, locally advanced tumors emerged in the 2000s
(37). Both procedures provoke
inflammation accompanied by cell death in the tumor and neighboring
peripheral tissue. Thus, it is likely that recent surgically
resected tumors contain inflammation induced by extrinsic stress
along with naturally occurring intrinsic cell death. Although
external stress naïve tumors can be analyzed using biopsy samples,
such samples contain only small and limited amounts of tissue,
making the capture of the overall tumor environment difficult.
Analysis of naïve tumors collected by excisional biopsy essentially
eliminates locally advanced tumors since this method is typically
only used in early stage small-sized tumors. Thus, in order to
understand the association of vessel inflammation and TAM
infiltration in intrinsic tumor inflammation, the present study
specifically targeted surgically resected, procedure-naïve breast
tumor tissue samples collected between 1986 and 1988, when needle
biopsy was not yet broadly adopted as the standard of care.
The involvement of E-selectin in cancer has long
been recognized, as evidenced by histopathological studies;
however, its clinical implications have been controversial
(11,12,38). The
results of the present study revealed that 88.7% of procedure-naïve
breast tumors expressed E-selectin in the vessels within the tumor.
Previously, the prevalence of E-selectin-positive vessels has been
reported as 55.7% (n=113) (11) and
77.6% (n=22) (12) in frozen breast
tumor tissue sections. A previous study by Charpin et al
(11) demonstrated a positive
association between E-selectin expression level, and vascular cell
adhesion molecule-1, very late antigen-2 and CD44 expression
levels, and a negative association with E-cadherin expression
level. However, the latter was postiviely associated with
ER-negative breast cancer, which may be due to the release of
higher expression levels of IL-1 and TNF-α from ER-negative
compared with ER-positive breast cancer cells. The present study
and the study by Charpin et al (11) did not determine an association between
E-selectin expression and ER status, but both studies identified an
association between E-selectin, and CD68+ TAMs or
CD44+ immune infiltrates, a common marker for immune
cells.
TAMs are classified as either pro-inflammatory M1 or
pro-tumorigenic M2 macrophages (36),
although it is yet to be determined which of these is more
clinically important for prognosis (37,39).
Unlike T-lymphocytes, whose phenotypes are classified by
differentiation, the TAM phenotype is plastic and determined by its
surrounding microenvironment (40).
For example, the M1 phenotype can switch to M2 in response to T
helper 2-released cytokines, including IL-13 and IL-4 (41). Accordingly, the overall composition
and balance of immune subsets determines the pro-tumorigenic
potential and fate of the tumor. TAMs were present in all
procedure-naïve invasive breast carcinoma samples in the present
study and their spatial distribution pattern, as well as abundance,
differed among cases. TAMs were a predominant component near the
necrotic core, in adipose tissue adjacent to the tumor and in the
tumor stroma. Further investigation of TAM accumulation and
phenotypic distribution at different locations may improve the
understanding of their clinical implications.
E-selectin turnover is short, and it is shed into
the circulation as a circulating form of E-selectin, soluble
(s)E-selectin (42). sE-selectin has
been used as a surrogate marker for vessel inflammation since
sE-selectin expression levels appear to be associated with the
vascular E-selectin present on the surface of the endothelial cells
(43–48). For example, the sE-selectin expression
level was significantly higher among patients with metastatic
breast cancer compared with that of healthy counterparts (33.5 vs.
21.8 ng/ml; P<0.01), as well as in patients with liver
metastasis compared with those without (55.3 vs. 26.0 ng/ml;
P<0.0001). Thus, increased expression levels of sE-selectin were
associated with reduced overall survival in breast cancer (49). Similarly, pre-surgical sE-selectin
expression levels were higher in patients with colorectal cancer
(43 ng/ml) compared with patients with benign diseases (43 ng vs.
31 ng/ml) and were positively associated with carcinoembryonic
antigen tumor marker and poorer prognosis (both P<0.001)
(50). However, a study of microarray
data from 1,809 breast cancer patients with no previous treatment
history revealed that E-selectin expression level was associated
with longer survival times (HR, 0.67; CI 95%, 0.54–0.83; P=0.001)
(51). In the present study,
E-selectin expression level in breast tumor tissue samples was more
abundant in females >60 years compared with those ≤60. Elevated
E-selection expression level among elderly females may be
attributed to age-associated inflammation due to comorbidities,
since sE-selectin expression levels are reported to be high in
chronic inflammatory conditions, including arthritis (52,53),
diabetes (52), atherosclerosis
(54) and alcoholism (55). However, E-selectin expression level in
the tumor was not associated with OS following age adjustment in
the present study (data not shown). The survival implications of
E-selectin expression may require integration of area-specific
expression (tumor or necrosis vs. stroma), type of survival
(overall vs. disease specific) and comorbidity status. In
conclusion, tumor inflammation and E-selectin expression levels
were identified to be positively correlated with TAMs, and the
abundance of TAMs present in the tumor was an independent
prognostic factor in invasive breast tumors.
Acknowledgements
The present study was supported by the National
Institutes of Health (grant no. 1R01CA160271-01A1). The authors
would like to thank Lynsie Morris for the technical assistance
provided.
References
|
1
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: Cancer incidence and mortality worldwide: sources, methods
and major patterns in GLOBOCAN 2012. Int Agency Res Cancer.
2014.
|
|
4
|
Newman EA and Newman LA: Lymphatic mapping
techniques and sentinel lymph node biopsy in breast cancer. Surg
Clin North Am. 87353–364. (viii)2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Redig AJ and McAllister SS: Breast cancer
as a systemic disease: A view of metastasis. J Intern Med.
274:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rakoff-Nahoum S: Why cancer and
inflammation? Yale J Biol Med. 79:123–130. 2006.PubMed/NCBI
|
|
11
|
Charpin C, Bergeret D, Garcia S, Andrac L,
Martini F, Horschowski N, Choux R and Lavaut MN: ELAM selectin
expression in breast carcinomas detected by automated and
quantitative immunohistochemical assays. Int J Oncol. 12:1041–1048.
1998.PubMed/NCBI
|
|
12
|
Nguyen M, Corless CL, Kräling BM, Tran C,
Atha T, Bischoff J and Barsky SH: Vascular expression of E-selectin
is increased in estrogen-receptor-negative breast cancer: A role
for tumor-cell-secreted interleukin-1 alpha. Am J Pathol.
150:1307–1314. 1997.PubMed/NCBI
|
|
13
|
Müller AM, Weichert A and Müller KM:
E-cadherin, E-selectin and vascular cell adhesion molecule:
Immunohistochemical markers for differentiation between
mesothelioma and metastatic pulmonary adenocarcinoma? Virchows
Arch. 441:41–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Staal-van den Brekel AJ, Thunnissen FB,
Buurman WA and Wouters EF: Expression of E-selectin, intercellular
adhesion molecule (ICAM)-1 and vascular cell adhesion molecule
(VCAM)-1 in non-small-cell lung carcinoma. Virchows Arch.
428:21–27. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhaskar V, Law DA, Ibsen E, Breinberg D,
Cass KM, DuBridge RB, Evangelista F, Henshall SM, Hevezi P, Miller
JC, et al: E-selectin up-regulation allows for targeted drug
delivery in prostate cancer. Cancer Res. 63:6387–6394.
2003.PubMed/NCBI
|
|
16
|
Eichbaum MH, de Rossi TM, Kaul S and
Bastert G: Serum levels of soluble E-selectin are associated with
the clinical course of metastatic disease in patients with liver
metastases from breast cancer. Oncol Res. 14:603–610.
2004.PubMed/NCBI
|
|
17
|
Leek RD, Landers RJ, Harris AL and Lewis
CE: Necrosis correlates with high vascular density and focal
macrophage infiltration in invasive carcinoma of the breast. Br J
Cancer. 79:991–995. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tozeren A, Kleinman HK, Grant DS, Morales
D, Mercurio AM and Byers SW: E-selectin-mediated dynamic
interactions of breast- and colon-cancer cells with
endothelial-cell monolayers. Int J Cancer. 60:426–431. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsutsui S, Yasuda K, Suzuki K, Tahara K,
Higashi H and Era S: Macrophage infiltration and its prognostic
implications in breast cancer: The relationship with VEGF
expression and microvessel density. Oncol Rep. 14:425–431.
2005.PubMed/NCBI
|
|
20
|
Leek RD and Harris AL: Tumor-associated
macrophages in breast cancer. J Mammary Gland Biol Neoplasia.
7:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leek RD, Talks KL, Pezzella F, Turley H,
Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC and Harris AL:
Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha)
expression in tumor-infiltrative macrophages to tumor angiogenesis
and the oxidative thymidine phosphorylase pathway in human breast
cancer. Cancer Res. 62:1326–1329. 2002.PubMed/NCBI
|
|
22
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berg EL, Robinson MK, Mansson O, Butcher
EC and Magnani JL: A carbohydrate domain common to both sialyl
Le(a) and sialyl Le(X) is recognized by the endothelial cell
leukocyte adhesion molecule ELAM-1. J Biol Chem. 266:14869–14872.
1991.PubMed/NCBI
|
|
25
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luster AD, Alon R and von Andrian UH:
Immune cell migration in inflammation: Present and future
therapeutic targets. Nat Immunol. 6:1182–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Welply JK, Keene JL, Schmuke JJ and Howard
SC: Selectins as potential targets of therapeutic intervention in
inflammatory diseases. Biochim Biophys Acta. 1197:215–226. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zetter BR: Adhesion molecules in tumor
metastasis. Semin Cancer Biol. 4:219–229. 1993.PubMed/NCBI
|
|
29
|
Phillips ML, Nudelman E, Gaeta FC, Perez
M, Singhal AK, Hakomori S and Paulson JC: ELAM-1 mediates cell
adhesion by recognition of a carbohydrate ligand, sialyl-Lex.
Science. 250:1130–1132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Picker LJ, Kishimoto TK, Smith CW, Warnock
RA and Butcher EC: ELAM-1 is an adhesion molecule for skin-homing T
cells. Nature. 349:796–799. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimizu Y, Shaw S, Graber N, Gopal TV,
Horgan KJ, Van Seventer GA and Newman W: Activation-independent
binding of human memory T cells to adhesion molecule ELAM-1.
Nature. 349:799–802. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomas W: Catch bonds in adhesion. Annu
Rev Biomed Eng. 10:39–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hidalgo A, Peired AJ, Wild MK, Vestweber D
and Frenette PS: Complete identification of E-selectin ligands on
neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44.
Immunity. 26:477–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsuura N, Narita T, Hiraiwa N, Hiraiwa
M, Murai H, Iwase T, Funahashi H, Imai T, Takagi H and Kannagi R:
Gene expression of fucosyl- and sialyl-transferases which
synthesize sialyl Lewisx, the carbohydrate ligands for E-selectin,
in human breast cancer. Int J Oncol. 12:1157–1164. 1998.PubMed/NCBI
|
|
35
|
Lester SCI, Bose S, Chen YY, Connolly JL,
de Baca ME, Fitzgibbons PL, Hayes DF, Kleer C, O'Malley FP, Page
DL, et al: Protocol for the examination of specimens from patients
with invasive carcinoma of the breast. Arch Pathol Lab Med.
133:1515–1538. 2009.PubMed/NCBI
|
|
36
|
Martinez FO and Gordon S: The M1 and M2
paradigm of macrophage activation: Time for reassessment.
F1000Prime Rep. 6:132014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH,
Wang XZ, Zhao YW and Wei YQ: Prognostic significance of
tumor-associated macrophages in solid tumor: A meta-analysis of the
literature. PLoS One. 7:e509462012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei J, Cui L, Liu F, Fan Y, Lang R, Gu F,
Guo X, Tang P and Fu L: E-selectin and Sialyl Lewis X expression is
associated with lymph node metastasis of invasive micropapillary
carcinoma of the breast. Int J Surg Pathol. 18:193–200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016.PubMed/NCBI
|
|
40
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeNardo DG, Barreto JB, Andreu P, Vasquez
L, Tawfik D, Kolhatkar N and Coussens LM: CD4(+) T cells regulate
pulmonary metastasis of mammary carcinomas by enhancing protumor
properties of macrophages. Cancer Cell. 16:91–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pigott R, Dillon LP, Hemingway IH and
Gearing AJ: Soluble forms of E-selectin, ICAM-1 and VCAM-1 are
present in the supernatants of cytokine activated cultured
endothelial cells. Biochem Biophys Res Commun. 187:584–589. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cowley HC, Heney D, Gearing AJ, Hemingway
I and Webster NR: Increased circulating adhesion molecule
concentrations in patients with the systemic inflammatory response
syndrome: A prospective cohort study. Crit Care Med. 22:651–657.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fassbender K, Mössner R, Motsch L, Kischka
U, Grau A and Hennerici M: Circulating selectin- and
immunoglobulin-type adhesion molecules in acute ischemic stroke.
Stroke. 26:1361–1364. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gearing AJ, Hemingway I, Pigott R, Hughes
J, Rees AJ and Cashman SJ: Soluble forms of vascular adhesion
molecules, E-selectin, ICAM-1, and VCAM-1: Pathological
significance. Ann N Y Acad Sci. 667:324–331. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gearing AJ and Newman W: Circulating
adhesion molecules in disease. Immunol Today. 14:506–512. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koch AE, Turkiewicz W, Harlow LA and Pope
RM: Soluble E-selectin in arthritis. Clin Immunol Immunopathol.
69:29–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leeuwenberg JF, Smeets EF, Neefjes JJ,
Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ and Buurman WA:
E-selectin and intercellular adhesion molecule-1 are released by
activated human endothelial cells in vitro. Immunology. 77:543–549.
1992.PubMed/NCBI
|
|
49
|
Hebbar M, Révillion F, Louchez MM, Vilain
MO, Fournier C, Bonneterre J and Peyrat JP: The relationship
between concentrations of circulating soluble E-selectin and
clinical, pathological, and biological features in patients with
breast cancer. Clin Cancer Res. 4:373–380. 1998.PubMed/NCBI
|
|
50
|
Ferroni P, Roselli M, Spila A,
D'Alessandro R, Portarena I, Mariotti S, Palmirotta R, Buonomo O,
Petrella G and Guadagni F: Serum sE-selectin levels and
carcinoembryonic antigen mRNA-expressing cells in peripheral blood
as prognostic factors in colorectal cancer patients. Cancer.
116:2913–2921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gyorffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Çakar M, Balta Ş, Şarlak H, Akhan M,
Demirkol S, Karaman M, Ay SA, Kurt Ö, Çayci T, İnal S and Demirbaş
Ş: Arterial stiffness and endothelial inflammation in prediabetes
and newly diagnosed diabetes patients. Arch Endocrinol Metab.
59:407–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Klimiuk PA, Fiedorczyk M, Sierakowski S
and Chwiecko J: Soluble cell adhesion molecules (sICAM-1, sVCAM-1,
and sE-selectin) in patients with early rheumatoid arthritis. Scand
J Rheumatol. 36:345–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kvasnicka T, Kvasnicka J, Ceská R, Grauova
B and Vrablík M: Increasing plasma levels of soluble cell adhesion
molecules (sE-Selectin, sP-Selectin and sICAM-1) in overweight
adults with combined hyperlipidemia. Sb Lek. 102:473–477.
2001.PubMed/NCBI
|
|
55
|
Sacanella E, Estruch R, Badía E,
Fernández-Sola J, Nicolás JM and Urbano-Márquez A: Chronic alcohol
consumption increases serum levels of circulating endothelial
cell/leucocyte adhesion molecules E-selectin and ICAM-1. Alcohol
Alcohol. 34:678–684. 1999. View Article : Google Scholar : PubMed/NCBI
|